Abstract
This study evaluated the histopathological changes in gills and liver of Prochilodus lineatus inhabiting the Salado River basin. Fish were collected in four different sampling stations. The histological lesions in the tissues were examined under light microscopy and evaluated with quantitative analyses. The morphometric analysis of the gills showed a significant shortening of secondary lamellae and a lower percentage of area for gas exchange in fish from station 1 (an urban area, located near the mouth of the Salado River) in comparison with fish gills from the reference site (station 4, a relatively pristine area). Moreover, a significantly higher area occupied with necrotic foci and the occurrence of an important inflammatory response were observed in fish liver of station 1 than the samples caught from other stations. Thus, histopathological evidences showed differences among sites, which could be related to different environmental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to urban, industrial and agricultural activities, freshwater sources are dumped with different kinds of chemicals that affect the inhabiting biota. In order to evaluate the adverse effects of these complex chemical mixtures on aquatic organism, there is a worldwide trend to complement chemical and physical parameters with biomarkers in aquatic pollution monitoring (van der Oost et al. 2003; Au 2004).
The use of histopathological markers has already been tested and proposed as an efficient and sensitive method to monitor fish health and environmental pollution in natural water bodies (Au 2004; Ayas et al. 2007; Camargo and Martinez 2007; Costa et al. 2009, 2011; Leonardi et al. 2009). Such biomarkers might indicate acute or chronic exposure to contaminants and facilitate the detection of fish physiological responses, thereby establishing a more realistic diagnosis for evaluating environmental health (Silva et al. 2009).
The assessment of morphological changes in target organs is recommended as a useful tool to determine the effect of pollution in fish (Bernet et al. 1999). The gills and the gut are the main entry routes for toxic agents in teleost fish, which turns these organs important targets for such chemicals. The large respiratory surface of lamellae as well as the extensive epithelium outlining the filaments represents an important area of contact between animals and ambient water. This facilitates an efficient gas and ion respiratory exchange, but at the same time forms a large and sensitive target area for toxicants (Wendelaar Bonga and Lock 2008). Gills are highly susceptible to adverse environmental conditions, and damage in gill epithelia has been considered as a good indicator of xenobiotics effects on fish (Oropesa-Jiménez et al. 2005; Ballesteros et al. 2007; Sensini et al. 2008; Korkmaz et al. 2009).
On the other hand, teleost liver is the primary organ for biotransformation of organic xenobiotics, excretion of harmful trace metals, food digestion and storage, and metabolism of sex hormones (Health 1995; Hinton et al. 2001). Liver is highly affected by environmental pollution. As many toxic compounds tend to accumulate in this organ, the magnitude of liver exposure to contaminants is greater than that of the environment, or in other organs (Health 1995). Histo-cytopathological changes in livers of fish exposed to a wide range of organic compounds and heavy metals have been reported (Rabitto et al. 2005; Sarkar et al. 2005, Roy and Bhattacharya 2006; Mela et al. 2007).
The lower Salado River basin is a large area subjected to various sources of anthropogenic disturbances, receiving agricultural, industrial and domestic effluents. An eutrophication process and presence of heavy metal (cadmium, copper, chromium, lead) in water and sediment have been indicated for this basin (Gallo et al. 2006; Gagneten et al. 2007; Marchese et al. 2008).
Water quality of Salado River basin has been recently assessed by using physico-chemical analyses and multiple biomarkers (hematological, biochemical and physiological parameters) in the native fish Prochilodus lineatus (Cazenave et al. 2009).
According to this study, this river is characterized by a high conductivity, sulfates, turbidity and significant amounts of total dissolved solid, as well as high ammonia concentration in some sites. However, water quality assessment did not show marked differences among sampling sites, but biomarkers responses were key to contribute to discrimination of sites (i.e., biomarkers showed a clear separation among different studied areas) (Cazenave et al. 2009).
