Abstract
Aging negatively affects individual survival and reproduction; consequently, characterizing the factors behind aging can enhance our understanding of fitness in wild populations. The drivers of biological age are diverse, but often related to factors like chronological age or sex of the individual. Recently, however, environmental factors have been shown to strongly influence biological age. To explore the relative importance of these influences on biological aging in a free-ranging and long-lived vertebrate, we quantified the length of telomeres—highly conserved DNA sequences that cap the ends of eukaryotic chromosomes and a useful molecular marker of biological age—for black bears sampled throughout Colorado, and measured a variety of environmental variables (habitat productivity, human development, latitude, elevation) and individual characteristics (age, sex, body size, genetic relatedness). Our extensive sampling of bears (n = 245) revealed no relationships between telomere length and any individual characteristics. Instead, we found a broad-scale latitudinal pattern in telomere length, with bears in northern Colorado possessing shorter telomeres. Our results suggest that environmental characteristics overwhelm individual ones in determining biological aging for this large carnivore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related differences in fitness can influence the dynamics of populations. Older individuals tend to experience reduced physical stamina, cognitive function, and immunocompetence (Cichoń et al. 2003; Punzo and Chavez 2003). Such aspects of senescence are exhibited by a diversity of wild animals (Nussey et al. 2013), ranging from fruit flies (Mackenzie et al. 2011) to elephants (Robinson et al. 2012). Consequently, older animals typically exhibit decreased reproductive (Broussard et al. 2003) and survival rates (Bryant and Reznick 2004). Thus, understanding such age-related differences in individual condition can be important for developing conservation and management strategies (Tarlow and Blumstein 2007). Tools to estimate individual condition and predict survival in wild populations are diverse, including field techniques using body condition indices (Stevenson and Woods 2006), physiological measures of stress hormones (Bonier et al. 2009), and genetic markers like MHC (e.g. Bonneaud et al. 2004). Such approaches are limited though, because they either capture relatively brief periods of an individual’s life, or a very limited aspect of condition. Telomeres, however, have emerged as a molecular marker to quantify biological age (Aydos et al. 2005; Houben et al. 2008; Monaghan 2010a; Pauliny et al. 2006; Young et al. 2015), and consequently capture accumulated life stress (Finkel and Holbrook 2000), which can provide a broader insight into individual condition and fitness.
Telomeres are repetitive and highly conserved DNA sequences (T2AG3)n (Monaghan and Haussmann 2006; Meyne et al. 1989) that cap the ends of eukaryotic chromosomes, providing chromosomal stability and an elegant solution to the “end replication problem” (Watson 1972). Telomeric repeats are lost during cellular replication, and attrition increases due to DNA damage, particularly oxidative damage (Epel et al. 2004; Kotrschal et al. 2007; von Zglinicki 2002). Telomerase, a reverse transcriptase, counteracts this degradation in the germline, but is far less active in somatic cells, likely evolved to be a barrier against developing cancer-causing “immortal cells” (Gomes et al. 2011). Consequently, telomeres tend to shorten with cellular replication and organismal age (Haussmann et al. 2003; Pauli et al. 2011).
However, in most species telomere length is still highly variable within age groups (Monaghan and Haussmann 2006). Besides chronological age, individual characteristics can drive telomere dynamics (Benetos et al. 2011). For example, the sex of an individual often explains some of this variation due to differing life histories; among mammals, females tend to have longer telomeres, potentially due to ameliorating effects of estrogen on telomere attrition (Barrett and Richardson 2011; Olsson et al. 2011). Telomere length is also partially heritable, though the strength of its heritability varies across species (Horn et al. 2011). Variation in telomere length can sometimes be attributed to body size (Ringsby et al. 2015; Scott et al. 2006)—larger animals tend to have shorter telomere lengths, which has been attributed to lower telomerase activity (Seluanov et al. 2007). Much of our research and understanding of telomere dynamics have been focused on these characteristics inherent to an individual, regardless of its environment.
