Abstract
Most screening programs for familial pancreatic cancer are currently based on endoscopic ultrasonography and/or magnetic resonance imaging (MRI). Cystic lesions, especially those suspicious for small intraductal pancreatic mucinous neoplasms (IPMNs) of the branch ducts, can be visualized in up to 40 % of individuals at risk, but their pathological importance in the setting of FPC is yet not well established. Individuals at risk from a prospective screening program for familial pancreatic cancer with small “imaging” IPMNs of the branch-duct type (BD-IPMN) who underwent pancreatic resection were analysed regarding clinico-pathological data and the locations of pancreatic lesions. Five of 125 individuals at risk who underwent screening had multiple small (size 2–10 mm) unicystic lesions and/or multicystic single lesions in the pancreatic body and tail suspicious for BD-IPMNs upon MRI imaging and decided to undergo surgical resection after interdisciplinary counselling, although none fulfilled the consensus criteria for IPMN resection. Histological examination revealed BD-IPMNs with low or moderate dysplasia of the gastric type in combination with multifocal PanIN2 and PanIN3 lesions in 4 individuals. The remaining patient had only tiny ductectasias in the pancreatic tail with multifocal PanIN 2 lesions in the entire gland and one PanIN3 lesion in the pancreatic head. Intriguingly, the location of the most dysplastic histological lesions (PanIN3) did not correspond to the preoperatively detected lesions and were not visible in preoperative imaging. In the setting of FPC, the presence of multiple small “imaging” BD-IPMNs may indicate the presence of high-grade PanIN lesions elsewhere in the pancreas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial pancreatic cancer (FPC) defines families with at least two first degree relatives with confirmed pancreatic ductal adenocarcinoma that do not fulfill the criteria of other inherited tumor syndromes with an increased risk for the development of pancreatic cancer. FPC is mostly autosomal dominant inherited with a heterogeneous phenotype [1, 2]. The major gene defect(s) is yet to be identified, although germline mutations in the BRCA2, PALB2 and ATM gene are associated with the disease in some FPC families [3–7]. The risk of pancreatic cancer in FPC is sufficiently high to consider an appropriate screening in high risk individuals, using a multidisciplinary approach [8]. The detection of high-grade precursor lesions, especially intraductal papillary mucinous neoplasms (IPMN) with high grade dysplasia and pancreatic intraepithelial neoplasia (PanIN) with high-grade dysplasia (PanIN3) are considered as success of screening [9].
Most screening programs are currently based on endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI) [reviewed in 10]. Although PanIN lesions cannot be detected reliably with current imaging methods, cystic lesions, often suspicious for IPMNs, can be visualized in up to 40 % of high risk individuals of FPC families [11]. IPMN is a distinct clinical and pathologic entity. One of the first description was given by Ohashi et al. [12] in 1982 who called it a mucin producing tumor of the pancreas, characterized by cystic dilatation of a pancreatic duct, mucous production and intraductal papillary growth. According to their location IPMNs are classified as main duct type (MD)-IPMN or branch duct (BD)-IPMN [13]. There exist four histological subtypes of IPMN, encompassing the gastric, intestinal, oncocytic and pancreatobiliary subtypes, respectively. The gastric type corresponds to the BD-IPMN. Growing controversy revolves around issues of natural history, management of small BD-IPMNs, ability to predict malignancy and/or progression, and surveillance strategies [14]. It has been shown that concomitant pancreatic carcinoma was found synchronously or metachronously in 8–9.2 % of patients with sporadic BD-IPMNs [15, 16]. The pathological importance of BD-IPMNs in the setting of FPC is yet not well established [17]. Here we report 5 individuals at risk with small (≤1 cm) unicystic or multiple cystic lesions (potential BD-IPMNs) who decided to undergo pancreatic resection, although this was not in line with the international consensus guidelines for the management of IPMNs [13]. Intriguingly, all 5 patients revealed multifocal PanIN2 or 3 lesions on pathological examination that were not visible in preoperative imaging and did not correspond to the preoperatively visualized lesions. This observation suggests that multiple small imaging BD-IPMN in the setting of FPC might be an indicator for high-grade PanIN-lesions developing distinct from IPMNs elsewhere in the gland.