Prochilodus lineatus is a widely distributed neotropical fish, which represents a large part of the total ichthyomass of our aquatic systems (Bonetto et al. 1970). Its ecological characteristics (detritivorous), economic value for local fisheries and availability make it a sentinel species suitable for freshwater contamination biomonitoring (Colombo et al. 2000, 2007a, b; Camargo and Martinez 2006; Cazenave et al. 2009; Lombardi et al. 2010).
Our present work is aimed at assessing histopathological changes in gills and liver of P. lineatus collected from four distinct stations of the Salado River basin to establish the real health state of the organisms, which could reflect the environmental conditions of such aquatic system.
Materials and methods
Sampling stations and fish collection
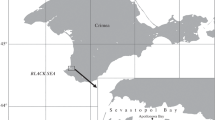
Four stations were selected in the Salado River basin, according to different land uses and the location of potential sources of pollutants (Fig. 1). Station 1 (31°31′S, 60°45′O) is an urban area located in the city of Santo Tomé, near the mouth of this river. Station 2 (31°22′S, 60°54′O) is located downstream of an industrial area, next the city of Esperanza. Station 3 (30°44′S, 60°37′O), is located near the city of San Justo, which is mainly an agricultural area. As reference site we selected El Bonete lagoon (station 4) (29°23′, 60°33′W), located in the upper portion of the basin, which is part of the Golondrinas–Calchaquí system, a tributary of the Salado River. This last site is a relatively pristine area, without industrial or urban influences, being extensive livestock the main activity of the region.
Two sampling of each station were carried out during non-reproductive season and during a dry hydrological season (May–August 2007). A total of 35 adult specimens (total length, 42.75 ± 2.58 cm; total weight, 1,207.29 ± 171.57 g) of P. lineatus were collected for histopathological analyses, using gill nets.
Histological preparation and assessment
After collection, fish were anesthetized (Parma de Croux 1990) and killed. Small pieces of livers and gills were immediately removed and fixed in 10% formalin fluid. Then, samples were washed with tap water and dehydrated through a graded series of ethanol, cleared in xylene, and embedded in paraffin. Paraffin sections of tissues were cut into 5 μm thickness and stained with hematoxylin and eosin (H&E).
The histopathological lesions in the tissues were examined in ten randomly selected sections of each tissue from each fish. All samples were examined under a light microscope (Nikon YS100), and photomicrographs were taken with a digital camera (Sony Cyber-shot) at its highest resolution (6.0 megapixels).
Photomicrographs of gill tissue were taken at random under medium-power magnification (100×) in order to carry out the following morphometric analysis: secondary lamellar length (SLL) and width (SLW), interlamellar distance (ID), and basal epithelial thickness (BET), according to Nero et al. (2006a). The proportion of the secondary lamellae available for gas exchange (PAGE) was averaged for each filament of an individual and calculated according to Nero et al. (2006a) as:
Photomicrographs of liver tissue were taken randomly under 400× magnification. Areas exhibiting melano-macrophage centers (MMCs), fibrosis and necrosis in each field were calculated.
Morphometric measurements in both tissues (i.e., length, area) were taken using the free UTHSCSA Image Tool program (developed at the University of Texas Health Science Center at San Antonio). The dimensions (in pixels) were calibrated using a common microscope stage graticule.
Histopathological condition of the gills and liver was estimated using a semiquantitative protocol proposed by Bernet et al. (1999) Firstly, gill and liver alterations were classified into four major reaction patterns: circulatory disturbances, regressive changes and progressive changes, and inflammation. Circulatory disturbances result from a pathological condition of blood and tissue fluid flow. Regressive changes are processes that terminate in a functional reduction or loss of an organ. Progressive changes are processes that lead to an increased activity of cells or tissues. Inflammatory changes are often associated with processes belonging to other reactions patterns.
