Abstract
The osmoregulation capabilities of 7-month-old juvenile Chinese sturgeon (Acipenser sinensis Gray) (128.8 ± 15 g) transferred directly from fresh water (0‰, 46 mOsmol kg−1) to brackish water (10‰, 273 mOsmol kg−1) were studied over a 20-day period. Changes in serum osmolarity, chloride (Cl−), sodium (Na+), potassium (K+) and calcium (Ca2+) ion concentrations, as well as gill and spiral valve Na+,K+-ATPase activities were measured at 3, 12, 24, 72, 216 and 480 h after transfer to BW. The serum osmolarity and ion concentrations (Na+, Cl− and Ca2+) increased immediately after the transference to BW, reaching maximum at 24 h and returned to a new steady state at 216 h, while the FW control group maintained basal levels which showed lower (P < 0.05) than the BW group. Gill Na+,K+-ATPase activity of BW group exhibited an abrupt decrease in the first 3 h after transfer, but began to increase at 3 h, reaching a peak value at 24 h, and returned to a new steady state at 216 h. The differences between gill Na+,K+-ATPase activity of BW and FW fish were significant (P < 0.05) after 12 h. In contrast, Na+,K+-ATPase activity of the spiral valve showed transient increase after transference from FW to BW, and then decreased rapidly at 3 h, reaching the lowest at 24 h after transference. At 216 h after exposure to BW, Na+,K+-ATPase activities of the spiral valve increased slowly to the levels of FW control. The results of our study indicate the existence of hyposmoregulatory adaptive mechanisms in 7-month-old juvenile Chinese sturgeon which enable this fish to acclimate itself successfully to brackish water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chinese sturgeon (Acipenser sinensis Gray), a class I endangered species by the Chinese government (Wei et al. 1997), is an anadromous species that presently only remains in the Yangtze (Changjiang) River, China (Hubei Institute of Hydrobiology 1976). Adult Chinese sturgeon, which are a maximum of 400 cm total length (TL) and 452 kg body weight (BW), are one of the largest fish to enter fresh water (Chang and Cao 1999). Juveniles hatched in the Yangtze River migrate to the sea and return to spawn at the age of 8–10 years (CARSGS 1988). As a result of overfishing and construction of Gezhouba Dam in 1981 at Yichang, Hubei Province, 1,766 km from the river estuary, which blocked the spawning migration of Chinese sturgeon to the Yibin spawning reach, populations of Chinese sturgeon have greatly declined in abundance (Wei et al. 1997). Some successful spawning occurs in the short reach below the dam (Gezhouba spawning site) as verified by the capture of early-life stages in 1982 (Yu et al. 1986) and during 1996–1999 by Wei et al. (personal communication). Although commercial fishing for Chinese sturgeon was prohibited after 1983, as many as 100 adults have been removed annually to support a culture and restocking program (Xiao et al. 1999).
Research on Chinese sturgeon has focused on artificial culture, spawning, and population dynamics. Zhao et al. (1986) and Yi (1994) investigated the distribution and abundance of juveniles from the Yichang reach to the estuary. They found juveniles concentrated at the river estuary during the period May–September, and almost all the young fish found in the estuary were younger than 1 year old. Juveniles develop their capacity for hyposmoregulation by salinity challenge at the river estuary. The physiological mechanisms involved in maintaining the plasmatic hydromineral equilibrium under brackish water condition, however, are unknown. Sturgeon and teleosts that move between fresh water and salt water must maintain rather tight control of serum water and ion concentration for efficient physiological function (Natochin et al. 1985; Krayushkina et al. 1995). Regulatory mechanisms are similar in sturgeon and teleosts (Krayushkina et al. 1995). In elevated salinities, osmotic water, lost across the integument, is replaced by drinking sea water (Krayushkina et al. 1995). Excess sodium and chloride is removed from blood serum by chloride cells located in the gill epithelium while calcium and potassium are excreted via the kidneys (Krayushkina et al. 1995; Cataldi et al. 1995a; McCormick et al. 1996). In the anadromous white sturgeon (A. transmontanus), these mechanisms provide stable serum osmotic and ionic concentrations during movement between fresh water and sea water (McEnroe and Cech 1985).
