Abstract
This study examined the effects of a probiotic, protexin, on the growth performance and hematological parameters in an ornamental fish, the Oscar Astronotus ocellatus fingerlings. A completely randomized experimental design was applied with three experimental diets each with three replicates. A commercial food, BioMar, was supplemented with protexin at levels of 0.15, 0.5, and 1.0 g kg−1 dry food and fed three times a day for 60 days. The control diet was prepared with no protexin supplementation. The experimental fish were biometried every 15 days to compare their growth rates at each treatment. For hematological assays, blood samples were prepared every 30 days to measure such parameters as red and white blood cells, hemoglobin, hematocrit, and percentages of lymphocytes, monocytes, neutrophiles, basophiles, and eosinophiles. Based on the results, the fingerlings fed a 0.15 g kg−1 supplemented food were significantly different from the fish in the other treatments and in the control, with the highest mean of both final weight (35.07 ± 1.19) and body weight gain (30.17 ± 1.08). Significant differences in both hemoglobin concentration and mean red and white blood cells were found between the experimental groups and the control within 2 months. The highest hemoglobin concentration and also red and white blood cells was observed in the fish-fed 0.15 dietary protexin in both months. The results of this study show that the probiotic, protexin, at a level of 0.15 g kg−1 dry food could have measurable effects on the growth and hematological parameters in the Oscar A. ocellatus fingerlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivation of ornamental fish species, in addition to the aquaculture of food fish species, has gained ground nowadays, and research projects in this field have become of great interest. Since the economic importance of aquarium fish is not less than that of the food fish, it is, therefore, important to investigate various aspects of their cultivation including growth and survival as well as ways of increased resistance of ornamental fish against diseases. One important aspect of aquaculture is nutrition, to which special attention should be paid by fish farmers as it accounts for a high portion of cultivation expenses. By applying the knowledge of nutrition, aquaculturists try to enhance such factors as reduction in feed conversion ratio, increase in growth rate, immunology level and disease resistance, and fish survival, all of which will ultimately result in a high economic efficiency.
Science of nutrition has always been in progress and research in this field presents at times new and valuable findings, which are beneficial for consumers and also contribute to the public health. With respect to this, an important scientific finding is achievement of the probiotics. The first definition of the word “probiotic” by Parker (1974) describes them as organisms and substances that establish a balance in the intestine microbial flora. Other refined interpretations of probiotics include: live, food-additive microbes which are useful to the host because of a balance in the intestinal microbial flora (Fuller 1989) and functional bacteria causing a stability in the intestinal microbial flora (Wang et al. 2008a). Moreover, probiotics produce digestive enzymes that, by promoting food assimilation, cause a higher utilization of ingested food and eventually result in an enhanced growth and production (Bairagi et al. 2002; Ramiez and Dixon 2003). Hence, they can be appropriately substituted for antibiotic growth accelerators, the consumption of which is being limited (Irianto and Austin 2002a; Wang et al. 2008a). In teleost fish, morphological and functional analyses of alimentary canal have revealed the role of gut microbiota in the health and growth of the fish (Dimitroglou et al. 2011).
Besides recognition of the physiology of a fish species, identification of the hematological parameters is a specific, distinguishing characteristic of any species. This is not only important in species identification but can also be practical in disease recognition and as a determining tool for health status of fish. Blood is an important biological fluid tissue, the composition of which is affected by a variety of physiological and pathological situations. Accordingly, data on natural levels and the quality of changes in hematological parameters are important for recognizing a number of diseases (Jain 1986).
The application of probiotics as growth accelerators in the farmed food fish as well as for the improvement of disease resistance of these fishes has been frequently investigated (Wang et al. 2008a; Gatesoupe 1994; Gildberg et al. 1997; Abd El-Rhman et al. 2009; Avella et al. 2010a; Dimitroglou et al. 2011). The ornamental fish, on the other hand, have been rarely studied in that regard (Gosh et al. 2007, 2008; Avella et al. 2010b). The Oscar A. ocellatus (family Cichlidae) has been turned out as a popular ornamental fish due to its unique beauty and colorfulness. Being a carnivore, this species is a predator hunting small fish, insects, and aquatic invertebrates in nature (Firouzbakhsh and Aliasghari 2009). Oscar is considered as an ever-hungry fish showing interest in food uptake even at satiation. Therefore, reductions in feed intake as well as the farming period to attain desired weight and ultimately decreasing the fish-farming costs were the incentives in the study of this species.