Then, reaction and organ indexes were calculated based on two factors: the extension of a pathological change (score value) and its pathological importance (importance factor). Scores (1–6) were assigned based on the percentage of tissue exhibiting a certain alteration (occurrence for each alteration). For each alteration, an importance factor (1–3) was assigned, as proposed by Bernet et al. (1999), based on the biological significance of the lesion for fish health. The importance factor reflects the ability of the alteration to be reversible following removal of the stressor.
Reaction index of an organ was calculated by the sum of the multiplied importance factors and score values of the alteration of the corresponding reaction patterns. The sum of the four reaction indices of an organ is equivalent to the organ index (I org).
where org = organ (constant); rp = reaction pattern; alt = alteration; a = score value; and w = importance factor.
Statistical analysis
All data are reported as mean ± standard error. Morphometric data and indexes values were first tested for normality and homoscedasticity using Shapiro–Wilk’s and Levene’s tests, respectively. Comparison of each parameter among stations was performed using ANOVA (P < 0.05). Tukey’s posteriori test was used, where necessary, to distinguish among stations. When variables could not be normalized, the Kruskal–Wallis test was used.
Results
Gills
A number of histopathological differences were observed among fish caught in the four sampling stations. Prochilodus lineatus from station 1 had significantly shorter and thinner secondary lamellae than fish from other stations (Table 1). Secondary lamellae of fish from stations 2 and 3 were also shorter than lamellae of fish from reference site (station 4). In addition, fish gills from station 1 showed also lower percentage of area for gas exchange (PAGE) (Table 1).
The main histological alterations in gills of fish caught in the sampling stations are shown in Fig. 2. The observed pathological changes were classified into three reaction patterns: circulatory disturbances (hemorrhage, aneurysm, edema), regressive changes (epithelial lifting, lamellar disorganization, rupture of the pillar cells, bifurcation, desquamation) and progressive changes (hypertrophy of mucous cells and hyperplasia in both epithelium and supporting tissue).
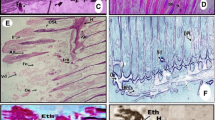
Cross-sections of gills of P. lineatus from the Salado River basin. a Normal gill structure showing primary and secondary lamellae without lesions; b hyperplasia of the epithelial cells, showing total (arrow) and partial fusion (arrow head) of gill lamellae; shortening of secondary lamellae is also showed; c hypertrophy of mucous cells (arrows); d epithelial lifting; e lamellar aneurysm (arrows)
The commonest anomalies found in fish gills from all sites were epithelial lifting, aneurysm and rupture of the pillar cells. Alterations such as lamellar disorganization, lamellar fusion and hyperplasia of lamellae and filament showed a higher prevalence in fish gills from stations 1, 2 and 3 (>80%) than those from station 4 (<25%). Additionally, alterations such as bifurcation were only detected in one fish from station 1.
In general, gill histopathological lesions were more frequent in fish collected from station 1, and less frequent in those caught in station 4. Similarly, total gill pathological index showed the same trend (Fig. 3), but no statistically significant differences were detected.
Liver
The main histopathological lesions recorded in the liver of fish from the Salado River basin are shown in Fig. 4. The observed changes were classified into three reaction patterns: circulatory disturbances (hemorrhage), regressive changes (plasma alterations, deposits, vacuolar degeneration, nuclear alteration, fibrosis, necrosis) and inflammatory processes (leukocyte infiltration, activation of macrophages).
Liver tissue from all examined fish showed a high prevalence of nuclear pyknosis (>70%) and vacuolar fatty degeneration (>60%). A high prevalence of necrosis (90%) was found in fish liver of station 1, while a medium one was observed in those from sites 2 and 3 (50 and 40%, respectively) and the lowest in station 4 (25%).
The area occupied with necrotic foci was significantly higher in fish livers from station 1 (Fig. 5). Besides, the occurrence of an important inflammatory response (leukocyte infiltration) was also observed in 90% of fish liver in this station. MMCs, normally present in fish liver, occupied larger areas in fish from sites 1, 2 and 3 than in site 4 (Fig. 5). On the other hand, fibrosis was evident in a few individuals from stations 1, 2 and 3 (prevalence of 10–40%), but it was not detected in liver of fish from the reference station. No significant differences of the calculated indices among sites were found in liver (Fig. 6).
