Abstract
To examine attitudes toward childbearing and prenatal genetic testing among individuals at risk for Lynch Syndrome (LS), the most common type of hereditary colorectal cancer. Individuals undergoing clinical genetic testing for mismatch repair (MMR) gene mutations completed written questionnaires before and after testing. 161 of 192 (84%) eligible individuals participated in the study. Mean age was 46 years (range 20–75), 71% were female, 53% had a personal diagnosis of cancer, and 68% had children. Eighty percent worried about their children’s risk for developing cancer; however only 9% reported their decision to have children was affected by their family history of cancer. When asked whether providing prenatal testing to carriers of MMR gene mutations was ethical, 66% (86/130) of respondents agreed/strongly agreed, 25% (32) were neutral and 9% (12) disagreed/strongly disagreed. Of 48 individuals planning to have children in the future, 57% (27) intended to have children regardless of their genetic test result. If found to carry a MMR gene mutation that confirmed LS, 42% (20) would consider prenatal testing for a future pregnancy and 20% (7/35) of women would consider having children earlier in order to have prophylactic surgery to reduce their risk for gynecologic cancers. Individuals undergoing genetic testing for LS may utilize test results to make reproductive decisions. Clinicians should be prepared to discuss options of reproductive genetic technologies during counseling of LS patients of childbearing age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lynch Syndrome (LS), also known as Hereditary Nonpolyposis Colorectal Cancer (HNPCC), is the most common type of hereditary colorectal cancer (CRC). Individuals with LS have a high lifetime risk of CRC which approaches 70% in the absence of colonoscopic surveillance and are also predisposed to other extracolonic tumors, including endometrial and ovarian cancers [1–4]. LS is caused by germline mutations in DNA mismatch repair (MMR) genes and genetic testing for MLH1, MSH2, MSH6, EPCAM and PMS2 genes is now commercially available. Genetic testing is used to identify at-risk individuals who require specialized cancer surveillance, which can reduce cancer incidence and improve survival among individuals with familial CRC syndromes [5–7].
Prenatal testing for genetic conditions has been performed for decades and is routinely offered to screen for inherited diseases which cause significant morbidity and mortality in children, such as cystic fibrosis [8–10] and Tay-Sachs [10, 11]. Prenatal testing technologies currently available include preimplantation genetic diagnosis (PGD) and prenatal diagnostic testing (PND), which can identify whether the gene mutation is present in the embryo before or after uterine implantation, respectively [9].
As clinical genetic testing has become more available, interest in assisted reproductive technologies has grown among individuals affected with familial cancer syndromes [3, 12–14]. Prenatal testing has been used in highly penetrant autosomal dominant cancer syndromes including Li-Fraumeni syndrome (LFS), [15–17]familial adenomatous polyposis (FAP), [12, 18–21]and multiple endocrine neoplasia, [22] as well as in variably penetrant syndromes such as hereditary breast ovarian cancer (HBOC), [23–26] and hereditary retinoblastoma [27–30]. In this study we sought to examine the attitudes toward childbearing and prenatal genetic testing among individuals undergoing genetic evaluation for Lynch syndrome (LS).
Methods
Individuals undergoing clinical genetic testing for LS at the Dana-Farber Cancer Institute (Boston, MA) between November 2003 and November 2009, were invited to participate in a longitudinal questionnaire study examining cancer risk awareness, health behaviors, and attitudes toward genetic testing. Patients were considered eligible for the study if they were at least 18 years of age and had a personal or family history meeting clinical criteria for genetic evaluation for LS [31]. Study participants completed a series of questionnaires prior to genetic testing (pre-test), at 3 months and 1 year after genetic testing.
Study instruments
The study questionnaires were developed at the Dana-Farber Cancer Institute and included questions used in previous studies with individuals with LS, HBOC syndrome and other populations with inherited conditions [32–41]. The pre-test questionnaire elicited standard demographic data as well as information about personal and family history of cancer, health and cancer screening behaviors and cancer risk perception. In addition, the instrument contained questions assessing individuals’ motivations for undergoing genetic testing, plans for having children and whether genetic test results would have an impact on decisions regarding childbearing.
