Abstract
We have evaluated the homology of the electrophile-responsive element (EpRE) core sequence, a binding site for the Nrf2 transcription factor, in the proximal promoters of the mouse and zebrafish glutathione-S-transferase (gst), glutamate cysteine ligase catalytic subunit (gclc) and heat shock protein 70 (hsp70) genes. The EpRE sites identified for both species in the three analyzed genes showed a high similarity with the putative EpRE core sequence. We also produced a transgenic zebrafish model carrying a transgene comprised of the luciferase (luc) reporter gene under transcriptional control of a mouse EpRE sequence. This transgenic model was exposed to copper sulfate, and the reporter gene was significantly activated. The endogenous gst, gclc and hsp70 zebrafish genes were analyzed in the EpRE-Luc transgenic zebrafish and showed an expression pattern similar to that of the reporter transgene used. Our results demonstrate that EpRE is conserved between mouse and zebrafish for detoxification-related genes and that the development of genetically modified models using this responsive element to drive the expression of reporter genes can be an important tool in understanding the action mechanism of aquatic pollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food metabolism and the respiratory process generate reactive electrophiles that can, directly or indirectly, affect the physiological function of macromolecules, such as DNA, proteins and lipids (Halliwell 2001). Cells have acquired, during evolution, a complex defense mechanism against the electrophile-induced toxicity through a very coordinated induction of many genes and proteins (Talalay et al. 2003). This response is regulated, mainly, by the antioxidant-responsive element (ARE) or the electrophile-responsive element (EpRE),both of which are present in the regulatory region of genes encoding for enzymes of phase II (for review, see Itoh et al. 2004). The EpRE core sequence (5′-RTGAYnnnGC-3′) was characterized as a minimal sequence required for basal and xenobiotic inducible expression by Rushmore et al. (1991). The EpRE is recognized by the nuclear transcription factor erythroid 2 (NF-E2)-related factor 2 (Nrf2). Under non-induced conditions, Nrf2 is retained in the cytoplasm by the protein Keap1, which is associated to the actin filaments. An increase in electrophile/oxidative stress results in the dissociation of Nrf2 from Keap1, allowing translocation of Nrf2 to the nucleus and binding to EpRE and a consequent activation of target genes (Lee and Surh 2005; Nguyen et al. 2003). The main genes regulated by EpRE/Nrf2 include genes encoding phase II enzymes, such as NAD(P)H:quinone oxidoreductase (NQO1) (Rushmore et al. 1991), the glutathione S-transferase Ya subunit (Gsta1) (Frilling et al. 1990), heme oxygenase 1 (HO-1) (Alam et al. 1995) and γ-glutamate cysteine ligase (Gcl) (Mulcahy et al. 1997). Additionally, chaperone proteins, such as heat shock protein (Hsp70), have also been identified as being regulated by EpRE-mediated mechanisms (Kwak et al. 2003), which together with the ubiquitin/proteasome system play an essential role in the response to stress (Beckmann et al. 1990; Parsell and Lindquist 1993).
The EpRE/Nrf2 signaling pathway seems to be conserved among vertebrates. A comparison between zebrafish (Danio rerio) and mouse (Mus musculus) Nrf2 proteins has demonstrated high similarity among critical domains, which are very important for Nrf2 to exert its transcriptional regulatory function (Kobayashi et al. 2002). Six Neh (Nrf2-ECH homology) domains are shared by both mouse and zebrafish Nrf2, which implies a common mechanism of gene regulation (Itoh et al. 1995). Indeed, it has been demonstrated by gene knockdown analysis that Nrf2 is essential for some phase II enzymes in zebrafish (Kobayashi et al. 2002; Suzuki et al. 2005). In addition, Carvan et al. (2000, 2001) have produced zebrafish reporter cell lines transfected with transgenes containing EpRE from the mouse gsta1 promoter. The exposure of these reporter cell lines to several classes of environmental pollutants resulted in a dose-dependent transgene induction, which suggests a conserved mechanism since the zebrafish Nrf2 seems to be able to recognize an EpRE sequence of mammalian origin. These authors have proposed the utilization of transgenic zebrafish carrying a reporter gene under EpRE control as sentinel for aquatic pollution.