To date, little information has been gathered concerning the osmoregulatory physiology of the Chinese sturgeon. However, such information would provide a better understanding of the life history of this species, and could be used to develop conservation strategies. The main objective of this study was to obtain detailed information on development of osmoregulatory mechanisms, and to examine alterations in the short term in juvenile Chinese sturgeon transferred directly from fresh water to brackish water. Gill and spiral valve Na+,K+-ATPase activity, serum osmolarity and serum ion concentration were the main parameters investigated.
Materials and methods
Fish acclimation and experimental procedure
Eighty Chinese sturgeon juveniles (7 months old) were obtained from the Institute of Sturgeon Researches in Yichang, China, as yolk-sac larvae produced by induced spawning of wild fish. The fish were transferred to the Aquaculture Laboratory (East China Sea Fisheries Research Institute, Shanghai, China) and reared indoors in 2-m-diameter circular fiberglass tanks (2.5-m3), filled with approximately 1,800 l of dechlorinated tap water (0‰, 46 mOsmol kg−1), and aerated by air stones. The fish were acclimated for 15 days. At the end of this period fish body weight was 128.8 ± 15 g (mean ± SE). The stock was randomly divided into two groups (n = 12 fish for each group), and re-distributed in tanks (6 fish for each tank) with the same characteristics as those described above. One group was transferred directly from fresh water (0‰) to brackish water (BW, 10‰, 273 mOsmol kg−1). The other was also transferred, from fresh water to fresh water (FW, Control fish). Each treatment had three replicates (0.43 ± 0.05 kg m−3 initial density). Six fish (two from each replicate tank) were randomly collected and sacrificed from both experimental groups (BW-exposed and control fish) at 3, 12, 24, 72, 216 and 480 h after transfer. Before the transfer, eight fish were sampled. The desired salinity (10‰) was obtained by diluting sea water (SW) at 37‰ with dechlorinated tap water. During the acclimation and experimental period, the fish were fed twice a day at 1.5% of tank biomass with a dry commercial pellet diet (45% protein, 12% lipid, 10% ash and 3.5% carbohydrate; Haima, Seahorse Feed, Fuzhou, China). The tanks were cleaned twice a day in order to remove uneaten feed and feces. In each tank, half the water volume was renewed every 4 days to assure water quality. Throughout the experimental period, water salinity, dissolved oxygen concentration, pH and temperature were checked daily. Water salinity was adjusted with dechlorinated tap water or 37‰ SW when needed. Water temperature, pH and dissolved oxygen were 29 ± 1, 7.8 ± 0.02 and 6.2 ± 0.06 mg l−1 (mean value of all treatment groups ± SE), respectively. The fish were exposed to a 12-h light-dark photoperiod using overhead fluorescent lights. There were no mortalities during the experiment.
Sampling and analytical procedures
The fish were anaesthetized by immersion in Tricaine Methanesulphonate (MS-222) at 200 mg l−1, and their body weight was measured in order to evaluate the effect of water osmolarity on growth performance. Approximately 1.5–2.0 ml blood was drawn from the caudal vessels, just behind the anal fin. Blood was allowed standing for 15 min at room temperature to achieve complete clotting, then centrifuged at 15,000g for 15 min. Serum was decanted from red blood cells and kept refrigerated (4°C) until analysis. Within 4 h of sampling, concentrations of Na+, K+, Cl−, Ca2+ in serum were measured on the Automatic Biochemistry Analyser Beckman LX20 (Beckman, Brea, Calif., USA) by using ion selective electrode assay, while osmolarity (mOsmol kg−1) was determined in a VAPRO® 5520 vapor pressure osmometer (Wescor, Logan, Utah, USA). After blood collection, the fish were decapitated, the spiral valve and the second gill arch from the fish left side were carefully dissected, placed in ice-cold sucrose buffer (0.25 M, pH 7.4) to remove blood, and quickly frozen in liquid nitrogen and stored at −70°C until the measurement of Na+,K+-ATPase was made. Na+,K+-ATPase activity, expressed as μmol phosphate (Pi) mg prot−1 h−1, was measured according to the method described by Penefsky and Bruist (1984). Ouabain-sensitive hydrolysis of ATP is coupled through the action of pyruvate kinase and lactate dehydrogenase to the oxidation of NADH, which is measured spectrophotometrically. In this process, ATP is regenerated and the accumulation of ADP is equimolar to the oxidation of NADH. The concentration of the total protein was determined by the method of Lowry et al. (1951), using crystalline bovine albumin as standard. The effect of osmolarity on fish growth was measured by examining the final body weight and the specific growth rate (SGR). The SGR was approximated in accordance with Dabrowski et al. (1985): SGR (% day−1) = 100 × (ln W t − ln W o)/t; where W t and W o represent final and initial mean body weights and t is the growth period in days.
