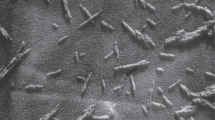
A process for preparing a dispersion of nanocrystalline cellulose (NCC) particles from various types of plant raw materials that is based on the use of preliminary radiation-chemical treatment, hydrolysis, and ultrasound treatment is described. The experiments produced dispersions in which the NCC particles had thin rod-like shapes of length 100–500 nm and width 25–50 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Wood is of interest to nanotechnology because it is an available and renewable natural nanosystem. The expression “That which other nanotechnologists have to create, wood nanotechnologists can just investigate and use” applies to it [1]. The inner hierarchical structure of wood comprises several dimensions. Whole wood is measured in meters; tree diameters, in centimeters (heartwood, core, sapwood, bark); rings (tree age), in millimeters; cell anatomy, in tens of micrometers; layered cell-wall structure, in micrometers; shape of cellulose fibrils in hemicellulose and lignin matrix, in tens of nanometers; and molecular structures of cellulose, hemicellulose, lignin, and their chemical reactions, in nanometers.
Cellulose is not only the most available natural polymer on earth but also one of the most interesting naturally occurring constructs. Unique 3D-networks resulting in a complicated structure formed by alternating nanocrystalline and amorphous regions are produced from a rather simple chemical structure (two glucose molecules represent the repeat unit of the macromolecule) through hydrogen bonds [2].
The crystalline structures typically have high physicomechanical properties and high chemical stability. From an industrial viewpoint, the supramolecular structure of available and renewable cellulose has the potential to become a candidate for the development and stable growth of innovative technologies for producing high-quality value-added materials based on application of structural elements dispersed to the nano-sized level [3].
The unique properties of nanocrystalline and nanofibrous celluloses, derivatives of renewable and well-managed forestry resources, are responsible for its excellent commercial potential, from improving paper quality to manufacturing bionanocomposites [4]. It should be noted that almost any cellulose-containing material can be viewed as a potential source for isolating nano-sized cellulose structures.
Controlled hydrolysis is an effective method for producing NCC [5]. It is combined in several instances with various physical treatments. However, the properties of the resulting nanodispersions (yield of final product, degree of dispersion, particle-size distribution of the nanoparticles) are determined to a large extent by the type of precursor, i.e., the starting cellulose-containing material and its processing conditions.
Technical bleached, semi-bleached, and unbleached cellulose produced by sulfate, sulfite, and organic-solvent pulping methods from coniferous and deciduous wood; microcrystalline cellulose (MCC), and plant raw material of nonwoody origin from beet pulp and cotton lint were used as starting material for preparing NCC (Table 1).
An aqueous dispersion of NCC particles was prepared by depolymerization of starting cellulose-containing material through a stepwise process of radiation-chemical pretreatment, acid hydrolysis at elevated temperature, and ultrasonic treatment of the resulting product. This process for producing NCC was implemented on an experimental line (Fig. 1). The presence of nanodisperse particles was evaluated visually from the Tyndall effect, which is observed by passing light through an optically heterogeneous medium to produce a lighted cone visible on a dark background.
Starting raw material was moistened, soaked in water with gentle agitation (1:4 ratio), and stored for at least two weeks. Ground cellulose of various origin and MCC were separated into individual fragments under the usual conditions. The lack of a Tyndall effect in the filtrate led to the conclusion that these particles were much larger than nano-sized.
The precipitate was filtered and subjected to ionizing radiation in a Gammatok-100 stand. When the irradiation was finished, the sample was diluted with water (1:4 ratio) and filtered.
The precipitate was subjected to acid hydrolysis in a 1-L reactor by the literature methods [6, 7]. For this, a given volume of H2SO4 (10% solution or conc.) and H2O2 were added. The pulp:solution ratio was adjusted to 1:4. The stirrer and heater were turned on. Hydrolysis was carried out at 85-98°C for 2 h. The resulting suspension was filtered while monitoring the filtrate for the presence of nanoparticles using the Tyndall effect. If a Tyndall effect was not observed, the precipitate was washed until neutral and hydrolyzed again.
The washed precipitate that formed after hydrolysis was processed in some instances. For this, NaOH solution (10%, 0.2 mL/g) was added in a 1:4 ratio. The processing was carried out in the same reactor at 80-85°C for 2 h. The resulting pulp was filtered, washed with hot water until the pH was 7, and diluted with water to a 1:4 ratio while agitating.
Suspensions obtained after hydrolysis and alkali treatment were subjected to ultrasonic treatment on units of power 200 W for 90–360 min and of power 1.5 kW for 30–60 min. Suspensions of starting raw material and precipitates after hydrolysis and alkali treatment were also processed separately using ultrasound. The aqueous dispersion was separated on a centrifuge at 5,000-6,000 rpm for 60 min. The precipitate obtained after centrifugation was in several experiments diluted with water to a 1:4 ratio and returned for repeated ultrasonic treatment. The cycle was repeated another two times in isolated instances. In those instances the yield of nanoparticles was calculated as the sum of nanoparticles from each cycle. Table 2 presents data for the nanoparticle yield and type of formed dispersion as functions of the processing conditions.