Discussion
It is well known that biomarkers in fish may reflect the integrated effects of all impacts on water body, and can be used to compare relative changes in water quality from site to site, or over a time period (Friedrich et al. 1992). In the present study, histological biomarkers have been used to assess the health state of Prochilodus lineatus inhabiting the Salado River basin, which could reflect environmental conditions of the four sampling stations.
This river is characterized by hard waters, with high conductivity and suspended material. In addition, heavy metals (Cu, Cd, Cr, Pb) in water and sediments have been reported in areas next to Esperanza (station 2) and San Justo (station 3) (Gallo et al. 2006; Gagneten et al. 2007; Marchese et al. 2008). In a previous work, Cazenave et al. (2009) reported that most of physical and chemical parameters measured were within normal ranges reported for freshwater systems throughout the studied basin. However, parameters such as conductivity, sulfates, turbidity and dissolved solids showed values not optimal for aquatic life preservation. Besides, water samples at stations 1–3 showed also higher ammonia concentrations.
On the other hand, water quality of the Salado River basin was assessed using a set of biomarkers in P. lineatus, which included hematological and biochemical parameters, as well as detoxication and oxidative stress markers (Cazenave et al. 2009). Based on the biomarker responses, which were related to functions such as metabolism (glucose, total protein), defense (white blood cells, antioxidant enzymes) and detoxification (glutathione S-transferase) as well as evident oxidative damage (lipid peroxidation) in both gill and liver, it was possible to establish that fish at site 1 were living under stress. It was also evident that the health status of fish inhabiting stations 2 and 3 was rather different (worse) than that corresponding to station 4.
In line with these findings, our current results indicate that fish inhabiting the Salado River basin, mainly station 1 fish, can be negatively affected at tissue level by pollution.
The morphometric analysis of the gills showed a significant shortening of secondary lamellae in fish from stations 1, 2 and 3, in comparison with fish gills from the reference site (station 4). In addition, station 1 fish also showed a decreased gill surface area (PAGE, 26% reduction compared to the reference station) (Table 1), which would inevitably impair the normal physiological functions. Similar findings were observed in fish exposed to oil sands, naphthenic acids and endosulfan (Nero et al. 2006a, b; Ballesteros et al. 2007).
Moreover, a higher prevalence of lamellar disorganization, hyperplasia and lamellar fusion was observed in gills of stations 1, 2 and 3 fish, than in those from station 4. There were some cases where the hyperplasia was more severe, resulting in the complete fusion of some secondary lamellae (Fig. 2b). This response has been frequently observed in field studies (Evans et al. 2000; Cerqueira and Fernandes 2002; Triebskorn et al. 2002; Moissenko et al. 2005; Camargo and Martinez 2007) and after exposure to different toxicants (Guimarães et al. 2006; Mishra and Mohanty 2008; Álvarez-Muñoz et al. 2009; Korkmaz et al. 2009). Despite an uncontrolled hyperplasia of the gill lamellae and filaments increases the diffusion distance between the respiratory blood and waterborne xenobiotics, it also increases the respiratory blood–dissolved oxygen distance for gaseous exchange due to the decreased surface area of the secondary lamellae (Banerjee 2007). Therefore, these defense responses can take place in detriment of the respiratory efficiency, which eventually will outweigh any protective effect against the uptake of pollutants (Cengiz 2006).
The observed gill histopathological changes in fish from the Salado River basin are, in general, responsive but non-specific to pollutant exposure. The literature indicates that a variety of toxicants (organochlorates, polycyclic aromatic hydrocarbons, organophosphate compounds, carbamates, miscellaneous herbicides, acidification, nitrogenous compounds and heavy metal salts) in the aquatic environment can affect gill structure and function (Metcalfe 1998; Wood 2001; Elahee and Bhagwant 2007; Sensini et al. 2008). Gill pathologies can result in the suppression or inhibition of physiological function, irrespective of whether the pathologies are caused by chemical, physical or secondary parasitic irritation (Schlacher et al. 2007). So, respiratory, osmoregulatory and excretory impairments are possible consequences, particularly if the reaction is diffuse, affecting the entire gill (Ferguson 1989).