Statistical analysis
Data are presented as means ± SE. Factorial analysis of variance (ANOVA) was used to test for treatment and time effects. Significant differences between means were identified using Duncan’s multiple-stage (P < 0.05).
Results
General observations and growth
No change in behavior or activity levels was observed in the fish during the course of the trial. At the end of the experiment, statistically significant differences were detected between the final body weight and SGR of fish kept in 0‰ and 10‰ media, while no significant differences were recorded in the initial body weight (Table 1). The final body weight and SGR values revealed that fish exposed to 10‰ media grew at a faster rate than those maintained in 0‰ media.
Osmolarity
Figure 1 shows the serum osmolarity over the experimental period. Serum osmolarity levels were affected by duration of brackish water exposure (P < 0.05). Serum osmolarity increased 13% in the BW in relation to FW during the first 12 h, and remained on a plateau until 24 h. Between 24 h and 216 h of brackish water exposure, serum osmolarity levels decreased significantly, reaching a new steady state which was higher (P < 0.05) than the fresh water control, which maintained its initial levels (264.14 ± 0.72 mOsmol kg−1) during the experiment (Fig. 1).
Electrolyte concentrations
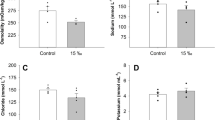
Serum Na+, Ca2+ and C1− concentrations displayed similar patterns of change in relation to duration of brackish water exposure. Following direct transfer from FW to BW, serum Na+, Ca2+ and C1− levels increased significantly to peak value during the first 24 h (P < 0.05). Between 24 h and 216 h of brackish water exposure, serum Na+, Ca2+ and C1− levels decreased significantly, reaching a plateau which was significantly higher (P < 0.05) than the levels of fresh water control, which maintained its initial levels during the experiment (Fig. 2).
Blood serum ions concentrations of Chinese sturgeon after transfer from FW to FW or to 10‰ BW. Data are presented as means ± SE (n = 6–8). Filled and empty symbols corresponded to values of BW and FW fish, respectively. Bars show the standard error. Different letters indicate significant differences (P < 0.05, Factorial ANOVA, Duncan’s multi-stage test)
Serum potassium levels of fish cultured at brackish water were unaffected by salinity at the beginning and end of the trial. Between 24 h and 216 h, a significant effect of salinity on serum Na+ level occurred; the increase was gradual and significant. Values reached a peak at 216 h after exposure to brackish water. At 24, 72 and 216 h, serum Na+ levels were significantly higher (P < 0.05) in the BW group than in the FW control (Fig. 2). On the other hand, no significant change (P > 0.05) in serum Na+ levels was observed in the FW control fish during the course of the trial (Fig. 2).
Na+,K+-ATPase activity
Na+,K+-ATPase activity of gill exhibited an abrupt decrease after transference from FW to BW, but began to increase at 3 h after transference. This parameter doubled in fish exposed to BW in comparison to those kept in FW at 24 h, remaining on a plateau until 72 h, and then decreased rapidly, reaching a new steady state after 216 h. Between 12 h and 480 h after transference, gill Na+,K+-ATPase activities were significantly higher (P < 0.05) in the BW group than in the FW control (Fig. 3). In contrast, Na+,K+-ATPase activity of the spiral valve showed transient increase after transference from FW to BW, and then decreased rapidly at 3 h, reaching the lowest at 24 h after transference. Minimum enzyme activity of the spiral valve was 0.12 ± 0.015 μmol Pi mg prot−1 h−1. At 216 h after exposure to BW, Na+,K+-ATPase activities of the spiral valve increased slowly to the levels of the FW control.
