Abstract
Baitfish such as golden shiners are subjected to stress during harvesting, grading, and transport. Their small size makes it difficult to measure the stress response with the biological indicator cortisol using conventional assay methods for plasma. This paper examines the development and validation of methods for whole-body cortisol extraction from individual baitfish. Three types of extracts were tested: (1) an ethyl ether unaltered extract (UA); (2) an extract reconstituted in phosphate buffered saline (PBS); (3) an extract that had been increased in volume by the addition of food-grade vegetable oil (VO). These extracts were evaluated using validation tests with radioimmunoassays (RIA) and enzyme-linked immunosorbent assays (ELISA). The UA extract produced inadequate volumes of extract for multiple assays and could not be used for the determination of cortisol in a single fish. The PBS reconstitution method failed the precision recovery of serial dilutions (62.3%), linearity (R 2: 0.7864), and parallelism validation tests. The VO volume-boosting method passed all validation tests [intra-assay coefficent of variation (%CV): 16.3 for ELISA and 5.9 for RIA; inter-assay %CV: 10.3; spiked recovery: 102.0%; dilution recovery: 93.0%; linearity R 2: 0.9435; log of serial dilutions was parallel] and provided enough extract for multiple assays from an individual baitfish. Based on these results, we conclude that the VO volume-boosting method presents a means for determining cortisol from individual baitfish using either RIA or ELISA assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baitfish production is a multi-million dollar industry in the United States, with Arkansas baitfish production alone totaling 20.3 million dollars (51 farms) in 2005 (NASS 2006). Baitfish, such as golden shiners (Notemigonus crysoleucas), are subjected to stress at various points during harvesting, grading, and transport to distribution points (Lochmann et al. 2003). The most common method of determining the stress response in fish is to measure plasma cortisol concentrations (Strange 1980; Tomasso et al. 1981; Wise et al. 1993, Sink and Strange 2004). However, there is currently no precise method for measuring the stress response in baitfish due to their small size and the associated difficulty in obtaining the minimum amount of plasma (typically 50–200 μl) to run a radioimmunoassay (RIA) or enzyme-linked immunosorbent assay (ELISA). Attempts to measure cortisol from golden shiners by conventional plasma techniques have produced highly variable results (Lochmann et al. 2003). Plasma samples from several fish are often pooled to obtain an adequate amount of sample for the cortisol determination assays, but the pooling of samples can lead to the dilution of treatment effects and the inability to detect individual responses to different treatments.
One probable solution to this problem is a whole-body cortisol assay. Published methods exist for whole-body cortisol assays of larval rainbow trout, Oncorhynchus mykiss (Barry et al. 1995), larval yellow perch, Perca flavescens (Jentoft et al. 2003), juvenile winter flounder, Pseudopleuronectes americanus (Breves and Specker 2003), juvenile summer flounder, Paralichthys denatus (Hinkle and Specker 2003), and adult zebrafish, Danio rerio (Ramsay et al. 2006). The methodology of the extraction procedure varies in these papers and is often incomplete in terms of the description of methods used during the extraction. Few attempts, other than the recovery of spiked samples (Breves and Specker 2003; Ramsay et al. 2006) or the recovery of dilutions (Jentoft et al. 2003), were made to validate the extraction process and determine if the extracts were appropriate for cortisol analysis.
We evaluated whole-body cortisol extraction procedures for use with golden shiners. We performed a series of whole-body extraction techniques adapted from previously published papers to test the suitability of the assay to measure stress response in baitfish. Validation tests were conducted to determine the suitability of extracts and their reconstitution procedures for detection by cortisol RIA and ELISA. The purpose of this study was to develop a technique in which cortisol, and the associated stress response, from an individual baitfish could be measured. This paper represents a technical update in the use of whole-body extraction procedures to evaluate stress response in industries that deal with small fish.
Methods
Fish
Stressed golden shiners (Notemigonus crysoleucas) were acquired from vats during grading (Lochmann et al. 2003) at a commercial baitfish production facility in Lonoke, Arkansas. The fish were seined and transported from the ponds to the vats approximately 20–24 h prior to grading, so they had undergone multiple stressors within a 24-h period that would increase plasma cortisol concentrations. The shiners were salt-“hardened”, un-sexed, arbitrarily selected from the vats and averaged 1.8 ± 0.19 g.