On the other hand, the main hepatic lesions observed in the present study were nuclear pyknosis, fatty degeneration, leukocyte infiltration, necrosis and fibrosis. Some of these pathologies were mainly observed in stations 1, 2 and 3 fish livers. Particularly, a high incidence of necrosis (estimated by both prevalence and occupied area) was observed in fish from station 1. The incidence of necrosis in the liver is generally typical in multiple-contaminant exposure, as described by other researchers in this field (Evans et al. 2000; Oliveira Ribeiro et al. 2002; Au 2004; Moissenko et al. 2005; Miranda et al. 2008; Korkmaz et al. 2009; Costa et al. 2011). According to some authors, the occurrence of necrosis is strongly associated with oxidative stress (Li et al. 2000; Avci et al. 2005) and is also a consequence of enzymatic inhibition, damages in the cellular membrane integrity and disturbances in the synthesis of proteins and carbohydrate metabolism (Mela et al. 2007). In line with these studies, Cazenave et al. (2009) reported a significant induction of hepatic oxidative stress markers as well as high levels of plasmatic protein and glucose mainly in P. lineatus from station 1, which could be associated with the high incidence of necrosis and a hepatic metabolic disorder, respectively. Additionally, a significant higher white blood cell count in P. lineatus from site 1 was reported (Cazenave et al. 2009), which may be attributed to an increased leukocyte mobilization to protect the body against infection in necrotic tissues.
In some areas, damaged tissue was accompanied by leukocyte infiltration and proliferation of connective tissue (fibrosis). Similar findings have been reported in field and laboratory studies (Takashima and Hibiya 1995; Moissenko et al. 2005; Rios et al. 2007; Miranda et al. 2008).
Furthermore, fish livers from stations 1, 2 and 3 showed an increased area occupied with MMCs. A higher presence of MMCs, as observed in the livers of fishes in other studies (Pacheco and Santos 2002; Camargo and Martinez 2007; Rios et al. 2007; Costa et al. 2011), is generally related to important hepatic lesion, such as degenerative and necrotic processes. The function of the MMCs in the liver of fishes remains uncertain, but some studies have suggested that it is related to destruction, detoxification or recycling of endogenous and exogenous compounds (Haaparanta et al. 1996).
Since liver is the primary organ for metabolism, detoxification of xenobiotics and excretion of harmful substances, unfavorable consequences to liver physiology may be expected in fish from the sampling stations.
It should be emphasized that all the histopathological lesions observed in gill and liver of P. lineatus are responsive to a variety of stressors (at least more than one) and are therefore only indicative of the general quality of the environment rather than specific types of pollutants (Au 2004). In biomonitoring programs, the integrated analysis of both histopathological and biochemical endpoints facilitate the observation of the physiological response in individuals, thereby establishing a more realistic diagnosis for evaluating environmental health (Akaishi et al. 2004; Ayas et al. 2007). Histopathological analyses of liver and gills of P. lineatus corroborate with the previous findings on biochemical markers (Cazenave et al. 2009), which revealed differences in the health state of fish among stations with clear signs of stress and pathology. According to both previous and present results, P. lineatus inhabiting station 1 may be clearly unhealthy. So, both histopathological and biochemical markers in P. lineatus should indicate that station 1 of Salado River shows the worse environmental conditions for fish health, while stations 2 and 3 are rather worse than the corresponding reference site (station 4). Thus, the use of biochemical markers together with histological parameters is a very realistic approach that was successfully applied in the Salado River basin for environmental monitoring.