In fact, fish have been considered to be a very useful model for ecotoxicology studies because these vertebrates are very sensitive to chemical toxicity and are valuable comparative animal models (Hill et al. 2005). The utility of fish as sentinels for environmental pollution has been expanded since transgenic fish have been designed to harbor reporter genes, such as luciferase (luc) or green fluorescent protein (gfp), driven by promoters that are responsive to chemical exposure. An additional advantage is that this technology can result in fish models carrying identical transgenes to those found in other organisms, thereby further increasing the possibility of extrapolation across species (Winn 2001).
We have compared, for the first time, elements of the EpRE core sequence in conserved regions of the zebrafish—gst, the gclc catalytic subunit and hsp70 promoters—with their mouse homologs. In addition, we produced transgenic zebrafish carrying a genetic construct comprised by the luc reporter gene under the transcriptional control of a mouse EpRE-containing promoter. This in vivo model was exposed to copper sulfate and the expression of the endogenous gclc, gst and hsp70 genes was compared to the induction of the reporter transgene.
Materials and methods
In the search for the EpRE core sequence in the analyzed genes, we identified sequences containing gst, gclc and hsp70 proximal promoters at GenBank (http://www.ncbi.nlm.nih.gov). For the mouse gst, gclc and hsp70, we used sequences AC159313, AC160124 and AF109906, respectively; for zebrafish gst, gclc and hsp70, we used sequences AL929536, CR376766 and BX511232, respectively. Within these sequences, the transcription starting point for each gene was identified and the upstream 1500 bp were selected. The potential transcription factor binding sites for Nrf2 (RTGAYnnnGC or CGnnnYAGTR) were localized using the MatInspector program (Quandt et al. 1995). The mEpREmt1 plasmid (hereafter named EpRE-Luc), kindly provided by Dr. M.J. Carvan III (Great Lakes WATER Institute, Milwaukee, WI), was used to produce the EpRE-mediated Luc. This plasmid contains a single EpRE from the mouse gsta1 enhancer region (−754 to −714) fused to the minimal mouse mt1 promoter and cloned into the pGL3-Basic (Promega, Madison, WI) firefly luciferase (luc) reporter gene construct (Carvan et al. 2000). Adult wild-type zebrafish obtained from a commercial supplier were kept in a closed water circulation system at 28°C under a 14/10-h (light/dark) photoperiod. Freshly fertilized eggs were collected for microinjection, and the EpRE-Luc plasmid was injected into fertilized eggs before the first cleavage. One-cell embryos were injected following the general protocol recommended by Vielkind (1992) using an IM-30 motorized picoinjector (Narishige, Japan) to inject approximately 300 pl of DNA solution, representing a total of 106 copies of the transgene per injected embryo. Microinjected embryos were incubated at 28°C until hatching. EpRE-Luc transgenic larvae were exposed to copper sulfate (Sigma, Brazil) 72 h after fertilization in 20 ml of acclimated tap water in an all-glass container. Three replicate treatments (3 × 20 larvae) were exposed to 0.02 mg l−1 copper sulfate for 24 h. After exposure, larvae were killed and immediately frozen at −80°C for RNA extraction.