Na+,K+-ATPase activities of Chinese sturgeon after transfer from FW to FW or to 10‰ BW. Data are presented as means ± SE (n = 6–8). Filled and empty symbols corresponded to values of BW and FW fish, respectively. Bars show the standard error. Different letters indicate significant differences (P < 0.05, Factorial ANOVA, Duncan’s multi-stage test)
Discussion
A fish’s capacity to adapt to different levels of environmental salinity ultimately depends on its capacity to regulate the uptake and excretion of ions, and to maintain its hydromineral equilibrium. The present study shows an increase of serum osmolarity and ion concentrations (Na+, Cl− and Ca2+) immediately after the transference of juvenile Chinese sturgeon from FW to BW. At 24 h after transition to BW of 10‰, serum osmolarity and ion concentrations (Na+, Cl− and Ca2+) had begun to decrease, and reached a new steady state after 216 h. These results are consistent with other studies in several sturgeon species (A. oxyrinchus, Altinok et al. 1998; A. güeldenstäedti, Natochin et al. 1985; A. transmontanus, McEnroe and Cech 1985; A. brevirostrum and A. oxyrhynchus, Krayushkina 1998; A. naccarii, Cataldi et al. 1995b, 1999; McKenzie et al. 1999; Martínez-Álvarez et al. 2002). These changes can be attributed to changes in the water content in the blood, caused by the change in environmental salinity (Plaut 1998). Like other sturgeon, juvenile Chinese sturgeon are hyperosmotic to fresh water and hyposmotic to sea water. Thus, at the beginning of exposure to a hyperosmotic/isosmotic environment, the fish would lose water passively, and thereby undergo increases in the concentrations of serum ions. Afterwards, the compensatory increase in water ingestion would provide a transitory dilution of the blood parameters. Finally, these would return to new steady values as a result of the rest of the osmoregulatory mechanisms (Martínez-Álvarez et al. 2002).
In euryhaline fish, abrupt transfer from SW to BW or from BW to SW induces changes in osmotic plasma parameters and the consequent activation of osmoregulatory system to try to recover the original values. In this process, two periods were described: (1) the adaptative period, with changes in osmotic parameters, and (2) the chronic regulatory period, where these parameters again reach homeostasis (Holmes and Donaldson 1969; Maetz 1974). In agreement with this, our study shows that the acclimation of Chinese sturgeon to BW involves two different physiological periods, the crisis period and the stabilization period. The crisis period, when the serum osmolarity and ion concentrations (Na+, Cl− and Ca2+) exhibit transient increase, occurs in the first 24 h after transference to BW. Immediately post-transfer to BW, the critical problem faced by sturgeon is dehydration, caused by osmotic removal of water in the gills (Cataldi et al. 1999; McKenzie et al. 1999; Martínez-Álvarez et al. 2002). This crisis period occurs in Gulf of Mexico sturgeon (A. oxyrinchus) at 24 h after transition to BW (Altinok et al. 1998). The subsequent 24–216 h marks the beginning of the stabilization period, when the serum osmolarity and ion concentrations (Na+, Cl− and Ca2+) start to decrease. Altinok et al. (1998) also reported a strong increase of the osmolarity and ion concentrations after the transference to BW in A. oxyrinchus, peaking at 24 h, followed by a decline to basal level.
Ion regulation maintains stable concentrations of electrolytes in extracellular fluids. Generally, extracellular Na+, Cl− and Ca2+ concentrations are elevated while K+ concentrations are depressed relative to the intracellular medium. This unequal distribution of ions across cell membranes results in the establishment of membrane potential. As regulatory capacity is lessened, electrolyte concentrations are altered, disrupting the membrane potential. As the cell is no longer excitable, function is lost, and the cell dies (Holmes and Donaldson 1969). Ion regulations in sturgeon are thought to be similar to those of teleosts (Krayushkina et al. 1995). Na+,K+-ATPase plays a critical role in sodium and water balance in both marine and fresh water fishes because it is involved in active sodium transport (Borgatti et al. 1992). In the gill, Na+,K+-ATPase drives the active ion transport processes of the “chloride” or “mitochondria-rich” cells (Marshall and Bryson 1998). Na+,K+-ATPase similarly activates the ion pumps in the intestine. In sea water fish, the intestine actively absorbs Na+ and Cl− until intestine fluid become hyposmotic to plasma, at which time water moves passively into the plasma, thereby maintaining overall osmotic balance (Kirsch et al. 1985; Karnaky 1998).