The obtained dispersions of NCC were reminiscent visually of gels. However, they exhibited flowability and differed both externally and in composition and properties. A transparent gelFootnote 1 (Table 2, No. 1) had a low concentration of NCC, a low yield, and poor stability to the process conditions. A sour-cream-like gel (No. 2) had an elevated concentration of NCC, higher yield relative to the starting weight, and high stability.
According to the results, the NCC yield doubled if sulfite cellulose was used with increased concentrations of H2SO4 and H2O2 and a simultaneous increase of ultrasonic treatment power, despite a much shorter duration. A higher NCC yield (gel No. 1) was also achieved if deciduous cellulose sulfate was used without alkali treatment even under milder hydrolysis conditions than for sulfite cellulose.
The NCC yield (gel No. 1) approached or exceeded those obtained for various types of cellulose if MCC was used as the precursor under hydrolysis conditions similar to those typical for cellulose and ultrasonic treatment of shorter duration. It is interesting that increasing the H2SO4 and H2O2 concentrations during MCC treatment caused a sharp drop in the NCC yield as a more highly concentrated gel (gel No. 2).
Although alkali treatment can be used as an isolation step for NCC [8], the results indicated that the NCC yield decreased as a result of such treatment. Obviously this was the result of an increased percentage of low-molecular-weight fractions and their dissolution. The result obtained from hydrolysis and ultrasonic treatment of beet pulp was unexpected. The NCC yield (gel No. 2) was almost the same as the cellulose content in the starting material.
It should be noted that ultrasonic treatment gave a high NCC yield (gel No. 2) if Mg-bisulfite coniferous cellulose that was taken from an industrial stream, not dried, and even not hydrolyzed was used. However, like when beet pulp was used as the precursor, a large amount of impurities and the dark color of the product limited substantially its area of application.
A 1:4 solid:liquid ratio, hydrolysis temperature not exceeding 85°C, and H2SO4 concentration in the 3-4% (by mass) range should be used during hydrolysis of fibrous raw material and ultrasonic treatment to prepare gel No. 1. The suspension obtained by observing these processing parameters was separated on a centrifuge into transparent gel No. 1 and a precipitate that required further processing. Gel No. 2 was obtained under more rigorous conditions. The ratio was less than 1:4; hydrolysis temperature, 90-95°C; and H2SO4 concentration, higher.
It was found that preliminary treatment of plant raw material using ionizing radiation shortened significantly the time for hydrolysis and MCC production under the given conditions. It also reduced energy consumption during subsequent production of the NCC hydrogels. The reduction in the processing time was from 20 to 35% depending on the preliminary processing conditions.
This method could produce an aqueous dispersion of NCC with particle structures (Fig. 2) of the following dimensions: Gel No. 1, 100–500 nm length; 25–50 nm width; gel No. 2, 100–900 nm length, 25–120 nm width.
The resulting NCC dispersions were intended for use as binders, a nanostructured component in multifunctional composites, and rheological modifiers (e.g., in drilling and cement solutions). They are useful in the manufacture of biodegradable polymers, thickeners, viscosity modifiers, and water-latex paint and emulsion stabilizers and in the pharmaceutical, medical, food, perfume, and other industrial sectors [9].
Notes
According to the formulation given in an electronic encyclopedia (http://en.wikipedia.org/wiki/Nanocellulose/), aqueous dispersions of NCC are called nanocellulose hydrogels.
References
D. Klemm, Macromol. Symp., No. 280, 60–71 (2009).
V. I. Azarov, A. V. Burov, and A. V. Obolenskaya, Chemistry of Wood and Synthetic Polymers [in Russian], SPbLTA, St. Petersburg (1999).
T. Zimmermann, E. Pohler, and P. Schwaller, Adv. Eng. Mater., 7, No. 12, 1156–1161 (2005).
K. Oksman, A. P. Mathew, et al., Compos. Sci. Technol., 66, 2776–2784 (2006).
V. Favier, H. Chanzy, and J. Cavaille, Macromolecules, 28, 6365–6367 (1995).
A. A. Polyutov, R. Z. Pen, and A. V. Byvshev, New Celluose Intermediates [in Russian], Krasnoyarsk (2004).
I. A. Vshivkova, in: Proceedings of the All-Russian Scientific-Practical Conf. Dedicated to the 80 th Anniversary of the Siberian State Technological University [in Russian], Krasnoyarsk (2010), Vol. 2, pp. 56–59.
B. N. Kuznetsov, V. G. Danilov, et al., Zh. Sib. Gos. Univ., Khim., No. 2, 156–164 (2009).
I. Kvien, B. S. Tanem, and K. Oksman, Biomacromolecules, 6, 3160–3165 (2005).
Author information
Authors and Affiliations
Additional information
Translated from Khimicheskie Volokna, No. 2, pp. 3–6, March-April, 2011.
Rights and permissions
About this article
Cite this article
Voskoboinikov, I.V., Konstantinova, S.A., Korotkov, A.N. et al. Process for preparing nanocrystalline cellulose. Fibre Chem 43, 125–128 (2011). https://doi.org/10.1007/s10692-011-9318-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10692-011-9318-z