The use of multi-species probiotics as feed complements is probably more influential than mono-species probiotics in terms of more diversity in antimicrobial compounds and higher adhesion on the gut mucus (Lahtinen and Ouwehand 2009; Nayak 2010). It has recently been shown that a mixture of Bacillus probiotics has significantly increased growth rate and body weight in the sea bream Sparus aurata (Avella et al. 2010a). Protexin, a commercial probiotic used in this study, is a multi-species probiotic, consisting mainly of a variety of lactic acid bacteria and also yeast and fungi species. This investigation aims at study of the effects of different dietary levels of the probiotic protexin on growth response and likely changes in hematology and blood cell indices as well as on differential count of white blood cells in an ornamental fish, the Oscar A. ocellatus fingerling.
Materials and methods
The fish
The Oscar A. ocellatus fingerlings with mean initial weights of 4.92 ± 0.12 g were obtained from a local center for ornamental fish propagation located in the study area (Sari, Iran). The fingerlings, packed in water-filled, aerated plastic bags, were transferred to an aquarium room at the Sari Agricultural Sciences and Natural Resources University, Sari, Iran. For adaptation purposes, the fingerlings were reared in the new aquarium conditions for 2 weeks.
Water parameters
The rearing water was supplied from a well. Water temperature (28 ± 0.5°C) was provided by an automatic heater in each aquarium (55 (L) × 40 (W) × 50 (H) cm). The aquaria were aerated by a central pump. To control water quality during the experiment, water temperature and dissolved oxygen were measured daily, and ammonia, hardness, and pH were determined weekly (Table 1). To maintain water quality throughout the study, an under-gravel filter was used, and 25% of the water was replaced once every 3 days.
The probiotic
In this study, a probiotic with the trade name, protexin (Probiotics International Ltd., Lopen Head, Somerset, TA13 5JH, UK) was used. Protexin is a multi-strain probiotic, consisting of seven bacteria species and two species of yeasts and fungi (Table 2).
The experimental design
The fish were divided into four experimental groups with three replicates for 60 days. The Oscar fingerlings were completely random partitioned into the aquaria (12 fingerlings per aquarium). A commercial food, BioMar (BioMar SAS, 60, Rue Pierre-Georges Debouchaud, Zone Industrielle, FR-16440 Nersac, France) was fed to the fingerlings based on 4% of their biomass (Ghosh et al. 2008) at three intervals (6 a.m., 12, and 6 p.m.). After the 2-week adaptation period, all groups were fed daily lasting for 60 days. The dietary protexin was supplemented at levels of 0.15 (T 1), 0.5 (T 2), and 1.0 (T 3) g kg−1 dry food for the three experimental groups. The control (C) group received no protexin supplement. Different levels of protexin sprayed into the diets (10 ml of sterile distilled water kg−1); the diets were air-dried under a sterile hood for 1 h and stored at 4° C until use. The control diet did not receive any protexin during spraying.
Estimation of growth criteria
In order to analyze the growth indices of the Oscar fingerlings and to compare effects of the different treatments, the fingerlings in all groups were biometried once every 15 days during the 60-day experiment. The fingerlings were weighed by a digital scale (bearing: 0.01 mg) after they had been anesthetized using Tricaine Methanesulfonate (0.1 ppm). The average weight of the fingerlings was then calculated. Based on the results of the biometry, the daily ration of the fish in the supplemented groups and in the control was determined. Some of the growth parameters were also measured by the following equations:
Measuring hematological parameters
Blood samples were prepared at two times, in the end of the 1st and 2nd month of the experiment. For each preparation, 16 fingerlings were randomly selected from each group and anesthetized, and then, blood samples were taken through dissecting their peduncles. Red and white blood cells (RBC & WBC) diluted using the Natt & Herrick’s stain solution were counted under a Neubaur hemocytometer (Bullis 1993). Hemoglobin was assessed by a spectrophotometer (unico UV-2150) at 540 nm through the method cyanmethemoglobin (Drobkin 1945). Hematocrit was measured by the procedure microcentrifuge with heparinated tubes (Svetina et al. 2002). Differential recognition of the WBC was based on blood films preparation and Gimsa staining. Blood cell indices (MCH, MCV, and MCHC) were assessed according to the following standard calculations (Campbell and Ellia 2007):
Statistical analysis
One-way ANOVA was applied to analyze the data by the SPSS (Ver. 17) software. The average values were compared using the LSD test at 5% confidence.