The questions pertaining to prenatal diagnosis (PND) and preimplantation genetic diagnosis (PGD) had been used in previous studies with individuals with FAP and patients undergoing genetic testing for HBOC [40, 42]. A brief introductory description of PND and PGD was provided (Table 1). Participants were asked to rank (on a five-point Likert scale) the degree to which they agreed/disagreed with statements that it is ethical to provide PGD or PND to individuals with a known genetic mutation for LS (Table 2). Participants were asked whether a “positive” genetic test result (confirming the diagnosis of Lynch Syndrome) would affect their decision to have children (response choices included ‘yes’, ‘no’, ‘I don’t know’ and ‘N/A- I am not considering having children in the future’). Participants who indicated they were considering (more) children were asked whether receiving a positive genetic test result would change their decision to have children or consider adoption and whether they would consider prenatal testing using either PGD or PND (Table 3). Responses were selected from a five-point Likert scale ranging from ‘strongly agree’ to ‘strongly disagree’.
The study was approved by the Institutional Review Board of the Dana-Farber Cancer Institute/Harvard Cancer Center and informed consent was obtained from all subjects.
Statistical analysis
Questionnaire data were scanned and incorporated into computerized data sets for analyses using statistical software programs (SAS, Version 9.1). Responses to the question “Do you think it is ethical to provide PGD or PND to individuals with a known genetic mutation for LS?” were dichotomized as ‘agree/strongly agree/neutral’ versus ‘not agree’ (disagree or strongly disagree). Responses to questions of whether participants would consider PGD or PND if their test result showed they carried a genetic mutation associated with LS were dichotomized as ‘agree/strongly agree’ versus ‘not agree’ (neutral, disagree or strongly disagree). Because of the small number of subjects considering having children in the future, individuals who answered agree/strongly agree that they would consider either PGD and/or PND were grouped together and classified as considering prenatal testing. The associations of individual dichotomous variables (subject characteristics including gender, personal history of cancer, having children) with the outcomes (including interest in prenatal testing and opinion on ethics of prenatal testing) were explored using Fisher’s Exact test and quantified using odds ratios (OR). A P value of < 0.05 was considered statistically significant.
Results
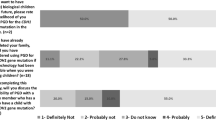
Out of 192 individuals invited to participate in the study, 161 (84%) completed the study questionnaire prior to genetic testing. The overall mean age of participants was 46.1 years with an age range of 20–75 years. 115 (71%) subjects were women. Nearly all (95%) subjects classified their race as white. Half (53%) had previously been diagnosed with cancer and 3 (1.8%) were undergoing genetic testing for a LS mutation previously identified in a family member. 129/161 (80%) responders said they were worried about their children’s cancer risk given their own family history. 109/161 (68%) responders already had children and only 14/161 (9%) said they had decided not to have (more) children because of their familial cancer risk.
Baseline attitudes regarding prenatal testing for lynch syndrome
130 of 161 (80%) participants who completed baseline questionnaires answered the questions regarding prenatal testing. Although the 31 non-responders were significantly older than responders (mean age 53.2 vs. 45.6 years, P < 0.05), there were no other demographic differences between subjects who did and did not answer the questions in this section. Before receiving the results of their genetic test, subjects were asked whether they felt it would be ethical to provide some type of prenatal testing for individuals with mutations associated with Lynch Syndrome and 86 (66%) responders strongly agreed/agreed, 32 (25%) were neutral and only 12 (9%) disagreed/strongly disagreed. There were no significant differences in demographic characteristics between those whose response was “agree” or “neutral” and those who disagreed that providing prenatal testing was ethical (Table 4). Of those who believed providing prenatal testing was ethical, 72/86 (84%) agreed with offering both PND and PGD, while 9 (10%) agreed with providing PND but not PGD and 5 (6%) agreed with providing PGD but not PND.