In our experiment, we detected a progressive increase in gill Na+,K+-ATPase activity of fish inhabiting brackish water, peaking at 24 h after transference and remaining at a new steady state which was significantly higher than the levels of fresh water control fish (Fig. 3). The increase in plasma osmolality reflects the challenge of the hyperosmotic medium against the body fluids of the fish, whereupon greater Na+,K+-ATPase activity occurs—that is, the branchial hypo-osmoregulatory mechanisms participating in the re-establishment of the normal values of blood concentration were activated. Greater activity of Na+,K+-ATPase in the gills of teleosts during acclimation to sea water has been reported (Kirschner 1980; Johnston and Cheverie 1985; Boeuf et al. 1985; Fuentes et al. 1997). According to McCormick (1995), in almost all the teleosts studied (several dozen species), stronger salinity provoked high activity levels of this transporter enzyme. In sturgeons, increased activity of this enzyme has also been demonstrated during the transition from fresh water to salt water. In the Siberian sturgeon A. baerii, this enzyme increased when exposed to an iso- and hyperosmotic medium (Rodríguez et al. 2002, 2003). McKenzie et al. (1999), also working with A. naccarii, found a significant increase in branchial Na+,K+-ATPase in fish acclimated to brackish water (23‰).
When fish were transferred from FW to BW of 10‰, there was a marked, short-term (3 h after transfer to brackish water) drop in the gill Na+,K+-ATPase activity compared with that of the fish kept in fresh water. In contrast, the spiral valve Na+,K+-ATPase activity increased rapidly. These changes in the spiral valve and the gill Na+,K+-ATPase activities might be attributed to the hyperosmoregulatory mechanism used to adapt to hyposmotic environment, i.e., active uptake of Na+ and Cl− ions, and producing a large amount of dilute urine (Jobling 1995). Na+-K+-ATPase activity in the spiral valve fell rapidly at 3 h, reaching the lowest at 24 h after transference. From 24 h to 480 h, a significant increase in the spiral valve Na+-K+-ATPase activity was recorded in fish exposed to 10‰ water salinity, and at the end of the period no statistically significant differences were recorded between fish kept in 0‰ and 10‰ media. This decrease in the spiral valve Na+,K+-ATPase activity might be the mechanism used to adapt to isosmotic/hyperosmotic environments, i.e., reducing the ionic Na+ and Cl− inflow. These changes of the spiral valve Na+-K+-ATPase activity indicate that Chinese sturgeon exposed to isoosmotic media may ingest water in order to compensate for the osmotic water losses, as Kirsch et al. (1984) reported for euryhaline teleost species. This reduction in the spiral valve Na+,K+-ATPase activity, however, also compromised intestinal nutrient absorption, since the spiral valve is the main site of intestinal absorption in sturgeons (Gawlicka et al. 1995), directly affecting fish nutrition and growth performance (Morgan and Iwana 1991). Rodriguez et al. (2002) showed that A. baerii exposed to isosmotic and hyperosmotic media were unable to grow normally and had actually lost weight in hyperosmotic media. Allen and Cech (2007) also reported an age/body size of fish effect in hyperosmotic adaptability. Our data do not coincide with the results reported by Rodriguez et al. (2002). The final body weight and SGR values in our study revealed that fish exposed to isosmotic media grew at a faster rate than those kept in hyposmotic media. These differences might be due to species-specific morpho-physiological mechanisms for salinity adaptation and tolerance, which would be directly related to the natural history of this species. The Siberian sturgeon is semi-anadromous, spending a significant portion of its life in brackish water habitats (Rodriguez et al. 2002), and Chinese sturgeon is truly anadromous, spending a significant portion of its life in full-strength oceanic sea water (Zhuang et al. 2002).
Na+,K+-ATPase activities in the gills and spiral valve, SGR values and new steady states of plasma osmolality and electrolyte concentrations of fish kept in 273 mOsmol kg−1 all seem to indicate that Chinese sturgeon juveniles (7 months old) were able to regulate their hydromineral content satisfactorily during the 20-day period. However, further research is needed to evaluate the long-term growth of juvenile Chinese sturgeon exposed to a plasmatic isosmotic/hyperosmotic medium. The results of our study indicate the existence of hyposmoregulatory adaptive mechanisms in 7-month-old juvenile Chinese sturgeon which enable this fish to acclimate itself successfully to brackish water.