Results
Growth parameters
Table 3 shows the measured growth parameters of the Oscar fingerlings fed the dietary protexin for 60 days. The experimental groups (T 1, T 2, and T 3) were significantly different (P < 0.05) in both average final weights and weight gains from the control fish. The highest averages of final weight and weight gain were observed in the T 1; it was statistically different (P < 0.05) from T 2, T 3, and the control fingerlings. The T 1 also showed significant difference (P < 0.05) in reduction in FCR compared to T 2, T 3, and the control, whereas this parameter was not significantly different (P > 0.05) between T 2 and T 3, and also in comparison with the control. Specific growth rate was not different (P > 0.05) between the experimental groups T 2 and T 3, but the differences between the T 2 and T 3 and between each of these groups versus the control were statistically significant (P < 0.05). The T 1 showed the highest SGR, which was significant (P < 0.05) compared to the control and also to the dietary-supplemented groups.
Hematological factors
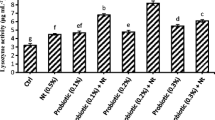
Tables 4 and 5 present the hematological factors and differential counting of leukocytes, respectively, found in the Oscar fingerlings supplemented various levels of dietary protexin. All protexin-supplemented groups were different from the control (P < 0.05) in hemoglobin density and numbers of both RBC and WBC within the 2 months of the study period. The highest hemoglobin density within months 1 and 2, RBC and WBC was observed in the T 1.
In the 1st month, hematocrit increased in the T 1, and in the 2nd month, both T 1 and T 2 showed an increased hematocrit, all of which differed statistically from the control (P < 0.05). The T 1 was the only group in the 1st month that showed a significant rise (P < 0.05) in MCV compared to the control and even to the T 2 and T 3; the latter groups were not different from the control in the 1st month. In the 2nd month, all groups were different in MCV content from each other and also from the control (P < 0.05). The results of differential counting of leukocytes (Table 5) showed significant rise (P < 0.05) in lymphocyte numbers in all groups compared to the control. Among the experimental groups, the T 1 presented the highest count of lymphocytes in comparison with the control in months 1 and 2. However, the percentages of both monocytes and neutrophils showed a marked decrease (P < 0.05) in the experimental fingerlings compared to the control within months 1 and 2.
Discussion
The effects of probiotics on growth parameters have been studied in a variety of farmed fish and other aquatic species (Douillet and Langdon 1994; Ghosh et al. 2003; Carnevali et al. 2004; Wang et al. 2008b; Abdel-tawwab et al. 2008; Abd El-Rhman et al. 2009; Avella et al. 2010a; Dimitroglou et al. 2011). Nonetheless, research on the effects of probiotics upon growth and hematology parameters of ornamental fish is very scarce (Gosh et al. 2008; Avella et al. 2010b). A relatively large body of literature is concerned with the effects of various probiotic microorganisms on both terrestrial and aquatic animals; yet, lactic acid bacteria (LAB) and bacilli have received the highest number of scientific documents (Dimitroglou et al. 2011). It has been shown that Lactobacillus probiotics enhance growth and survival, for instance, in the farmed rainbow trout Oncorhynchus mykiss (Kesarcodi-Watson et al. 2008). Similarly, Carnevali et al. (2004) found that Lactobacillus fructiorance and L. plantarum could considerably increase growth of the sea bream S. aurata. Carnevali et al. (2006) also noticed that, when the European sea bass juveniles (Dicentrarchus labrax L.) were fed on Lactobacillus delbrueckii for 59 days, it showed elevated body weight; the weight gain appeared after 25 days when compared with the control.