Cortisol extraction
The standard method was an ethyl ether extraction adapted from procedures described in Breves and Specker (2003) and Hinkle and Specker (2003). The shiners were euthanized with 150 mg/l of tricaine methane sulfonate and immediately frozen at −20°C. Each fish was then weighed, sliced into small sections, and placed into a disposable stomacher bag with 2 ml of phosphate buffered saline (PBS; 0.26 g KH2PO4, 2.17 g Na2HPO4−7H2O, 8.71 g NaCl adjusted to 1 l with de-ionized water at pH 7.4). The sample was then placed in a stomacher for 6 min. The contents were transferred to a 50-ml beaker, and 1 ml of PBS was used to rinse the remaining contents of the bag into the beaker.
Aliquots of 5 ml of laboratory grade ethyl ether were added to the contents of the beaker. The contents of the beaker were then transferred to a 10-ml screw top disposable test tube. Two milliliters of ethyl ether was used to rinse the remaining contents of the beaker into the tube and the tube was capped. The tube was vortexed for 1 min to ensure mixing of the tissue with the ethyl ether for cortisol extraction. The test tube was centrifuged for 10 min at 3000 rpm to condense the tissue at the bottom of the tubes. The tube was then immediately frozen at −20°C for 2 h, and the unfrozen portion (ethyl ether containing cortisol) was decanted into a fresh 10-ml test tube. The ethyl ether was evaporated under a gentle stream of nitrogen for 2 h, yielding a lipid extract containing the cortisol. The extract was stored at −20°C until the RIA and ELISA were conducted on the samples.
Three protocols were evaluated for use in extracting the cortisol in baitfish. An unaltered lipid extract (UA method) obtained from the extraction procedure was the first method used for determination of the cortisol. In the second method, the lipid extract was reconstituted with 1 ml of PBS (PBS method) to increase the volume, as described in Ramsay et al. (2006). The third method involved the addition of 100 μl vegetable oil (food grade) per gram of body weight to increase the volume of the extract (VO method) before adding the homogenized sample and ethyl ether. The extraction procedure was then completed as described above.
The vegetable oil was previously analyzed in our laboratory to ensure that it contained no cortisol. Oils from all-plant sources (soybean, canola, cottonseed, etc.) should not contain cortisol, but it should be assayed prior to use to confirm that no contamination has occurred. Cortisol from vegetable oil was not detectable by RIA or EIA. The recovery of vegetable oil from the extraction procedure was greater than 96% in the three trials. We therefore conclude that this presents a standardized method of increasing the extract volume based on fish weight.
Cortisol ELISA
Cortisol extracts were thawed (allowed to reach ambient temperature) and used in conjunction with an Assay Designs (Ann Arbor, Mich.) cortisol ELISA kit (no. 900-071) that had been previously validated for use in golden shiners in our laboratory. Validation procedures were similar to those used in Barry et al. (1993) to validate a cortisol ELISA for use in rainbow trout (Oncorhynchus mykiss) and lake trout (Salvelinus namaycush). This is a comparative assay designed for determining differences in relative cortisol concentrations in between treatments in the same study. When evaluated against a standard RIA, the ELISA produces cortisol readings that are approximately 25% less (1:0.75) than those of the RIAs. The first method of extraction (unaltered extract) had to be pooled (three shiners) to produce a sufficient volume for all of the validation procedures.
Extracts from all three extraction methods were used for the validation. All samples were run in duplicate for the validation procedures. Validation tests followed the 'Validation of Analytical Procedures: Methodology' (FDA CVM 1999). The accuracy of cortisol detection was tested by calculating the recoveries from samples spiked with known amounts of cortisol (50, 25, and 12.5 ng/ml) as well by comparing the results from the ELISA and RIA methods. The precision of the method was tested by comparing the results from repeated assays of samples (ten times) and by serial dilutions (0, 25, 50, and 75%) made by adding ELISA buffer to the samples and subsequently calculating the coefficient of variation (%CV). Spiked and diluted sample recovery percentage limits of within 90–110% were defined as meeting validation criteria. A %CV of ≤20.0 was set as the acceptable limit for the intra- and inter-assay %CV (Pifat 2006).