The expression of the luc, gst, gclc and hsp70 genes was evaluated by semiquantitative reverse transcriptase (RT)-PCR from EpRE-Luc transgenic larvae. Following copper sulfate exposure, larvae were pooled in 1.5-ml microtubes (20 larvae per tube), and total RNA was extracted using TRIzol reagent (Invitrogen, Brazil) according to the protocol suggested by the manufacturer. RNA concentration was quantified on a Qubit Fluorometer (Invitrogen, Brazil). The Quant-iT RNA Assay kit (Invitrogen, Brazil) was used, and calibration was performed using a two-point standard curve. Approximately 2 μg of total RNA from each pool was utilized as the template for the RT-PCR with the AP primer [5′-GGCCACGCGTCGACTAGTAC(T)17-3′; Invitrogen, Brazil]. The complementary DNA (cDNA) synthesis was carried out using the enzyme RT SuperScript III (Invitrogen) according to the protocol suggested by the manufacturer. All PCR reactions were carried out in a 12.5-μl reaction volume containing 1.25 μl 10× PCR buffer, 0.2 μM of each primer, 0.2 mM of each dNTP, 0.75 mM MgCl2, 0.5 U Platinum Taq DNA polymerase (Invitrogen) and 1 μl cDNA solution. In order to be able to normalize the data, we used β-actin gene expression as an internal control. Specific primers, fragment length and annealing temperature for each gene are listed in Table 1. Amplification products were separated on a 1% (w/v) agarose gel and stained with Sybr Safe (Invitrogen). Band densitometry was performed with 1Dscan EX software ver. 3.1 (Scanalytics, Rockville, MD).
Differences between controls and treated samples in terms of gene expression were determined using Student’s t test for statistical comparisons. The statistical test used a fixed type I error of 5% (α = 0.05).
Results and discussion
The EpRE-mediated gene expression represents an important factor in the cellular detoxifying process. Considering that this responsive element is present in promoter regions of several genes coding for phase II enzymes and chaperones, such as Hsp70, it seems obvious that this system includes very intricate and coordinated mechanisms. The EpRE sequence has been recognized as a primary binding site for Nrf2, which is a very conserved transcription factor among vertebrates (Kobayashi et al. 2002). Within this context, we have demonstrated that the EpRE sequence is a common element within different promoters of gst, gclc and hsp70 genes. In order to determine the position of the EpRE core sequences, the proximal promoters (1500 bp) from mouse and zebrafish gst, gclc and hsp70 genes were analyzed. The results obtained are shown in Table 2. Two EpRE core sequences were identified for the mouse gsta1 promoter, as previously described by Prestera et al. (1993) at position −743 to −734 and −728 to −719, respectively. However, the promoter analysis carried out in our study recognized two additional EpRE sequences at position −913 to −904 and −148 to −139, respectively. For the zebrafish ortholog gene, we found only two EpRE sequences—at positions −1331 to −1322 and −749 to −740, respectively.
Analysis of the mouse gclc proximal promoter revealed an EpRE site at position −731 to −722, while in the zebrafish ortholog two EpRE sites at −453 to −444 and −355 to −346, respectively, were found. Our analysis of the mouse hsp70 promoter revealed an EpRE at −502 to −493, while in the zebrafish ortholog, this element appears at −690 to −681. The EpRE sites identified for both species in all three genes compared showed a high similarity with the putative EpRE core sequence that could be a potential binding site for the Nrf2 transcription factor (Table 2). This observation suggests that the EpRE/Nrf2 signaling pathway is a shared detoxifying mechanism for all genes analyzed here. Nevertheless, it is interesting to notice that the comparison between the mouse and zebrafish gclc proximal promoter identified two EpRE sites for zebrafish while the mouse only has one. Conversely, the gst promoter analysis indicated four mouse EpRE sequences against only two for the zebrafish orthologous promoter. This loss and gain of transcription factor binding sites probably has to do with the evolution of these genes in terms of their role within the cellular complex detoxification mechanism in mouse and zebrafish.
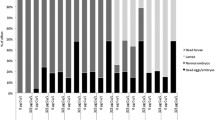
In addition to comparing proximal promoters in order to establish the relationship of the EpRE/Nrf2 signaling pathway between mouse and zebrafish for the different proteins involved with detoxification mechanisms, we also produced a transgenic zebrafish model carrying a transgene comprised of reporter genes of luciferase under transcriptional control of a mouse EpRE sequence. As expected, all genes (luc, endogenous gst, gclc and hsp70) were induced in terms of mRNA transcript quantity after metal exposure. This observation suggests that the gst, gclc and hsp70 genes are under the same regulatory system mediated by the EpRE sequence located at the proximal promoters. No mortality was observed in any of our experiments. In comparison to the control group, which was not exposed to copper, exposure to copper increased the level of Luc mRNA (Fig. 1a) by about 1.39-fold (P < 0.05), that of the endogenous zebrafish gclc gene (Fig. 1b) by 1.23 fold (P < 0.05); there was an even higher induction for the zebrafish gst gene—3.38 fold (P < 0.05) (Fig. 1c). The hsp70 gene expression analysis showed an increase in the mRNA level (Fig. 1d) of about 1.59 fold that of the control group (P < 0.05). These results indicate a specific binding of the zebrafish Nrf2 transcription factor to the EpRE of mouse origin and corroborate the hypothesis that this signaling pathway is fully conserved among vertebrates (Kobayashi et al. 2002). Carvan et al. (2001) have already demonstrated that zebrafish transcription factors are able to recognize the mouse EpRE sequence in a dose-dependent manner in zebrafish cells exposed to several pollutants.