Patients and methods
Since 1999 FPC families have been prospectively collected in the German National Case Collection of Familial Pancreatic Cancer of Germany (FaPaCa) [18]. The diagnosis of FPC was based on the presence of two or more first degree relatives with a confirmed diagnosis of pancreatic adenocarcinoma (PC). In addition, individuals with BRCA2 or PALB2 mutations and familial clustering of PC (primary tumor burden in the family) were included in the FPC cohort. Individuals with two first degree relatives with PC were classified as moderate risk (fivefold to tenfold), individuals with three or more first degree relatives with PC or with a BRCA2 or PALB2 mutations were classified as high risk (> tenfold). Individuals at risk were encouraged to participate in a prospective screening program that has been conducted since 2002 [19, 22]. The screening started 10 years before the earliest age of onset of PC in the family or age 40, whichever was earlier. The FaPaCa registry was approved by the ethic committee of the Philipps University of Marburg (36/1997, last amendment 2009) and all participants provided provided written informed consent.
The prospective annual screening program consisted of both MRI with MRCP and EUS. MRIs were evaluated by a radiologists with a high expertise in pancreatic imaging (JHT). In case of an abnormal finding, either close follow-up with MRI/MRCP and EUS or surgery was advised by a multidisciplinary team. Detailed information regarding follow-up and MRI technique were described previously [19].
Based on imaging, preferably MRI with MRCP, cystic lesions were further classified as multicystic single lesions consisting of multiple small cysts, single or multiple unicystic lesions. An IPMN was suspected if the cystic lesion(s) originated from pancreatic ducts. Those “imaging” IPMNs were classified as main-duct (MD)-IPMN or branch-duct (BD)-IPMNs according to their location.
In the case of a pancreatic cystic lesion upon imaging, the findings were reviewed by an interdisciplinary board, consisting of surgeons, gastroenterologists and pathologists. Generally criteria to recommend surgery included cystic lesions >3 cm, potential main duct type IPMN, cystic lesions of any size with a substantial solid component, cystic lesions with irregular boundaries, a significant change in size, number or morphology during follow-up according to international consensus guidelines, so called Sendai-criteria [13]. However, some patients had a strong cancer anxiety and demanded surgery with histological clarification for lesions detected by imaging though not fulfilling the Sendai criteria. In addition, surgery as therapeutical option was discussed and considered in individuals with a strong family history (three or more first degree relatives) and potential BD-IPMNs, since it has been recently shown that almost 25 % of Sendai negative BD-IPMNs showed malignant features (invasive carcinoma or carcinoma in situ) upon histological examination of the surgical specimen [20].
If surgery was indicated, the patient was offered two options depending on the number and morphology of the suspected lesion(s), either partial pancreatectomy or total pancreatectomy with potential secondary autotransplantion of islet cells. In case of a planned partial pancreatectomy, first the part of the pancreas that contained the suspicious lesion(s) was resected and total pancreatectomy was only performed, if a pancreatic cancer or multifocal high-grade PanIN2/3 or IPMN with high-grade dysplasia was histopathologically confirmed on frozen section.
Pancreatic surgical specimens were assessed by two experienced pancreas pathologists (G.K., I.E.). The whole resected pancreas was cut into 5 mm sections and stained with H&E. PanINs were classified as PanIN1-3 according to the grade of dysplasia, whereas PanIN3 corresponds to carcinoma in situ. IPMNs were classified as main duct or branch duct type with low grade, moderate or high grade dysplasia. Histological subtypes of IPMNs including gastric, intestinal, oncocytic, and pancreatobiliary types were classified according to histomorphology and immunohistochemical expression patterns of MUC1, MUC2 and MUC5AC. The presence of atypical flat lesions (AFL), recently described putative PC precursors, was also evaluated [21].