The impact of lactic acid from a bacterial probiotic on the Atlantic cod Gadus morhua fingerlings was investigated by Gildberg et al. (1997); they reported a significant decrease in FCR. In the present study, a protexin supplement of 0.15 g kg−1 dry food (group T 1) resulted in a significant decrease in FCR in comparison with other treatments and also the control. In the same way, use of the probiotic Bacillus subtilis efficiently raised growth and survival rate with significantly diminished FCR in live-bearing ornamental fishes (Gosh et al. 2008). Avella et al. (2010a) also demonstrated that a combination of three Bacillus species (B. subtilis, B. licheniformis, and B. pumilus) fed both via rotifer and Artemia nauplii and through addition to the water could significantly influence the growth rate and body weight at larval and juvenile stages of the sea bream S. aurata. The findings of this study confirm a growth preference together with reduced FCR in the Oscar fingerlings-fed dietary protexin at 0.15 g kg−1 dry food than at 0.5 and 1.0 g kg−1 dry food.
The influence of probiotics on enzymatic activities and, as a result, on increased digestive process is emphasized in some studies (Jafarian et al. 2007; De Silva and Anderson 1995). Many probiotic bacteria contain extracellular enzymes such as amylase, lipase, and protease that, through stimulating appetite and enhancing microbial metabolism, promote host feeding (Gildberg et al. 1997). By increasing digestibility and enhanced absorption of ingested food, these bacteria increase feeding efficiencies leading to a high growth in fish (Gatesoupe 1999). Through provision of essential nutrients (such as vitamins and short-chain fatty acids) and enzymes, probiotics can promote food digestion and absorption (Gatesoupe 1999). Elevated digestibility, therefore, together with antibacterial properties of probiotics may entrain survival and increased fish weights (Gosh et al. 2008). Accordingly, it can be concluded that the improved values of body weight, FCR, and SGR of the protexin-fed fish in this study may have arisen from the useful effects mentioned above.
Considerable increase in fish performance and durability due to supplementation of probiotics (Ghosh et al. 2003; Hevroy et al. 2005) might be because of either killing harmful bacteria by useful (probiotic) bacteria, or secretion of compounds such as bacteriocins, which prevent growth of other microorganisms (Kesarcodi-Watson et al. 2008). Altogether, according to the results of this study, it can be concluded that addition of the probiotic protexin as a dietary complement with an effective dose of 0.15 g kg−1 dry food efficaciously improves growth performance in A. ocellatus fingerlings.
It has been noted that benefiting from appropriate probiotic species with adequate dosage to be of great importance in order to avoid either overdose or lower dose ultimately leading to economic loss (Lee 2009); these consequences may also depend on the fish species (Nayak 2010). Although most of related studies have utilized different doses of probiotics with contrasting consequences (e.g., 109 & 1012 cfu/g/feed: Nikoskelainen et al. 2001; 106, 108 & 1010 cfu/kg/feed: Son et al. 2009), the rationale for which are not definitely provided (Gosh et al. 2008). The present study also applied different concentrations of protexin with the lowest dose showing the best growth performance in the Oscar fingerlings. Altogether, it seems to be the only point to note that the highest doses consumed would not necessarily result in the best performances. And the required concentrations of probiotics in order to induce favorable effects and to prevent diseases in various fish species at differing situations remain to be further questioned.
Hematology parameters are considered as proper indices for tracking health status of fish and their response to environmental stresses (Schuett et al. 1997). The impacts of nutrition and food additives on blood factors have been questioned in a number of studies (Rawling et al. 2009; Merrifield et al. 2010). In this study, the hematological analyses of A. ocellatus fingerlings fed a dietary protexin complement of 0.15 g kg−1 dry food signified marked changes (P < 0.05) in hematological parameters as opposed to the control fish. The dietary supplementations of 0.5 and 1.0 g protexin kg−1 dry food also represented non-significant differences compared to the control (P > 0.05). In the same way, Abd El-Rhman (2009) observed a rise in the number of RBC in Nile tilapia following the use of Micrococcus luteus showing no significant difference as opposed to the control. On the other hand, the use of probiotic in rainbow trout caused significant increase in the number of RBC (Irianto and Austin 2002b).