Linearity and parallelism were tested using serial dilutions of extracts. Duplicates were run of extract dilutions (0, 25, 50, and 75%) made by adding ELISA buffer. An R 2 > 0.90 was set as the passing limit for the linearity test. The log of two cortisol concentrations must not cross during the range of the dilutions in order to pass the parallelism test.
Cortisol RIA
The same cortisol extracts used in the ELISA validation were shipped frozen to the University of Tennessee’s Clinical Endocrinology Service for RIA cortisol analysis by an independent laboratory. A competitive assay was used for quantitative analysis of the cortisol (hydrocortisone) in the fish extracts. All samples were analyzed in duplicate. A %CV ≤ 10.0 was set as the acceptable limit for the intra- and inter-assay %CV for the RIA (Pifat 2006).
Statistical analysis
Regression of standardized sample values for the standard curve and linearity dilutions and the coefficient of variation for the unknown samples were calculated automatically by a calibrated microplate reader and the gamma counter software. All other regression, recovery, and inter-assay %CVs were calculated using SPSS ver. 11.0 (SPSS, Chicago, Ill.) statistical software.
Results
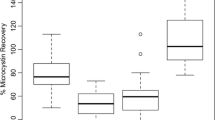
The results from the RIA and ELISA in terms of determining cortisol concentrations (Table 1) and %CVs for the UA and VO methods were similar (P > 0.05) to the previously noted assay ratio of 1: 0.75 for the two methods (1: 0.74, and 1: 0.71, respectively). The PBS method varied more between the two assays (1: 0.39). However, precision as determined by the intra-assay %CV (Table 1) was within the set parameters for all treatments in both assays. The inter-assay %CV was conducted for the ELISA only and was acceptable for all three methods (UA: 6.3%, PBS: 15.9%, and VO: 10.3%).
The mean detection accuracy of spiked samples was within the predetermined limits for all three methods (UA: 105.9%, PBS: 92.3%, and VO: 102.0%). The mean recovery of diluted samples was within the set limits for the UA (100.0%) and VO (93.0%) treatments, but the PBS treatment failed this test of precision, with mean recoveries of 62.3%. The UA and VO treatments passed the linearity test (R 2: 0.9837 and 0.9435, respectively), but the PBS treatment was non-linear with an R 2 of 0.07864. In addition, the UA and VO treatments passed the parallelism test, remaining parallel throughout the course of the dilutions; in contrast, the PBS treatment failed the set parameters for parallelism as the dilutions were not parallel to the standard curve due to the failed linearity test. The UA and VO treatments passed all of the validation criteria, while the PBS treatment failed three tests (precision recovery of dilutions, linearity, and parallelism).
Discussion
The purpose of this study was to develop a technique in which cortisol from an individual baitfish could be measured. All three methods produced an extract that contained measurable levels of cortisol. The UA extract from an individual fish did not produce enough volume to allow repeated measures, thereby necessitating the pooling of extracts from three shiners. The two volume enhancement methods – either the utilization of PBS to reconstitute the sample extract or the addition of vegetable oil (VO) to the sample – were more appropriate methods as they produced volumes large enough to conduct multiple cortisol assays from a single shiner.
The extraction process removes not only cortisol from the sample but also various lipids and other cholesterol-derived hormones such as testosterone, estradiol, progesterone, pregnenolone, and aldosterone. Because the extract is oil based, reconstitution with an aqueous solution such as PBS does not produce an emulsified product. Even with constant vortexing, the lipid extract forms minute oil droplets in suspension, and the extract is lost due to the visible adhesion of extract to the sides of the tube. This may be why the PBS treatment failed the precision recovery, linearity, and parallelism tests as extract is lost to the sides of the tubes during the dilution process. This also explains why each dilution produced less cortisol than expected, even though the intra-assay %CV remained acceptable.
The VO volume enhancement method is the best of the three extraction methods tested as it passed all of the validation tests and can be used with a single fish. It is standardized by the addition of oil based on fish weight, which is important as previous authors (Breves and Specker 2003; Jentoft et al. 2003) have added a set volume of PBS or other buffered diluents to each sample regardless of fish weight. This results in different dilution effects, as the fish yield different quantities of extract based on the size and lipid content of the individual. Also, no standard method of extract reconstitution exists, as Breeves and Specker (2003) and Hinkle and Specker (2003) used 500 μl of diluent, while Jentoft et al. (2003) used 250 μl of diluent and Ramsay et al. (2006) used 1 ml of diluent to reconstitute samples.