Results
The 5- (2002–2007) and 7 (2002–2009) years screening results of the FaPaCa registry have been reported previously [19, 22], including the histopathological results of 9 individuals at risk (IAR) who underwent pancreatic surgery, since they fulfilled established criteria for the resection of IPMNs or had indeterminate pancreatic lesions [22]. The current report focuses on five IAR, who either demanded resection of cystic pancreatic lesions due to cancer fear or because of our recommendation, although current consensus criteria for IPMN resection were not fulfilled. The histological results of two patients were reported earlier [22], but not a detailed association between imaging and pathology results. These five patients provided an exceptional possibility for a detailed clinicopathological analysis, which showed highly interesting results:
IAR No. 1 (ID 25-4-48-206)
This 58 years old female belonged to a family with 2 first degree relatives affected with PC. Her brother died of PC at age 61 years and her monozygotic twin sister at age 57 years. No germline mutations of BRCA2, PALB2 or CDKN2a could be identified in the family. The patient was asymptomatic and had no history of diabetes mellitus. The laboratory parameters, including Ca 19–9, were in the normal range. At baseline screening the patient revealed 5 small 2–5 mm unicystic lesions in the body and tail and 1 single 8 × 5 mm sized multicystic lesion in the tail (Fig. 1), all suspicious for BD-IPMNs. EUS confirmed the cystic lesions in the body and tail with no evidence of intramural nodules. After counselling the patient demanded a total pancreatectomy, since her twin sister died of PC. A total spleen-preserving pancreatectomy with isolation of islet cells was performed. The postoperative course was uneventful, despite the obligate development of a pancreatoprive diabetes mellitus. Histopathological examination revealed one gastric type 3 mm sized BD-IPMN in the pancreatic tail and multifocal PanIN1 and 2 lesions throughout the gland (Fig. 1), the PanIN2 lesions were preferentially located in the pancreatic head. In addition, atypical flat lesions (AFL), recently described putative PC precursors, were identified in areas of lobulocentric atrophy [21]. Autotransplantation of islet cells was abandoned due to the multifocal PanIN2 lesions. 12 months postoperatively the imaging was unremarkable, but 4 months thereafter the patient developed jaundice caused by a Klatskin tumor Bismuth IIIb. The patient underwent an extended right hemihepatectomy in an outside hospital, but unfortunately died due to postoperative liver failure.
IAR No. 2 (ID 25-5-67-28)
This 51 year old female belonged to a family with 4 first degree relatives affected with PC, including her mother and her sister at ages 67 and 52 years, respectively. In addition her sister, one aunt and one female cousin had breast cancer. In the family an unclassified variant of BRCA2 (9203del126) was identified, but the patient carried wildtype BRCA2. The patient was asymptomatic and had no history of diabetes mellitus. The laboratory parameters, including Ca 19–9 were in the normal range. At baseline MRI and EUS screening in 2006, the patient revealed two small (< 5 mm) unicystic lesions in the pancreatic body and tail (Fig. 1), which were suspicious for BD-IPMNs. After 36 months follow-up the size of one cyst had minimally progressed to 7 × 3 mm. After counselling the patient required total pancreatectomy, since in the mean time her sister died of PC and she could not stand the psychological distress of developing PC. Therefore, a total spleen-preserving pancreatectomy with isolation of islet cells was performed. The postoperative course was uneventful, despite the development of a pancreatoprive diabetes mellitus. Histopathological examination revealed no IPMNs in the region of the preoperative detected lesions, but multifocal PanIN 1 and 2 lesions throughout the gland, AFLs and one 3 mm sized lesion in the pancreatic head corresponding to at least a PanIN3- lesion or even an early infiltrative PC (Fig. 1). No autotransplantation of islet cells was performed due to the high-grade PanIN lesions. The patient was well and with an unremarkable imaging 24 months postoperatively.
IAR No. 3 (ID 25-7-104-2)
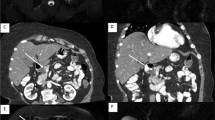
This 64 years old female belonged to a family with 2 relatives affected with PC, including her father and her cousin at ages 76 and 51 years. In addition, the IAR was operated on for breast cancer with adjuvant radiation therapy at age 60 years. No pathogenic germline mutations of BRCA2, PALB2 or CDKN2a could be identified in the family. The patient was asymptomatic and had no history of diabetes mellitus. The laboratory parameters, including CA 19-9, were in the normal range. At baseline MRI screening in 2007 the patient revealed one septed 13 × 10 mm unicystic lesion suspicious for serous cystadenoma in the pancreatic neck and multiple (> 10) small (2–10 mm) unicystic lesions in the pancreatic body and tail, which were classified as potential BD-IPMNs (Fig. 2). EUS confirmed the cystic lesion in the pancreatic neck. After 24 months imaging showed no progress in size of the lesions, but a subtle increase of their number on MRI imaging. Based on the experiences with the IAR mentioned above, we extensively discussed the results with the patient and finally recommended total pancreatectomy with the option of islet cell autotransplantation. She agreed and total spleen-preserving pancreatectomy with isolation of islet cells was performed. The postoperative course was uneventful, despite the development of a pancreatoprive diabetes mellitus. Histopathological examination revealed multiple gastric type BD-IPMNs with low to borderline dysplasia in the region of the preoperatively visualized cystic lesions. In addition, AFLs and multifocal PanIN 1/2 lesions were detected throughout the gland, and a one 5 mm sized PanIN3 lesion was present close to the Papilla of Vater in a pancreatic duct of second degree (Fig. 2). No autotransplantation of islet cells was performed due to the high-grade PanIN lesions. After a follow-up of 28 months the patient felt well and imaging was unremarkable.