Plans for future childbearing
Prior to genetic testing, subjects were asked if they were considering having children in the future and how receiving a positive genetic test result might affect their plans. Forty-eight subjects indicated that they were considering having children; of these 27 (56%) indicated they planned to have children regardless of the result of their genetic test. Among women considering a future pregnancy, seven of 35 (20%) said that if their genetic test showed they carried a mutation they would plan to have children earlier in order to proceed with prophylactic surgery to reduce their risk for gynecologic cancers associated with LS. Thirteen subjects (27%) indicated they would consider adoption in the setting of a positive genetic test result. Only five individuals (10%) said they would decide not to have children if they were found to carry a MMR gene mutation.
When asked about prenatal testing for Lynch Syndrome, 20/48 (42%) subjects considering a future pregnancy agreed/strongly agreed that they would consider prenatal testing if they were found to carry a MMR gene mutation (10 would consider PGD and/or PND, 9 would consider only PND, and 1 would consider only PGD). Twenty (42%) respondents indicated they would not consider prenatal testing and 8 (17%) were undecided or neutral. Comparison of characteristics of subjects who would and would not consider prenatal testing for Lynch Syndrome appears in Table 5. Individuals who would consider prenatal testing were significantly younger than those who would not (mean age 35 vs. 42 years, P = 0.02). Interest in prenatal testing was also higher among subjects who were not married and among those who reported annual household incomes below $50,000. Interest in prenatal testing appeared to be less among individuals who already had children when compared with those without children, although this difference did not achieve statistical significance (P = 0.08).
One-year follow up
Follow up questionnaires completed 1 year after disclosure of genetic test results were available for 35 of 48 (73%) subjects who were considering a future pregnancy. One individual who had tested negative for the familial MMR mutation reported the birth of a child. Two out of 9 (22%) mutation carriers indicated they were considering using PGD for a future pregnancy.
Discussion
Hereditary cancer syndromes affect families. Carriers of MMR gene mutations are at increased risk for developing cancer themselves, and have a 50/50 chance of passing on this inherited susceptibility to each of their children. Although most individuals with a family history of cancer would not decide to forego having children, the identification of a heritable gene mutation may influence reproductive decisions. Our findings demonstrate that if found to carry a gene mutation associated with Lynch Syndrome, 42% of individuals contemplating future pregnancies would consider using prenatal testing and one in five women would consider having children earlier in order to proceed with prophylactic surgery to reduce their risk for developing gynecologic cancers. Overall, the majority of individuals undergoing genetic testing for LS felt that it would be ethical to offer prenatal genetic testing (either PGD or PND) to those with pathogenic MMR gene mutations.
The American Medical Association (AMA) Code of Medical Ethics states that prenatal genetic testing is most acceptable “for women or couples whose medical histories or family backgrounds indicate an elevated risk of fetal genetic disorder” [43]. Prenatal testing for genetic conditions has been available for decades and preimplantation genetic diagnosis has been performed for a number of cancer syndromes including hereditary breast ovarian cancer, Li Fraumeni syndrome, neurofibromatosis 1 and 2, Von Hippel Lindau disease, hereditary retinoblastoma, familial adenomatous polyposis (FAP), and Lynch Syndrome, among others [44]. In May 2006, the United Kingdom’s Human Fertilisation and Embryology Authority (HFEA) added hereditary breast ovarian and bowel cancer syndromes as conditions for which PGD might be approved, while also noting that indications should be reviewed on a case by case basis [26].
In our previous survey of 20 individuals with FAP, we found that all but 1 indicated they would consider prenatal testing for future pregnancies [40].
The present study, which examines attitudes toward childbearing and reproductive decision-making in individuals at risk for LS, indicates that while most believe it would be ethical to offer prenatal testing to LS mutation carriers, only 42% of those contemplating a future pregnancy would consider using prenatal testing themselves. Our findings are similar to those of other studies conducted among patients at risk for HBOC. Fortuny et al. used our same study questionnaire in a Spanish cohort of 77 individuals undergoing genetic testing for BRCA1/2 mutations and found that 48% and 55% of subjects would consider PGD and PND, respectively [42]. In other surveys of women at high risk for HBOC Quinn et al. found that 57% thought PGD was an acceptable option; however only 33% would consider using PGD themselves [45]. Menon et. al. found 75% of BRCA mutation carriers considered PGD acceptable, but only 38% would have used it had it been available [46]. At present there are few data regarding the uptake of PGD and PND for cancer predisposition syndromes. In 2007 the 57 centers enrolled in the European ESHRE PGD consortium reported only 12 cycles for FAP, 1 for HNPCC, and 4 for BRCA1 [47]. The Regional Genetics Service in Manchester, UK reported that 1.8% of FAP and 1% of LS and HBOC carriers were referred to discuss PGD in 2009 [48].