References
Akaishi FM, Silva de Assis HC, Jakobi SCG, St-Jean S, Couternay SC, Lima E, Wagener ALR, Scofield AL, Oliveira Ribeiro CA (2004) Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Arch Environ Contam Toxicol 46:244–253
Álvarez-Muñoz D, Gómez-Parra A, Blasco J, Sarasquete C, González-Mazo E (2009) Oxidative stress and histopathology damage related to the metabolism of dodecylbenzene sulfonate in Senegaleses ole. Chemosphere 74:1216–1223
Au DWT (2004) The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 48:817–834
Avci A, Kaàmaz M, Durak I (2005) Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicol Environ Saf 460:101–105
Ayas Z, Ekmekci G, Ozmen M, Yerli SV (2007) Histopathological changes in the livers and kidneys of fish in Sariyar Reservoir, Turkey. Environ Toxicol Pharmacol 23:242–249
Ballesteros ML, Bianchi GB, Carranza M, Bistoni MA (2007) Endosulfan acute toxicity and histomorphological alterations in Jenynsia multidentata (Anablepidae, Cyprinodontiformes). J Environ Sci Health 42B:351–357
Banerjee TK (2007) Histopathology of respiratory organs of certain air-breading fishes of India. Fish Physiol Biochem 33:441–454
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34
Bonetto C, Cordiviola de Yuan E, Pignalberi C (1970) Nuevos datos sobre poblaciones de peces en ambientes leníticos permanentes del Paraná Medio. Physis 30:141–154
Camargo MMP, Martinez CBR (2006) Biochemical and physiological biomarkers in Prochilodus lineatus submitted to in situ tests in an urban stream in southern Brazil. Environ Toxicol Pharmacol 21:61–69
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a neotropical fish caged in an urban stream. Neotrop Ichthyol 5(3):327–336
Cazenave J, Bacchetta C, Parma MJ, Scarabotti PA, Wunderlin DA (2009) Multiple biomarkers responses in Prochilodus lineatus allowed assessing changes in the water quality of Salado River basin (Santa Fe, Argentina). Environ Pollut 157:3025–3033
Cengiz EI (2006) Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharmacol 22:200–204
Cerqueira CCC, Fernandes MN (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol Environ Saf 52:83–91
Colombo JC, Bilos C, Remes Lenicov M, Colautti D, Landoni P, Brochu C (2000) Detritivorous fish contamination in the Río de la Plata estuary: a critical accumulation pathway in the cycle of anthropogenic compounds. Can J Fish Aquat Sci 57:1139–1150
Colombo J, Cappelletti N, Migoya M, Speranza E (2007a) Bioaccumulation of anthropogenic contaminants by detritivorous fish in the Rio de la Plata estuary: 1-aliphatic hydrocarbons. Chemosphere 68:2128–2135
Colombo J, Cappelletti N, Migoya M, Speranza E (2007b) Bioaccumulation of anthropogenic contaminants by detritivorous fish in the Rio de la Plata estuary: 2-polychlorinated biphenyls. Chemosphere 69:1253–1260
Costa PM, Diniz MS, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Vale C, DelValls TA, Costa MH (2009) Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: a weighted indices approach. Aquat Toxicol 92:202–212
Costa PM, Caeiro S, Lobo J, Martins M, Ferreira AM, Caetano M, Vale C, DelValls TA, Costa MH (2011) Estuarine ecological risk based on hepatic histopathological indices from laboratory and in situ tested fish. Mar Pollut Bull 62:55–65
Elahee KB, Bhagwant S (2007) Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol Environ Saf 68:361–371
Evans CW, Hills JM, Dickson JMJ (2000) Heavy metal pollution in Antarctica: a molecular ecotoxicological approach to exposure assessment. J Fish Biol 57:8–19