IAR No. 4 (ID: 25-5-67-212)
The 69 years old female was the aunt of IAR No. 2. She herself carried wildtype BRCA2. The patient was asymptomatic and in an excellent physic condition. The laboratory parameters, including Ca 19–9, were in the normal range. She underwent regular MRI imaging at her home town since 2005. In summer 2011 for the first time cystic lesions suspicious for IPMN were visualized on MRI and the patient was referred for second opinion. Baseline MRI and EUS screening at our institution showed multiple (> 10) unicystic lesions with a size between 2 and 10 mm in the pancreatic body and tail, which were classified as potential BD-IPMNs (Fig. 3). The main pancreatic duct was not dilated. Since the patient was a high risk individual a spleen-preserving distal pancreatectomy until the level of the portal vein was recommended and performed after counselling of the patient. The postoperative course was uneventful. Histopathological examination of the specimen revealed multiple gastric type BD-IPMN (2–10 mm) with low grade dysplasia in the regions of the preoperative located cystic lesions. However, multifocal PanIN lesions, at most PanIN3, were also detected. The PanIN3 lesions were located separate from the IPMNs along the main pancreatic duct with an extension of 5 cm between body and tail (Fig. 3). Based on this result we recommended completion pancreatectomy of the pancreatic head which was performed 7 days after the first operation. Histopathological examination of the pancreatic head again revealed multifocal PanIN1 and 2 lesions and one gastric type IPMN with low grade dysplasia. At first follow-up at 6 months the patient felt well and imaging was unremarkable.
IAR No. 5 (ID: 25-4-46-1)
This was a 47 year old male from a family with high-risk background of FPC. Mother, maternal aunt and maternal grandmother died of PC. There was no BRCA2 or PALB2 mutation in the kindred. The man had no complaints, was healthy and in excellent condition when he entered the surveillance program. The laboratory parameters, including Ca 19–9, were in the normal range. The baseline MRI screening in 2011 revealed a 10 mm × 10 mm cystic lesion without a solid component in the pancreatic head (Fig. 3), suspicious for an IPMN of the Santorini duct. EUS confirmed the cystic lesion in the pancreatic head. The patient was counselled and surgery was recommended, since a main-duct IPMN could not be excluded. For personal reasons the patient decided to have the operation later and another follow-up was performed after 9 months. At this time the lesion was stable in size, but still suspicious possibly originating from a main duct. Therefore surgery was again recommended and the patient underwent a pylorus-preserving pancreaticoduodenectomy (PPPD). Histopathological examination of the specimen revealed a 10 mm in diameter sized gastric type BD-IPMN with low grade and moderate dysplasia in the region of the preoperative located cystic lesion. In addition, multifocal PanIN lesions, at most PanIN2, were present throughout the pancreatic head (Fig. 3). The postoperative course was uneventful, despite a type A pancreatic fistula, which resolved spontaneously. Follow-up is yet not available.
Discussion
The presented small case series demonstrates for the first time that multiple, small (<1 cm) “imaging” IPMNs in IAR of FPC families correspond to gastric type BD-IPMNs that are frequently associated with multifocal moderate to high grade PanIN lesions distinct from the IPMNs. Of note, the PanIN lesions could not be visualised on preoperative imaging, neither by MRI with MRCP nor EUS. These results are important for several reasons.
First, the presented results are in line with previous analyses of pancreatic specimens of FPC patients frequently detecting IPMNs with concomitant PanINs in familial cases [23], which was significantly more common than in sporadic cases [24]. It has also been shown that PanINs were significantly more common in familial cases (mean of 10.7 % of the duct profiles) than in specimens of sporadic pancreatic cancer patients (mean 1.9 % of the duct profiles, p < 0.01) [24]. Another study comparing 51 resected pancreata of FPC patients with 40 pancreata of patients with sporadic PC detected PanINs, especially PanIN3 lesions, more common in familial cases (RR 2.75, 95 %CI 2.05–3.7 for PanIN and RR 4.20 for PanIN3, 95 % CI 2.22–7.93) than in the sporadic cases [25].