Decisions regarding childbearing are very personal ones and may be influenced by an individual’s personal and family history of cancer. While most of the subjects in our study believed prenatal testing would be ethical, only a minority would consider it themselves. In weighing the implications of prenatal testing in the care of families affected by hereditary cancer syndromes, Offit et. al. have suggested the following framework [44] (1) Does the disease have onset in childhood, with risk of death or severe morbidity by early adulthood? (2) What is the penetrance of disease and how severe is the phenotype? (3) Can the risk be reduced through surveillance or preventive surgeries? In this context, our finding that the potential rate of uptake of prenatal testing for Lynch Syndrome is markedly lower than for FAP is not surprising. In contrast to FAP, Lynch syndrome rarely results in childhood morbidity, the penetrance is highly variable, and the risk for CRC can be reduced effectively through frequent colonoscopic surveillance.
Our study is among the first to demonstrate that genetic testing may influence reproductive decision-making for individuals at risk for Lynch Syndrome. However, we recognize our study has several limitations. Subjects were recruited from a single, tertiary-referral cancer genetics clinic and elected to participate in a longitudinal questionnaire study; consequently it is possible their opinions may not be generalizable to other individuals with LS. We did not collect information about participants’ religious affiliation, which might influence attitudes regarding prenatal testing and pregnancy termination. The description of PND and PGD provided to subjects was brief and did not include any technical details about the procedures such as potential complications, success rates, or financial cost, which might influence patient decision-making, nor did the questionnaire require subjects to provide reasons why they might favor PGD vs. PND. Finally, the number of subjects contemplating future pregnancies was small and the follow up period was too short to quantify actual uptake or success rates for PGD and PND.
Even so, our findings demonstrate that genetic testing for LS can affect patients’ decisions about childbearing and suggest that most feel it is ethical to offer the option of prenatal testing for MMR gene mutation carriers. Although ethical concerns have been raised in recent literature about the “slippery slope of trying to achieve genetic perfection” and “designer babies,” [23, 44, 49–51] there have also been “wrongful birth” lawsuits in which parents claim that they were deprived of the opportunity to terminate a pregnancy due to the physician’s failure to inform them of the risk of genetic illness in their offspring [44]. A recent survey of patients with FAP of childbearing age found that approximately 84% had no prior information about PGD or PND [52]. The widespread availability of prenatal testing for multiple disease conditions makes it imperative for clinicians to be aware of the existing technology and to be prepared to offer referrals for patients who are interested in learning more about options for prenatal genetic testing [53].
Conclusion
Our results suggest that a number of men and women at risk for Lynch Syndrome would utilize the information learned from genetic testing in making reproductive decisions. Only a small minority felt that offering prenatal testing for LS would not be ethical. Health care providers should be prepared to discuss the option of assisted reproductive technologies during genetic counseling of individuals with hereditary cancer syndromes, such as LS, who are of childbearing age.