References
Allen PJ, Cech JJ (2007) Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79:211–229
Altinok I, Sara MG, Frank AC (1998) Ionic and osmotic regulation capabilities of juvenile Gulf of Mexico sturgeon, Acipenser oxyrinchus de Sotoi. Comp Biochem Physiol 120:609–616
Boeuf G, Le Roux A, Gaignon JL, Harache Y (1985) Gill (Na+-K+)-ATPase activity and smolting in Atlantic salmon Salmo salar L. Aquaculture 45:73–81
Borgatti AR, Trigari G, Paflianari A, Ventrella V (1992) Ouabain-insensitive Na+ stimulation of microsomal Mg2+-ATPase in gills of sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol A 81:127–135
CARSGS (The Changjiang Aquatic Resources Survey Group, Sichuan) (1988) The biology of the sturgeons in Chiangjiang and their artificial propagation. Sichuan Science and Technology Publishing House, Chengdu, China (in Chinese)
Cataldi E, Ciccotti E, Di Marco P, Di Santo O, Bronzi P, Cataudella S (1995a) Acclimation trials of juvenile Italian sturgeon to different salinities: morpho-physiological descriptors. J Fish Biol 47:609–618
Cataldi E, Bronzi P, Ciccotti E, Di Marco P, Di Santo O, Monaco G, Cataudella S (1995b) Morphology of gills, digestive tract and kidney of Italian sturgeon, Acipenser naccarii in fresh and saline water: preliminary results. In: Gershanovich AD, Smith TII (eds) International Symposium on Sturgeons Proceedings, VNIRO, Moscow, pp 52–61
Cataldi E, Barzaghi C, Di Marco P, Boglione C, Dini L, McKenzie DJ, Bronzi P, Cataudella S (1999) Some aspects of osmotic and ionic regulation in Adriatic sturgeon Acipenser naccarii. I: ontogenesis of salinity tolerance. J Appl Ichthyol 15:57–60
Chang JB, Cao WX (1999) History and prospect of conservation on Chinese sturgeon in the Yangtze River. Acta Hydrobiol Sinica 23:713–720 (in Chinese)
Dabrowski K, Kaushik SJ, Fauconneau B (1985) Rearing sturgeon (Acipenser baeri, Brandt) larvae. I. Feeding trial. Aquaculture 47:185–192
Fuentes J, Soengas JL, Rey P, Rebolledo E (1997) Progressive transfer to seawater enhances intestinal and branchial Na+–K+-ATPase activity in non-anadromous rainbow trout. Aquac Int 5:217–227
Gawlicka A, Teh SJ, Hung SSO, Hinton DE, de la Noüe J (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol Biochem 14:357–371
Holmes WN, Donaldson EM (1969) The body compartments and the distribution of electrolytes. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 1. Academic Press, New York, pp 1–89
Hubei Institute of Hydrobiology (1976) Fishes of the Yangtze River. Science Press, Beijing (in Chinese)
Jobling M (1995) Osmotic and ionic regulation—water and salt balance. In: Jobling M (ed) Environmental biology of fishes. Chapman & Hall, London, pp 211–250
Johnston CE, Cheverie JC (1985) Comparative analysis of ionoregulation in rainbow trout (Salmo gairdneri) of different sizes following rapid and slow salinity adaptation. Can J Fish Aquat Sci 42:1991–2003
Karnaky KJ (1998) Osmotic and ionic regulation. In: Evans DH (ed) The physiology of fishes, 2nd edn. CRC Press, Boca Raton, Fla., pp 157–176
Kirsch R, Humbert W, Rodeau JL (1984) Control of the blood osmolarity in fishes, with references to the functional anatomy of the gut. In: Gilles R, Gilles-Baillien M (eds) Osmoregulation in estuarine and marine animals. Springer, Berlin, pp 67–92
Kirsch R, Humbert W, Simmoneaux V (1985) The gut as an osmoregulatory organ: comparative aspects and special references to fishes. In: Gilles R, Gilles-Baillien M (eds) Transport processes, iono-and osmoregulation. Springer, Berlin, pp 265–277
Kirschner LB (1980) Comparison of vertebrate salt-excreting organs. Am J Physiol 7:R219–R223
Krayushkina LS (1998) Characteristics of osmotic and ionic regulation in marine diadromous sturgeons Acipenser brevirostrum and A. oxyrhynchus (Acipenseridae). J Ichthyol 38:660–668
Krayushkina LS, Polls WTW, Gerasimov AA, Panov AA (1995) Peculiarities ionic regulation in young sturgeons (Acipenseridae) during adaption to sea water. In: Gershanovichm AD, Smith TII (eds) International Symposium on Sturgeons Proceedings, VNIRO, Moscow, pp 43–51