Increasingly, though, research is identifying environmental factors as relevant in driving telomere dynamics; factors such as habitat and forage quality (Angelier et al. 2013; Mizutani et al. 2013; Young et al. 2013, 2015), as well as behavioral correlates like hibernation (Turbill et al. 2012, 2013) and social status (Lewin et al. 2015). Because chronic life stressors can lead to increased oxidative stress (Patel et al. 2002), they can accelerate telomere attrition (Angelier et al. 2013; Cassidy et al. 2010; Shi et al. 2007) and amplify cellular aging (Buffenstein et al. 2008). As habitat quality and associated behaviors modify individual stress, these characteristics then result in changes in telomeres and individual condition or fitness (e.g. Angelier et al. 2013; Young et al. 2015). Research that concurrently examines both individual and environmental drivers of biological aging is currently uncommon, but can provide insight into the relative importance of each to fitness and aging.
To better understand how individual and environmental characteristics influence chronic stress and biological aging in a wild and long-lived vertebrate, we quantified relative telomere length (RTL) in American black bear (Ursus americanus) endothelial tissues sampled throughout Colorado. We examined telomere length in relation to chronological age, and other individual characteristics such as sex and body size. We further examined environmental characteristics of each sample location to ascertain relative influences on telomere length. Bears are long-lived, large-bodied hibernators that have evolved to survive with seasonal resource extremes, and show evidence of reproductive senescence (Schwartz et al. 2003). Increased time spent in torpor has recently been shown to slow biological aging (as measured by telomeres) in rodents using daily torpor or seasonal hibernation (Turbill et al. 2012, 2013), presumably due to reduced cellular turnover from lowered metabolic rates. However, it is unknown whether large hibernators will respond similarly. Unlike small hibernators, black bears reduce their metabolic rate independent of body temperature (Tøien et al. 2011). Additionally, bears show strong individual differences in daily activity and heart rate, indicating idiosyncratic behavioral and physiological strategies to hibernation (Laske et al. 2011). Bears also exhibit strong demographic differences with females providing all parental care. As opportunistic omnivores, black bears have plastic foraging strategies (Jacoby et al. 1999; Robbins et al. 2004), and food availability is the primary predictive factor for their behavior, particularly denning chronology and reproduction (Baldwin and Bender 2010; Costello et al. 2003; Hilderbrand et al. 1999; Noyce and Garshelis 1994; Rogers 1987). Across Colorado, black bears experience varying conditions of habitat quality, and previous work found bear diet correlated with aspects of human development (Kirby et al. 2016).
We hypothesized that telomere length should reflect not only characteristics unique to individual bears, but also be influenced by environmental conditions. We predicted that age and sex would strongly influence telomere lengths, like most mammals, with older and male bears having shorter telomere lengths. Further, we predicted that environmental characteristics, particularly those related to habitat and hibernation would also influence biological age. Specifically, bears with access to better habitat and food should be under less stress, and thus have relatively longer telomeres. Additionally, if the consequences of hibernation in bears are similar to those of small hibernators (Turbill et al. 2013), bears with longer and deeper hibernation bouts (presumably at higher elevations and latitudes) should experience attenuated telomere attrition. Because multiple factors may affect biological aging in black bears, we further evaluated the relative influences of each of these individual and environmental characteristics.
Materials and methods
Sample preparation
We opportunistically sampled guard hairs with intact follicles from hunter-harvested black bears (n = 245) throughout the state of Colorado during fall hunting season in 2011. Though telomere dynamics can vary with tissue type, endothelial tissues are correlated with other somatic tissues (e.g. erythrocytes) in humans and other mammals (Benetos et al. 2011; Daniali et al. 2013; Smith et al. 2011a). Collected samples were stored at −20 °C until we extracted DNA with standard procedures (QIAGEN DNeasy Blood and Tissue Extraction Kit; QIAGEN Inc., Valencia, CA). DNA concentration was determined with Qubit 2.0 Fluorometer (Life Technologies) and DNA quality assessed using gel-electrophoresis.