Secondly, all BD-IPMNs identified in our 5 FPC IARs revealed only low to moderate dysplasia, were smaller than 2 cm in size and their progression, at least on MRI imaging, was slow. This is in line with the characteristics of sporadic BD-IPMNs [26]. It has been reported that 8 % (5/60) of patients with sporadic BD-IPMN developed PC distinct from IPMN during a median follow-up of 87 months [15]. Another 2010 study showed that 9.3 % (22 of 236) with BD-IPMN had concomitant PC as independent synchronous or metachronous lesions [16]. In the present series 3 of 5 of IAR with BD-IPMN revealed a carcinoma in situ (PanIN3), suggesting a higher risk of developing PC in the setting of FPC, especially if one considers that the preoperative follow-up in our 5 IAR patients was at most 36 months. Interestingly none of 7 additional IAR in our series, who underwent pancreatic resection for solitary cystic or solitary indeterminable pancreatic lesions on imaging revealed multifocal PanIN2/3 lesions on histopathological analysis [19, 22].
Third, the presented data might be important for counselling and screening of IAR of FPC families. Given the yet available evidence, the guidelines for management of sporadic IPMNs [13] cannot be fully adopted to the familial setting. At a 2011 consensus conference (CAPS Summit) [9], great disagreement existed on the indication and type of surgery that should be offered to IAR with asymptomatic cystic and indeterminate solid pancreatic lesions. Most centres would nowadays agree that pancreatic resection in FPC is justified, if imaging reveals lesion(s) suspicious for precursors with high-grade dysplasia (e.g. MD-IPMN, PanIN3) or pancreatic cancer [18, 27–31]. If imaging BD-IPMNs is confirmed to be a reliable indicator for the presence of multifocal high-grade PanIN lesions in larger series, one has to discuss whether the indication for pancreatic resection should be extended to IAR with multiple imaging BD-IPMNs with an otherwise unremarkable pancreas on imaging. This holds especially true, if the detection of IPMNs and PanINs with high-grade dysplasia (PanIN3) or T1N0 pancreatic cancer is considered to be the true success of screening by most experts [9]. As reported here for the last two patients we now consider pancreatic resection for IAR, especially those with a strong family history, if imaging reveals multiple BD-IPMNs. However, this might be an overtreatment, since the progression time from a PanIN 2 or 3 lesions towards invasive cancer in the setting of FPC is not defined. A quantitative analysis of the timing of the genetic evolution of sporadic PC indicated at least a decade time span between the occurrence of the initiating mutation and the birth of the parental, non-metastatic founder cell and at least 15 years for the acquisition of metastatic ability [32]. These data suggest a broad window for the detection of early PCs (T1N0 tumors) on imaging during prospective FPC screening programs. However, IAR who reveal multiple BD-IPMNs on imaging should be counselled that this feature might indicate the presence of preinvasive cancerous lesions in other locations of the gland. Clearly, the multifocal nature of FPC suggests that regular surveillance of these patients is warranted after any type of partial pancreatectomy.
The presented study is clearly limited by the low number of patients, but the reported patients are extremely rare. It should be a major goal of the scientific familial cancer community to collect and analyse those patients worldwide for confirmation of our preliminary observations, since this confirmation of our observations would have a major impact on the management of FPC families.