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maetz J (1974) Aspects of adaptation to hypo-osmotic and hyperosmotic environments. In: Malins DC, Sargent JR (eds) Biochemical and biophysical perspectives in marine biology. Academic Press, London, pp 1–167
Marshall WS, Bryson SE (1998) Transport mechanisms of seawater teleost chloride cells: an inclusive model of a multifunction cell. Comp Biochem Physiol A 119:97–106
Martínez-Álvarez RM, Hidalgo MC, Domezain A, Morales AE, García-Gallego M, Sanz A (2002) Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol 205:3699–3706
McCormick SD (1995) Hormonal control of gill Na+/K+-ATPase and chloride cell function. Fish Physiol 14:285–315
McCormick SD (1996) Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar): interaction with cortisol. Gen Comp Endocrinol 101:3–11
McEnroe M, Cech Jr JJ (1985) Osmoregulation in juvenile and adult white sturgeon, Acipenser transmontanus. Env Uio Fish 14(1):23–30
McKenzie DJ, Cataldi E, Di Marco P, Mandlich A, Romano P, Ansferri S, Bronzi P, Cataudella S (1999) Some aspects of osmotic and ionic regulation in Adriatic sturgeon Acipenser. naccarii II: morpho-physiological adjustments to hyperosmotic environments. J Appl Ichthyol 15:61–66
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 48:2083–2094
Natochin YV, Lukianenko VI, Kirsanov VI, Lavrova EA, Mctolloy GF, Shakhmotova HJ (1985) Features of osmotic and ionic regulations in Russian sturgeon (Acipenser guldenstadtii Brandt). Comp Biochem Physiol 80A(3):297–302
Penefsky HS, Bruist MF (1984) Adenosinetriphosphatases. In: Beinheim HU (ed) Methods of enzymatic analysis, vol IV. Chemie, Bergmeyer, pp 324–328
Plaut I (1998) Comparison of salinity tolerance and osmoregulation in two closely related species of blennies from different habitats. Fish Physiol Biochem 19:181–188
Rodríguez A, Gallardo MA, Gisbert E, Santilari S, Ibarz A, Sanchez J, Castello-Orvay F (2002) Osmoregulation in juvenile Siberian sturgeon (Acipenser baerii). Fish Physiol Biochem 26:345–354
Rodríguez A, Gisbert E, Gallardo MA, Santilari S, Ibarz A, Sánchez J, Castelló-Orvay F (2003) Osmorregulación en el esturión Siberiano juvenil (Acipenser baerii). Proceedings of the IX Congreso Nacional de Acuicultura, Cádiz, pp 111–112 (in Spanish)
Wei Q, Ke F, Zhang J, Zhuang P, Luo J, Zhou R, Yang W (1997) Biology, fisheries, and conservation of sturgeons and paddlefish in China. Environ Biol Fish 48:241–255
Xiao H, Chang JB, Liu Y (1999) Evaluation on status of artificial propagation and releasing of Chinese sturgeon in the Yangtze River. Acta Hydrobiol Sinica 23:572–576 (in Chinese)
Yi JF (1994) Investigation on the resources of young Chinese sturgeon in the Yangtze River. J Gezhouba Hydropower 1:53–58 (in Chinese)
Yu ZT, Xu YG, Deng ZL, Zhou CS, Yang X (1986) Studies on the reproductive ecology of Chinese sturgeon below Gezhouba Dam. Trans Chinese Ichthyol Soc 5:1–16 (in Chinese)
Zhao Y, Huang X, Yu ZT (1986) Investigation on the status of young Chinese sturgeon in the Yangtze River. Reservoir Fish 6:38–41 (in Chinese)
Zhuang P, Kynard B, Zhang LZ, Zhang T, Cao WX (2002) Ontogenetic behavior and migration of Chinese sturgeon, Acipenser sinensis. Environ Biol Fish 65:83–97
Acknowledgements
This study is based on part of the thesis by Xugang He in partial fulfilment of the requirements for the degree of Doctor at Huazhong Agricultural University. This study was supported by the National Natural Science Foundation of China (Project no. 30490230 WP4-003). We thank the Chinese Sturgeon Research Institute, Denghong Liu for help with obtaining juveniles. The authors would like to acknowledge the help of Hongjie Tian, Jing Ma and Xiaorong Huang with tissue sampling and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Zhuang, P., Zhang, L. et al. Osmoregulation in juvenile Chinese sturgeon (Acipenser sinensis Gray) during brackish water adaptation. Fish Physiol Biochem 35, 223–230 (2009). https://doi.org/10.1007/s10695-008-9230-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9230-5