Major hematological changes in the protexin-supplemented group with 0.15 g kg−1 dry food include statistically noticeable rise of total numbers of RBC, WBC, and hemoglobin during the 60-day experimental period. The observed hematological changes in the 2nd month might be because of relatively long-term influence of the probiotic. This means that with the extension of protexin consumption, the above changes became discernible; even some minor changes in the 1st month appeared markedly in the 2nd month. On account of the identical experimental conditions for all groups in this study, the observed hematological changes are probably as a result of protexin supplementation for feeding the Oscar fingerlings. And no role could be depicted for such factors as the fish habitat and the governing conditions including temperature, feeds, and pollutants (Bullis 1993). The distinguished total number of RBC in the fingerlings fed 0.15 g protexin kg−1 dry food compared to the control apparently caused by a high, probiotic-driven metabolism with consequent increase in oxygen requirements. A rise in the number of RBC, therefore, intensifies the concentration of hemoglobin and eventually leads to a high oxygen-carrying capacity in the probiotic-fed fish. Such fishes, hence, may be more capable of supplying oxygen to tissues in situations where oxygen is highly required.
The contribution of probiotics in stimulating fish immune system in addition to their effects on fish survival and growth has greatly been studied in various fish species. Probiotics are able to multiply RBC, granulocytes, lymphocytes, and macrophages in fish similar to higher vertebrates (Irianto and Austin 2002b; Kumar et al. 2008). Also, probiotics show interactions with immune cells such as monocytes, macrophages, neutrophils, and lymphocytes in order to improve innate immune response (Nayak 2010). Irianto and Austin (2002b) presented evidence that use of probiotic could result in raised RBC and WBC, especially lymphocytes, in rainbow trout. The use of B. subtilis as a probiotic significantly increased leukocytes count in the Major carp (Nayak et al. 2007) and rainbow trout (Newaj-Fyzul et al. 2009) compared to the control.
Similarly, the current study revealed significant rise of average total number of WBC together with raised number of lymphocytes in the Oscar fingerlings fed a dietary protexin of 0.15 g kg−1 dry food. The lymphocytes outnumbered in WBC followed by neutrophils. Elevated number of WBC can be seen as a reinforcement of non-specific immune system resulting from probiotic consumption (Nayak 2010).
Effective rise of phagocytic cells leading to increased phagocytosis (Nayak 2010) has been investigated in a number of fish species as a result of probiotic supplementation (Salinas et al. 2005, 2006, 2008; Picchietti et al. 2007, 2009). Picchietti et al. (2009) showed that early bacterial colonization in the intestine of the sea bass (D. labrax) using probiotic bacteria (L. delbrueckii) isolated from the mature fish could stimulate the gut immune system and increase T cells. Salinas et al. (2005) also noticed significant increase in phagocytic activity of leukocytes in gilthead sea bream; after 2 weeks, the fish had been fed by the bacteria B. subtilis and L. delbrueckii. Similarly, elevated leukocyte phagocytic ability was found in gilthead seabream (S. aurata L.) fed with a mix of two inactivated bacteria (Díaz-Rosales et al. 2006).
Given that the protexin-supplemented A. ocellatus at 0.15 g kg−1 dry food have acquired a rather higher level of immunity, they are expected to be more resistant against stressors and diseases, for which further investigation is required. As lymphocytes were the most numerous in WBC, and because interactions of lymphocytes B and T as well as macrophages is necessary for an immune response to occur (Borges et al. 2004), it can, accordingly, be concluded that fish immune system can be substantially stimulated by elevation of lymphocytes following an increase in WBC. In other words, an appropriate dose of probiotic microorganisms entering the digestive tract of fish is considered as aliens, provoking the defense system and multiplying WBC and other immunizing compounds.
Hematological parameters, in general, are considerably affected by fish physiological conditions, pollution and disease, sex (Ghosh et al. 2003, 2007), sexual maturity and behavior, age (Speckner et al. 1989) season and environment (Nikoskelainen et al. 2003), food quantity and quality, diet differences (Rawling et al. 2009; Merrifield et al. 2010), diet formulation and type, purity and dose of consumed probiotic. The overall results of this study demonstrate that a dietary protexin supplement of 0.15 g kg−1 dry food would give rise to positive effects on hematological factors of the Oscar fingerlings, ultimately leading to increased growth and reduction in feed conversion ratio. Considering the fact that resources and practical information regarding hematological parameters of fish in general and ornamental fish in particular are limited, this field is apparently in its infancy demanding further research.