References
Bartsch DK (2003) Familial pancreatic cancer. Br J Surg 90:386–387
Bartsch DK, Sina-Frey M, Ziegler A, Hahn SA, Pryzpadlo E, Kress R, Gerdes B, Rieder H (2001) Update of familial pancreatic cancer in Germany. Pancreatology 1:510–516
Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA, Campra D, Grützmann R, Rehder H, Rothmund M, Schmiegel W, Nepoptolemus JP, Bartsch DK (2003) BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 95:214–221
Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, Hruban RA, Kern SE (2002) Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 117 %. Cancer Res 62:3789–3793
Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP (2009) Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 324:217–220
Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoplotemos JP, Greenhalf W, Bartsch DK (2010) PALB2 mutations in European familial pancreatic cancer families. Clin Genet 78:490–494
Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, Gallinger S, Schwartz AG, Syngal S, Cote ML, Axilbund J, Schulick R, Ali SZ, Eshleman JR, Velculescu VE, Goggins M, Vogelstein B, Papadopoulos N, Hruban RH, Kinzler KW, Klein AP (2011) ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2:41–46
Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI (2007) Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut 56:1460–1469
Canto MI, Harink F, Hruban RH, Offerhaus J, Poley J-W, Fockens P, Kamel IR, Nio CY, Schulick RD, Basel C, Kluijt I, Goggins MG, Bruno MJ (2012) On behalf of the International CAPS Consortium. International consensus recommendations on the management of patients with increased risk for familial pancreatic cancer (Cancer of the Pancreas Screening Consortium (CAPS) 2011 Summit), to be presented at the Digestive Disease Week (DDW), San Diego, California, USA, May 19–May 22
Bartsch DK, Gress TM, Langer P (2012) Familial pancreatic cancer-current knowledge. Nat Rev Gastroenenterol Hepatol, ahead of print
Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M (2012) American cancer of the pancreas screening (CAPS) consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142(4):796–804
Ohashi K, Murakami Y, Takekoshi T (1982) Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Progn Diagn Endosc 20:348–351
Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S (2006) International consensus guidelines for management of intraductal papillary mucinous neoplas and mucinous cystic neoplasms of the pancreas. Pancreatology 6:17–32
Bussom S, Saif MW (2010) Intraductal papillary mucinous neoplasia (IPMN). JOP 11:131–134
Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A (2008) Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 57:1561–1565
Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M (2010) Predictors of the presence of concomitant invasive ductal carcinoma in intraductal. Ann Surg 251:70–75
Sipos B, Frank S, Gress T, Hahn SA, Klöppel G (2009) Pancreatic intraepithelial neoplaia revisited and updated. Pancreatology 9:45–54
Bartsch DK, Sina-Frey M, Ziegler A, Hahn SA, Przypadlo E, Kress R, Gerdes B, Rieder H (2001) Update of familial pancreatic cancer in Germany. Pancreatology 1:510–516
Langer P, Gress TM, Bartsch DK (2010) Pancreatic cancer screening in individuals at risk- too early for a general implementation on a health care basis. Gut 59:1006–1007
Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Bundy BD, Büchler MW, Werner J (2012) Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg 256:313–320
Aichler M, Seiler C, Tost M, Siveke J, Mazur PK, Da Silva-Buttkus P, Bartsch DK, Langer P, Chiblak S, Dürr A, Höfler H, Klöppel G, Müller-Decker K, Brielmeier M, Esposito I (2012) Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol 226:723–734
Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E, Langer P, Bartsch DK (2011) German national case collection for familial pancreatic cancer (FaPaCa) ten years experience. Fam Cancer 10:323–330
Brune K, Abe T, Canto M, O’Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ, Kern SE, Goggins M, Hruban RH (2006) Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Path 30:1067–1076
Nehra D, Ovaride VM, Mino-Kenudson M, Thayer SP, Ferrone CR, Wargo JA, Muzikansky A, Finkelstein D, Warshaw AL, Castillo F (2012) Intraductal papillary mucinous neoplasms: does a family history of pancreatic cancer matter? Pancreatology 12:358–363
Shi C, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH (2009) Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res 15(24):7737–7743
Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, Pea A, Bassi C (2010) Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg 2:342–346
Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, Ali S, Richman J, Jagannath S, Kantsevoy SV, Kalloo AN (2006) Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 4:766–781
Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ (2009) The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 104:2175–2181
Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H (2010) Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 16:5028–5037
Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K, Haider MA, Gallinger S (2011) Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 30 [Epub ahead of print]
Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ, Morreau H, Bonsing BA, de Vos tot Nederveen Cappel WH (2011) Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 140:850–856
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–1147
Acknowledgments
We would like to thank all individuals who participated in the study. Supported by the Deutsche Krebshilfe (107259 to D.K.B and P.L).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartsch, D.K., Dietzel, K., Bargello, M. et al. Multiple small “imaging” branch-duct type intraductal papillary mucinous neoplasms (IPMNs) in familial pancreatic cancer: indicator for concomitant high grade pancreatic intraepithelial neoplasia?. Familial Cancer 12, 89–96 (2013). https://doi.org/10.1007/s10689-012-9582-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-012-9582-y