Effects of exposure to copper sulfate in terms of luciferase (luc, a),glutamate cysteine ligase catalytic subunit (gclc, b), glutathione S-transferase (gst, c) and heat shock protein (hsp70, d) gene expression on the electrophile-responsive element (EpRE)-Luc transgenic zebrafish larvae. I Graphic representation of the quantification of the bands shown below, normalized according to β-actin expression and analyzed by band densitometry. II Levels of mRNA for all genes were measured by reverse transcriptase PCR analysis following treatment with copper sulfate at a concentration of 0.02 mg l−1 for a period of 24 h. Data are expressed as mean ± standard error (n = 3). Asterisks (*) indicate significant differences (P < 0.05)
Even though there are no published studies on transgenic fish harboring a genetic construct comprised by a reporter gene under transcriptional control of the orthologous gclc promoter, there is a recent study also demonstrating that a mammalian hsp70 promoter can be recognized by fish transcription factors. Seok et al. (2006) produced transgenic zebrafish carrying a transgene comprised by a human hsp70 promoter driving the gene coding for enhanced green fluorescent protein (eGfp). These authors demonstrated that this model is sensitive enough to detect copper sulfate at low concentration (0.066 mg l−1), which is below the median lethal concentration established for zebrafish larvae (0.08 mg l−1). We detected induction of the luc reporter gene at an even lower level of copper sulfate (0.02 mg l−1). However, this discrepancy cannot be associated with the EpRE origin of the two transgenes used in both studies, but with the different methods for gene expression analysis applied. In our study, we used RT-PCR to quantify expression of reporter gene (luc), while Seok et al. (2006) only detected eGfp expression through the epifluorescence microscope. Blechinger et al. (2002) used a similar approach to monitor the effect of cadmium in a stable germline transgenic zebrafish containing a zebrafish hsp70-regulated eGfp construct. The authors demonstrated that the transgene responds in a dose-dependent manner and is sensitive enough to detect cadmium at doses below the median lethal concentration.
In conclusion, the results of our study demonstrate that EpRE is conserved between mouse and zebrafish in terms of the gst, gclc and hsp70 genes. We also produced transgenic zebrafish containing an EpRE-Luc construct and analyzed the endogenous gst, gclc and hsp70 zebrafish genes. The gene expression analysis quantified by RT-PCR showed an induction of the endogenous genes as well as the transgene used. Therefore, since EpRE is conserved between fish and mammals and is present in the regulatory region of genes encoding for detoxification-related proteins, the development of genetically modified models using this responsive element driving the expression of reporter genes can be an important tool towards gaining an understanding of the action mechanism of aquatic pollutants. The use of a reporter gene, such as GFP, could make this technology more cost-effective, since GFP can be detected without any external substrate and chemical or radioactive labels. This could permit a wider ecotoxicological approach in terms of pollutant concentration and exposure time.