References
Abd El-Rhman AM, Khattab YAE, Shalaby AME (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27:175–180
Abdel-Tawwab M, Abd El-Rahman AM, Ismael NEM (2008) Evaluation of commercial live baker’s yeast, Saccharomyces cervisiae as a growth and immunity promoter for fry Nile tilapia, Oreochromis niloticus challenged in situ with Aeromonas hydrophila. Aquaculture 280:185–189
Avella MA, Gioacchini G, Decamp O, Makridis P, Bracciatelli C, Carnevali O (2010a) Application of multi-species of Bacillus in sea bream larviculture. Aquaculture 305:12–19
Avella MA, Olivotto I, Silvi S, Place AR, Carnevali O (2010b) Effect of dietary probiotics on clownfish: a molecular approach to define how lactic acid bacteria modulate development in a marine fish. Am J Physiol Regul Integr Comp Physiol 298:359–371
Bairagi A, Chosh KS, Sen SK, Ray AK (2002) Enzyme producing bacterial flora isolated from fish digestive tracts. Aquacult Int 10:109–121
Borges A, Scotti LV, Siqueira DR, Jurinitz DF, Wassermann GF (2004) Hematologic and serum biochemical values for jundia′ (Rhamdia quelen). Fish Physiol Biochem 30:21–25
Bullis RA (1993) Clinical pathology of temperate freshwater and estuarine fishes. In: Stoskopf MK (ed) Fish medicine. W.B. Sanders. CO., Philadelphia, pp 232–239
Campbell TW, Ellia CK (2007) Avian and exotic animal hematology and cytology, 3rd edn, Blackwell Puplishing, Iowa, pp 93–112
Carnevali O, Zamponi MC, Sulpizo P, Rollo A, Nardi M, Orpianesi C, Silvi S, Caggiano M, Polzonetti AM, Cresci A (2004) Administration of probiotic strain to improve sea bream wellness during development. Aquacult Int 12:377–386
Carnevali O, De Vivo L, Sulpizio R, Gioacchini G, Olivotto I, Silvi S (2006) Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture 258:430e8
De Silva SS, Anderson TA (1995) Fish nutrition in aquaculture. Chapman and Hall, London, p 319
Díaz-Rosales P, Salinas I, Rodríguez A, Cuesta A, Chabrillón M, Balebona MC (2006) Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of head-inactivated potential probiotics. Fish Shellfish Immunol 20:482e92
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels G, Guroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production—a mediterranean perspective. Fish Shellfish Immunol 30:1–16
Douillet PA, Langdon CJ (1994) Use of a probiotic for the culture of pacific oyster (Crassostrea gigas Thunberg). Aquaculture 199:25–40
Drobkin DR (1945) Crystallographic and optical properties of human hemoglobin: a proposal for the standardization of hemoglobin. Am J Med Sci 209:268–270
Firouzbakhsh F, Aliasghari M (2009) An illustrative encyclopedia of freshwater aquarium fish. Partove vagheeh press, Tehran, pp 32–35
Fuller R (1989) Probiotic in man and animals. J Appl Bacteriol 66:365–378
Gatesoupe FJ (1994) Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio. Aquat Living Res 7:277–282
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Ghosh k, Sen Sk, Ray Ak (2003) Supplementation of an isolated fish gut bacterium, bacillus circulans, in formulated diets for Rohu, Labeo rohita fingerlings. Bamidgeh 55:13–21
Gildberg A, Mikkelsen H, Sandaker E, Ringø E (1997) Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod (Gadus morhua). Hydrobiologia 352:279–285
Gosh S, Sinha A, Sahu C (2007) Effect of probiotic on reproductive performance in female livebearing ornamental. Aquc Res 38:518–526
Gosh S, Sinha A, Sahu C (2008) Dietary probiotic supplementation in growth and health of live-bearing ornamental fishes. Aquac Nutr 14:289–299
Hevroy EM, Waagbo R, Sandness K, Rund M, Hermre GI (2005) Nutrition utilization in Atlantic salmon (Salmo salar) fed increased level of fish protein hydrolysate during a period of fast growth. Aquac Nutr 11:301–313
Irianto A, Austin B (2002a) Probiotic in aquaculture. J Fish Dis 25:1–10
Irianto A, Austin B (2002b) Use of probiotic to control furunclosis in rainbow trout. J Fish Dis 25:333–342
Jafarian H, Azari Takami G, Kamali A, Soltani M, Habibirezaei M (2007) The use of probiotic bacillus bioencapsulated with Artemia urmiana nauplii for the growth and survival in Acipenser persicus larvae. J Agric Sci Natur Resur 14:32–38