Ferguson HW (1989) Systemic pathology of fish. A text and atlas of comparative tissue responses in diseases of teleosts. Iowa State University Press, Ames
Friedrich G, Chaapman D, Beim A (1992) The use of biological material. In: Chapman D (ed) Water quality assessments. A guide to the use of biota, sediments and water in environmental monitoring. Chapman & Hall, London
Gagneten AM, Gervasio S, Paggi JC (2007) Heavy metal pollution and eutrophication in the Lower Salado River Basin (Argentina). Water Air Soil Pollut 178:335–349
Gallo M, Trento A, Alvarez A, Beldoménico H, Campagnoli D (2006) Dissolved and particulate heavy metals in the Salado River (Santa Fe, Argentina). Water Air Soil Pollut 174:367–384
Guimarães ATB, Silva de Assis HC, Boeger W (2006) The effect of trichlorfon on acetylcholinesterase activity and histopathology of cultivated fish Oreochromis niloticus. Ecotoxicol Environ Saf 68:57–62
Haaparanta A, Tellervo Valtonen E, Hoffmann R, Holmes J (1996) Do macrophages centres in freshwater fishes reflect the differences in water quality? Aquat Toxicol 34:253–272
Health AG (1995) Water pollution and fish physiology, 2nd edn. CRC Lewis Publishers, Boca Ratón
Hinton DE, Segner H, Braunbeck T (2001) Toxic responses of the liver. In: Schlek D, Benson WH (eds) Target organ toxicity in marine and freshwater teleosts. Taylor & Francis, Boca Raton, pp 224–268
Korkmaz N, Cengiz EI, Unlu E, Uysal E, Yanar M (2009) Cypermethrin-induced histopathological and biochemical changes in Nile tilapia (Oreochromis niloticus), and the protective and recuperative effect of ascorbic acid. Environ Toxicol Pharmacol 28:198–205
Leonardi M, Tarifeño E, Vera J (2009) Diseases of the Chilenean flounder, Paralichthys adspersus (Steindachner, 1867), as a biomarker of marine coastal pollution near the Itata River (Chile): part II. Histopathological lesions. Arch Environ Contam Toxicol 56:546–556
Li Q, Yeo MH, Tan BK (2000) Lipid peroxidation in small and large phospholipid unilamellar vesicles induced by water-soluble free radical sources. Biochem Biophys Res Commun 273:72–76
Lombardi PE, Peri SI, Verrengia Guerrero NR (2010) Trace metal levels in Prochilodus lineatus collected from the La Plata River, Argentina. Environ Monit Assess 160:47–59
Marchese M, Rodriguez AR, Pave PJ, Carignano MR (2008) Benthic invertebrates structure in wetlands of a tributary of the middle Parana River (Argentina) affected by hydrologic and anthropogenic disturbances. J Environ Biol 29:343–348
Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68:426–435. doi:10.1016/j.ecoenv.2006.11.013
Metcalfe CD (1998) Toxicopathic responses to organic compounds. In: Leatherland JF, Woo PTK (eds) Fish diseases and disorders, vol 2, Non-infectious disorders. CABI Publishing, New York, pp 133–162
Miranda AL, Roche H, Randi MAF, Menezes ML, Oliveira Ribero CA (2008) Bioaccumulation of chlorinated pesticides and PCBs in the tropical freshwater fish Hoplias malabaricus: histopathological, physiological, and immunological findings. Environ Int 34:939–949
Mishra AK, Mohanty B (2008) Acute toxicity impacts of hexavalent chromium on behavior and histopathology of gill, kidney and liver of the freshwater fish, Channa punctatus (Bloch). Environ Toxicol Pharmacol 26:136–141
Moissenko TI, Gashkina NA, Sharova YN, Pokoeva AG (2005) Ecotoxicological assessment of after-effects of the Volga River water contamination. Water Resources 32(4):369–383
Nero V, Farwell A, Lister A, Van Der Kraak G, Lee LEJ, Van Meer T, MacKinnon MD, Dixon DG (2006a) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377