References
Aarnio M, Sankila R, Pukkala E et al (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81:214–218
Aarnio M, Mecklin JP, Aaltonen LA et al (1995) Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer 64:430–433
Garber JE, Offit K (2005) Hereditary cancer predisposition syndromes. J Clin Oncol 23:276–292
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919–932
Jarvinen HJ, Aarnio M, Mustonen H et al (2000) Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 118:829–834
Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K et al (2009) Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol 27:4793–4797
Lindor NM, Petersen GM, Hadley DW et al (2006) Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. Jama 296:1507–1517
Handyside AH, Lesko JG, Tarin JJ et al (1992) Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med 327:905–909
Sermon K, Van Steirteghem A, Liebaers I (2004) Preimplantation genetic diagnosis. Lancet 363:1633–1641
Strom CM, Ginsberg N, Rechitsky S et al (1998) Three births after preimplantation genetic diagnosis for cystic fibrosis with sequential first and second polar body analysis. Am J Obstet Gynecol 178:1298–1306
Sermon K, Lissens W, Tarlatzis B et al (1992) Beta-N-acetylhexosaminidase activity in human oocytes and preimplantation embryos. Hum Reprod 7:1278–1280
Rechitsky S, Verlinsky O, Chistokhina A et al (2002) Preimplantation genetic diagnosis for cancer predisposition. Reprod Biomed Online 5:148–155
Offit K, Kohut K, Clagett B et al (2006) Cancer genetic testing and assisted reproduction. J Clin Oncol 24:4775–4782
Spits C, De Rycke M, Van Ranst N et al (2007) Preimplantation genetic diagnosis for cancer predisposition syndromes. Prenat Diagn 27:447–456
Guran S, Tunca Y (2005) Prenatal diagnosis history of a Li-Fraumeni syndrome family. Cancer Genet Cytogenet 157:191
Avigad S, Peleg D, Barel D, Benyaminy H, Ben-Baruch N, Taub E, Shohat M, Goshen Y, Cohen IJ, Yaniv I, Zaizov R (2004) Prenatal diagnosis in Li-Fraumeni syndrome. J Pediatr Hematol Oncol 26:541–545
Verlinsky Y, Rechitsky S, Verlinsky O et al (2001) Preimplantation diagnosis for p53 tumour suppressor gene mutations. Reprod Biomed Online 2:102–105
Ao A, Wells D, Handyside AH et al (1998) Preimplantation genetic diagnosis of inherited cancer: familial adenomatous polyposis coli. J Assist Reprod Genet 15:140–144
Dalton A, Shannon NL, Johnson M et al (1998) Prenatal diagnosis to exclude FAP in a high risk pregnancy. Prenat Diagn 18:756
Davis T, Song B, Cram DS (2006) Preimplantation genetic diagnosis of familial adenomatous polyposis. Reprod Biomed Online 13:707–711
Moutou C, Gardes N, Nicod JC et al (2007) Strategies and outcomes of PGD of familial adenomatous polyposis. Mol Hum Reprod 13:95–101
Huang SM, Tao BL, Tzeng CC, Liu HT, Wang WP (1997) Prenatal molecular diagnosis of RET proto-oncogene mutation in multiple endocrine neoplasia type 2A. J Formos Med Assoc 96:542–544
Wagner TM, Ahner R (1998) Prenatal testing for late-onset diseases such as mutations in the breast cancer gene 1 (BRCA1). Just a choice or a step in the wrong direction? Hum Reprod 13:1125–1126
Jasper MJ, Liebelt LJ, Hussey ND (2008) Preimplantation genetic diagnosis for BRCA1 exon 13 duplication mutation using linked polymorphic markers resulting in a live birth. Prenat Diagn 28:292–298
Braude P (2006) Preimplantation diagnosis for genetic susceptibility. N Engl J Med 355:541–543
London SoM, London. Human Fertilisation and Embryology Authority PGD Conditions Licensed by the HFEA. http://www.hfea.gov.uk/pgd-screening.html. Accessed on January 14 2010
Dommering CJ, Moll AC, Imhof SM et al (2004) Another liveborn after preimplantation genetic diagnosis for retinoblastoma. Am J Ophthalmol 138:1088–1089
Girardet A, Hamamah S, Anahory T et al (2003) First preimplantation genetic diagnosis of hereditary retinoblastoma using informative microsatellite markers. Mol Hum Reprod 9:111–116
Pierro L, Brancato R, Capoferri C (1993) Prenatal detection and early diagnosis of hereditary retinoblastoma in a family. Ophthalmologica 207:106–111
Xu K, Rosenwaks Z, Beaverson K et al (2004) Preimplantation genetic diagnosis for retinoblastoma: the first reported liveborn. Am J Ophthalmol 137:18–23