Jain NC (1986) Schalms veterinary hematology, 4th edn. Mosby Inc, St. Louis
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo rohita following oral administration of Bacillus subtilis. Fish Shellfish Immunol 24:168–172
Lahtinen S, Ouwehand A (2009) Mechanisms of probiotics. In: Lee Y, Salminen S (eds) Handbook of probiotics and prebiotics, 2nd edn. Wiley, Hoboken, pp 377–440
Lee YK (2009) Effective dosage for probiotic effects. In: Lee Y, Salminen S (eds) Handbook of probiotics and prebiotics, 2nd edn. Wiley, Hoboken, pp 52–58
Merrifield DL, Guroy D, Guroy B, Emery MJ, Liewellyn CA, Skill S, Davies SJ (2010) Assessment of Chlorogloepsis as a novel microbial dietary supplement for red tilapia (Oreochromis niloticus). Aquaculture 299:128–133
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14
Nayak SK, Swain P, Mukherjee SC (2007) Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian Major Carp, Labeo rohita. Fish Shellfish Immunol 23:892–896
Newaj-Fyzul A, Adesiyun AA, Mutani A, Ramsubhag A, Brunt J, Austin B (2009) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103:1699–1706
Nikoskelainen S, Ouwehand A, Salmine S, Bylund G (2001) protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture 198:229–236
Nikoskelainen S, Ouwehand AC, Bylund G, Salminen S, Lilius EM (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish Shellfish Immonol 15:443–452
Parker RB (1974) Probiotics, the other half of the antibiotics story. Anim Nutr Health 29:4–8
Picchietti S, Mazzini M, Taddei AR, Renna R, Fausto AM, Mulero V (2007) Effects of administration of probiotic strains on GALT of larval gilthead seabream: immunohistochemical and ultrastructural studies. Fish Shellfish Immunol 22:57–67
Picchietti S, Fausto AM, Randelli E, Carnevali O, Taddei AR, Buonocore F (2009) Early treatment with Lactobacillus delbrueckii strain induces rise in intestinal T cells and granulocytes and modulates immune related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol 26:368–376
Ramiez RF, Dixon BA (2003) Enzyme production by obligate intestinal anaerobic bacteria isolated from Oscars, Angelfish and souther flounder. Aquaculture 227:417–426
Rawling M, Merrifield DL, Davies SJ (2009) Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture 294:118–122
Salinas I, Cuesta A, Esteban MA, Meseguer J (2005) Dietary administration of Lactobacillus delbrueckii and Bacillus subtilis, single or combinated, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol 19:667–677
Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillon M, Mori_nigo AA (2006) Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet Immunol Immunopathol 111:279–286
Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D (2008) Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 25:114–123
Schuett DA, Lehmann J, Goerlich R, Hamers R (1997) Hematology of swordtail, Xiphiphorus helleri, blood parameters and light microscopy of blood cells. J Appl Ichthyol 13:83–89
Son VM, Changa CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the probiotics, Lactobacillus plantarum, enhanced the growth, innate immune responses and disease resistance of the grouper Epinephalus coioides. Fish Shellfish Immunol 26:691–698
Speckner W, Schindler JF, Albert C (1989) Age dependent changes in volume and hemoglobin content of erythrocyte in the carp. J Exp Biol 141:133–149
Svetina A, Matasin Z, Tofant A, Vucemilo M, Fijan N (2002) Hematology and some blood chemical parameters of young carp till the age of three years. Acta Vet Hung 50:459–467
Wang YB, Li JR, Lin J (2008a) Probiotic in aquaculture: challenges and outlook. Aquaculture 28:1–4
Wang YB, Tian ZQ, Yao JT, Li WF (2008b) Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 277:203–207
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Firouzbakhsh, F., Noori, F., Khalesi, M.K. et al. Effects of a probiotic, protexin, on the growth performance and hematological parameters in the Oscar (Astronotus ocellatus) fingerlings. Fish Physiol Biochem 37, 833–842 (2011). https://doi.org/10.1007/s10695-011-9481-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9481-4