References
Alam J, Camhi S, Choi AM (1995) Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcriptional enhancer. J Biol Chem 270:11977–11984. doi:10.1074/jbc.270.20.11977
Beckmann RP, Mizzen LE, Welch WJ (1990) Interaction of hsp70 with newly synthesized proteins: implications for protein folding and assembly. Science 248:850–854. doi:10.1126/science.2188360
Blechinger SR, Warren JT Jr, Kuwada JY, Krone PH (2002) Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect 110:1041–1046
Carvan MJ III, Solis WA, Gedamu L, Nebert DW (2000) Activation of transcription factors in zebrafish cell cultures by environmental pollutants. Arch Biochem Biophys 376:320–327. doi:10.1006/abbi.2000.1727
Carvan MJ III, Sonntag DM, Cmar CB, Cook RS, Curran MA, Miller GL (2001) Oxidative stress in zebrafish cells: potential utility of transgenic zebrafish as a deployable sentinel for site hazard ranking. Sci Total Environ 274:183–196. doi:10.1016/S0048-9697(01)00742-2
Frilling RS, Bensimon A, Tichauer Y, Daniel V (1990) Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA 87:6258–6262. doi:10.1073/pnas.87.16.6258
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716. doi:10.2165/00002512-200118090-00004
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86(1):6–19. doi:10.1093/toxsci/kfi110
Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M (1995) Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15:4184–4193
Itoh K, Tong K, Yamamoto M (2004) Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36(10):1208–1213. doi:10.1016/j.freeradbiomed.2004.02.075
Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M (2002) Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells 7:807–820. doi:10.1046/j.1365-2443.2002.00561.x
Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW (2003) Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278:8135–8145. doi:10.1074/jbc.M211898200
Lee JS, Surh YJ (2005) Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 224:171–184. doi:10.1016/j.canlet.2004.09.042
Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ (1997) Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem 272:7445–7454. doi:10.1074/jbc.272.11.7445
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260. doi:10.1146/annurev.pharmtox.43.100901.140229
Parsell DA, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496. doi:10.1146/annurev.ge.27.120193.002253
Prestera T, Holtzclaw WD, Zhang Y, Talalay P (1993) Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA 90:2965–2969. doi:10.1073/pnas.90.7.2965
Quandt K, Frech K, Karas H, Wingender E, Werner T (1995) MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23:4878–4884. doi:10.1093/nar/23.23.4878
Rushmore TH, Morton MR, Pickett CB (1991) The antioxidant responsive element, activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem 266:11632–11639
Seok SH, Park JH, Baek MW, Lee HY, Kim DJ, Uhm HM, Hong JJ, Na YR, Jin BH, Ryu DY, Park JH (2006) Specific activation of the human Hsp70 promoter by copper sulfate in mosaic transgenic zebrafish. J Biotechnol 126:406–413. doi:10.1016/j.jbiotec.2006.04.029
Suzuki T, Takagi Y, Osanai H, Li L, Takeuchi M, Katoh Y, Kobayashi M, Yamamoto M (2005) Pi-class glutathione S-transferase genes are regulated by Nrf2 through an evolutionarily conserved regulatory element in zebrafish. Biochem J 388:65–73. doi:10.1042/BJ20041860
Talalay P, Dinkova-Kostova AT, Holtzclaw WD (2003) Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzym Regul 43:121–134. doi:10.1016/S0065-2571(02)00038-9
Vielkind JR (1992) Medaka and zebrafish: ideal as transient and stable transgenic systems. In: Hew CL, Fletcher GL (eds) Transgenic fish. World Scientific, Singapore, pp 72–91
Winn RN (2001) Transgenic fish as models in environmental toxicology. ILAR J 42(4):322–329
Acknowledgements
The authors would like to thank Maíra Proietti for valuable corrections of the manuscript. This work was supported by FAPERGS (Proc. No. 0412405) and CNPq/CT-HIDRO (Proc. No. 556022/2006-8). D.V. Almeida and L.A. Geracitano are post-graduate student and post-doctoral fellows, respectively, financed by CAPES. J.M. Monserrat and D. Martí Barros are research fellows from CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almeida, D.V., da Silva Nornberg, B.F., Geracitano, L.A. et al. Induction of phase II enzymes and hsp70 genes by copper sulfate through the electrophile-responsive element (EpRE): insights obtained from a transgenic zebrafish model carrying an orthologous EpRE sequence of mammalian origin. Fish Physiol Biochem 36, 347–353 (2010). https://doi.org/10.1007/s10695-008-9299-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9299-x