Umar A, Boland CR, Terdiman JP et al (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96:261–268
Hadley DW, Jenkins J, Dimond E et al (2003) Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med 163:573–582
Markel DS, Young AB, Penney JB (1987) At-risk persons’ attitudes toward presymptomatic and prenatal testing of Huntington disease in Michigan. Am J Med Genet 26:295–305
Lerman C, Daly M, Masny A et al (1994) Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol 12:843–850
Croyle RT, Lerman C (1999) Risk communication in genetic testing for cancer susceptibility. J Natl Cancer Inst Monogr 25:59–66
Aktan-Collan K, Haukkala A, Mecklin JP et al (2001) Comprehension of cancer risk one and 12 months after predictive genetic testing for hereditary non-polyposis colorectal cancer. J Med Genet 38:787–792
Stoffel EM, Ford B, Mercado RC et al (2008) Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol 6:333–338
Stoffel EM, Mercado RC, Kohlmann W et al (2010) Prevalence and predictors of appropriate colorectal cancer surveillance in lynch syndrome. Am J Gastroenterol 105(8):1851–1860
DiGianni LM, Kim HT, Emmons K et al (2003) Complementary medicine use among women enrolled in a genetic testing program. Cancer Epidemiol Biomarkers Prev 12:321–326
Kastrinos F, Stoffel EM, Balmana J et al (2007) Attitudes toward prenatal genetic testing in patients with familial adenomatous polyposis. Am J Gastroenterol 102:1284–1290
Digianni LM, Rue M, Emmons K et al (2006) Complementary medicine use before and 1 year following genetic testing for BRCA1/2 mutations. Cancer Epidemiol Biomarkers Prev 15:70–75
Fortuny D, Balmana J, Grana B et al (2009) Opinion about reproductive decision making among individuals undergoing BRCA1/2 genetic testing in a multicentre Spanish cohort. Hum Reprod 24(1):000–006
American Medical Association (1994) Code of medical ethics. Arch Fam Med 3:633–642
Offit K, Sagi M, Hurley K (2006) Preimplantation genetic diagnosis for cancer syndromes: a new challenge for preventive medicine. Jama 296:2727–2730
Quinn G, Vadaparampil S, Wilson C et al (2009) Attitudes of high-risk women toward preimplantation genetic diagnosis. Fertil Steril 91:2361–2368
Menon U, Harper J, Sharma A et al (2007) Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum Reprod 22:1573–1577
Goossens V, Harton G, Moutou C et al (2009) ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod 24:1786–1810
Clancy T (2010) A clinical perspective on ethical arguments around prenatal diagnosis and preimplantation genetic diagnosis for later onset inherited cancer predispositions. Fam Cancer 9:9–14
Lancaster JM, Wiseman RW, Berchuck A (1996) An inevitable dilemma: prenatal testing for mutations in the BRCA1 breast-ovarian cancer susceptibility gene. Obstet Gynecol 87:306–309
Kalfoglou AL, Doksum T, Bernhardt B et al (2005) Opinions about new reproductive genetic technologies: hopes and fears for our genetic future. Fertil Steril 83:1612–1621
(2004) Preimplantation genetic diagnosis—for or against humanity? Lancet 364:1729–1730
Douma KF, Aaronson NK, Vasen HF et al (2010) Attitudes toward genetic testing in childhood and reproductive decision-making for familial adenomatous polyposis. Eur J Hum Genet 18:186–193
Crockin SL (2005) Reproduction, genetics and the law. Reprod Biomed Online 10:692–704
Acknowledgments
The authors would like to thank Bridget Neville, MS, for her assistance with statistical programming. The authors acknowledge the following sources of grant support: National Cancer Institute K07 CA 120448-04 (Dr. Stoffel), American Recovery and Reinvestment Act 3K07CA120448-03S1 (Dr. Stoffel), American College of Gastroenterology Junior Faculty Award (2004—Dr. Stoffel), National Cancer Institute K24 CA 113433 (Dr. Syngal).
Conflicts of interest
The authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dewanwala, A., Chittenden, A., Rosenblatt, M. et al. Attitudes toward childbearing and prenatal testing in individuals undergoing genetic testing for Lynch Syndrome. Familial Cancer 10, 549–556 (2011). https://doi.org/10.1007/s10689-011-9448-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-011-9448-8