Nero V, Farwell A, Lee LEJ, Van Meer T, MacKinnon MD, Dixon DG (2006b) The effects of salinity on naphthenic acid toxicity to yellow perch: Gill and liver histopathology. Ecotoxicol Environ Saf 65:252–264
Oliveira Ribeiro CA, Schatzmann M, Silva de Assis HC, Silva PH, Pelletier E, Akaishi FM (2002) Evaluation of tributyltin subchronic effects in tropical freshwater fish (Astyanax bimaculatus, Linnaeus, 1758). Ecotoxicol Environ Saf 51:161–167
Oropesa-Jiménez AL, García-Cambero JP, Gómez-Gordo L, Roncero-Cordero V, Soler-Rodríguez F (2005) Gill modifications in the freshwater fish Cyprinus carpio after subchronic exposure to simazine. Bull Environ Contam Toxicol 74:785–792
Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53:331–347
Parma de Croux MJ (1990) Benzocaine (Ethyl-p-Aminobenzoate) as an anaesthetic for Prochilodus lineatus, Valenciennes (Pisces, Curimatidae). J Appl Ichthyol 6:189–192
Rabitto IS, Alves Costa JRM, Silva de Assis HC, Pelletier É, Akaishi FM, Anjos A, Randi MAF, Oliveira Rivero CA (2005) Effects of dietary Pb(II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotoxicol Environ Saf 60:147–156
Rios FS, Donatti L, Fernandes MN, Kalinin AL, Rantin FT (2007) Liver histopathology and accumulation of MMCs in Hoplias malabaricus after long-term food deprivation and re-feeding. J Fish Biol 71:1393–1406
Roy S, Bhattacharya S (2006) Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol Environ Saf 65:218–229
Sarkar B, Chatterjee A, Adhikari S, Ayyappan S (2005) Carbofuran- and cypermethrin-induced histopathological alterations in the liver of Labeo rohita (Hamilton) and its recovery. J Appl Ichthyol 21:131–135
Schlacher TA, Mondon JA, Connolly RM (2007) Estuarine fish health assessment: Evidence of wastewater impacts based on nitrogen isotopes and histopathology. Mar Pollut Bull 54:1762–1776
Sensini C, Della Torre C, Corsi I, Focardi S (2008) First observations of histopathological effects of 2,4,6-trinitrotoluene (TNT) in gills of European eel Anguilla anguilla (Linnaeus, 1758). Cell Biol Toxicol 24(6):621–628
Silva CA, Oliveira-Rivero CA, Katsumiti A, Araújo MLP, Zandoná EM, Costa Silva GP, Maschio J, Roche H, Silva de Assiss HC (2009) Evaluation of waterborne exposure to oil spill 5 years after an accident in Southern Brazil. Ecotoxicol Environ Saf 73:400–409
Takashima F, Hibiya T (1995) An atlas of fish histology. Normal and pathological features, 2nd edn. Kodansha LTD, Tokyo
Triebskorn R, Adam S, Casper H, Honnen W, Pawert M, Schramm M, Schwaiger J, Köhler H (2002) Biomarkers as diagnostic tools for evaluating effects of unknown past water quality conditions on stream organisms. Ecotoxicology 11:451–465
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wendelaar Bonga SE, Lock RAC (2008) The osmoregulatory system. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, Boca Raton, pp 401–416
Wood CM (2001) Toxic responses of the gills. In: Schlek D, Benson WH (eds) Target organ toxicity in marine and freshwater teleosts. Taylor & Francis, Boca Raton, pp 1–89
Acknowledgments
This work was supported by grants from Agencia Nacional de Promoción Científica y Técnica (FONCYT/PICT-1764), Consejo de Investigaciones y Técnicas (CONICET), Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba and Universidad Nacional del Litoral (CAID-UNL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Troncoso, I.C., Cazenave, J., Bacchetta, C. et al. Histopathological changes in the gills and liver of Prochilodus lineatus from the Salado River basin (Santa Fe, Argentina). Fish Physiol Biochem 38, 693–702 (2012). https://doi.org/10.1007/s10695-011-9551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9551-7