The ethyl ether/VO method for whole-body cortisol extraction is a possible method for measuring the physiological stress response in baitfish. This method passed all validation tests and produced cortisol extracts suitable for use with either RIA or ELISA cortisol assays. Research on stress response in small fish will benefit from an effective method to measure cortisol in fish that do not yield enough plasma for conventional RIA or ELISA plasma methods. Pooled plasma samples have many drawbacks, as one fish in the sample can lead to misinterpretation of the results and inaccurate results. The ethyl ether/VO method for whole body cortisol extraction can detect distinct levels of cortisol and allow the reproducibility of single observations.
References
Barry T, Lapp A, Kayes T, Malison J (1993) Validation of a microtitre plate ELISA for measuring cortisol in fish and comparison of stress responses of rainbow trout (Oncorhynchus mykiss) and lake trout (Salvelinus namaycush). Aquaculture 117:351–363
Barry T, Malison J, Held J, Parrish J (1995) Ontogeny of the cortisol stress response in larval rainbow trout. Gen Comp Endocrinol 97:57–65
Breves J, Specker J (2003) Acute cortisol stress response of juvenile winter flounder (Pseudopleuronectes americanus, Linnaeus) to predation. Integr Comp Biol 43:857
FDA CVM (U.S. Food and Drug Administration's Center for Veterinary Medicine) (1999) Validation of analytical procedures: methodology. Available at: http://www.fda.gov/cder/guidance/1320fnl.pdf#search=‘Validation% 20of%20analytical%20procedures%3A%20methodology'. Cited 21 Sept. 2006
Hinkle L, Specker J (2003) Cortisol levels and glucocorticoid receptor expression in summer flounder (Paralichthys dentatus) subjected to osmotic stress. Integr Comp Biol 43:1048
Jentoft S, Held J, Malison J, Barry T (2003) Ontogeny of the cortisol stress response in yellow perch (Perca flavescens). Fish Physiol Biochem 26:371–378
Lochmann R, Davis K, Simco A (2003) Cortisol response of juvenile golden shiner Notemigonus crysoleucas fed diets differing in lipid content. Fish Physiol Biochem 27:29–34
NASS (National Agriculture Statistics Service) (2006) Value of aquaculture products sold by Type, by State and United States: 2005 and 1998. In: 2005 Census of Aquaculture. United States Food and Drug Administration Available via http://www.nass.usda.gov/Census_of_Agriculture/2002/Aquaculture/index.asp
Pifat D (2006) Assay Validation. U.S. Food and Drug Administration. Available At: http://www.fda.gov/cber/summaries/120600bio10.ppt#1
Ramsay J, Feist G, Varga Z, Westerfield M, Kent M, Schreck C (2006) Whole-body cortisol as indicator or crowding stress in adult zebrafish, Danio rerio. Aquaculture 258:565–574
Sink T, Strange R (2004) Linking stress to the increased susceptibility of channel catfish to enteric septicemia using cortisol. J Aquat Anim Health 16:93–98
Strange R (1980) Acclimation temperature influences cortisol and glucose concentrations in stressed channel catfish. Trans Am Fish Soc 109:298–303
Tomasso J, Davis K, Parker N (1981) Plasma corticosteroid dynamics in channel catfish, Ictalurus punctatus (Rafinesque), during and after oxygen depletion. North American J Aquac 65:162–164
Wise D, Schwedler T, Otis D (1993) Effects of stress on susceptibility of naive channel catfish in immersion challenge with Edwardsiella ictaluri. J Aquat Anim Health 5:92–97
Acknowledgements
The authors thank Kenneth Davis and Matthew McEntire of the United States Department of Agriculture’s Agriculture Research Service for their support in bringing this line of research to our attention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sink, T.D., Kumaran, S. & Lochmann, R.T. Development of a whole-body cortisol extraction procedure for determination of stress in golden shiners, Notemigonus crysoleucas . Fish Physiol Biochem 33, 189–193 (2007). https://doi.org/10.1007/s10695-007-9130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-007-9130-0