Quantitative PCR assay
Primer optimization
We quantified relative length of telomeres using real-time quantitative polymerase chain reaction (qPCR) (Cawthon 2002). This approach has been found to be accurate, in particular for within species comparison (Cawthon 2002; Nakagawa et al. 2004). Although relative telomere length estimates from qPCR quantify both terminal and interstitial telomere repeats, other studies have shown them to be robust and correlated with mean telomere length as estimated using terminal restriction fragment analysis (Bize et al. 2009). The method determines relative telomere length by comparing the ratio of telomere repeat copy number (T) to single copy gene number (S) in a particular DNA sample. Relative differences in telomere length between individuals then, is exhibited by contrasting the T/S ratio of one individual to that of another (RTL). Any reliably amplified single copy (or non-variable copy) gene sequence can be employed for standardization (Olsen et al. 2012). We performed conventional PCRs on each primer set to assess amplification via gel electrophoresis, and then performed a series of qPCR reactions to test primer concentrations, annealing temperatures and template DNA concentrations. We tested three single copy gene primer sets previously applied in multiple taxa: 36B4 (Callicott and Womack 2006), albumin (Cawthon 2009), beta-globin (Cawthon 2009), and three primer sets specifically used in black bears: GAPDH (Gilbert et al. 2007), IRBP (Yu et al. 2004), and HNRPF (Fedorov et al. 2009). We also tested both sets of telomere primers developed by Cawthon (2002, 2009). To select the best single copy gene for this study, we assessed melting curves and correlations between each primer pair, as suggested by Smith et al. (2011b). Although albumin and HNRPF were correlated and both exhibited appropriate single-peak melting curves, the most consistently and reliably amplified single copy primer pairs were for HNRPF: HNRPF-f (CAAAGCCACAGAGAACGACA) and HNRPF-r (ACCCGTCACTCTTCCATCAG). The telomere primers developed by Cawthon (2009), telg (ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT) and telc (TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA), generate a short, fixed length product, and also showed reduced variability within sample replicates. These telomere and HNRPF primer sets were used for all analyses, and are hereafter referred to as “telomere” and “single copy.”
qPCR reaction conditions
Telomere and single-copy gene PCR were conducted on separate 96-well plates, with identical preparation except for primers. Immediately prior to reaction setup, samples were diluted to 3 ng/µl. Each reaction then contained 8 µl sample DNA, 10 µl SYBR Select Master Mix (Life Technologies—Applied Biosystems), telomere primers (250 nM each final concentration) or single copy gene primers (500 nM each final concentration), and distilled water to total 20 µl reaction volume. Samples were analyzed in triplicate within a plate and the average used in subsequent statistical analyses (each set of telomere and single copy plates here is referred to as a “batch”). Real-time PCR was conducted with an Eppendorf Mastercycler ep realplex, with the following thermocycling conditions: 50 °C for 2 min, 95 °C for 5 min, followed by 2 cycles of 94 °C for 15 s and 49 °C for 15 s, and then 35 cycles of 95 °C 15 s, 62 °C 10 s, 74 °C 15 s (telomere) or 95 °C 15 s, 62 °C 15 s, 72 °C 45 s (single copy); both protocols ended with a melting curve from 60 to 95 °C with a resolution of 0.5 °C.
Quantitative methods
We initially examined amplification curves visually in the Eppendorf Mastercycler ep realplex software, and then performed baseline correction on raw fluorescence data in the program LinRegPCR (Ruijter et al. 2009) using its automatic strict baseline correction. After baseline correction, we quantified telomere and single copy genes using three methods from Pfaffl (2001), Ruijter et al. (2009), and comparative Cq (Olsen et al. 2012). We ran 76 samples in triplicate within plate and across 2–3 separate batches (coefficient of variations for T: within-plate = 13%, between-plate = 19%; S: within-plate = 11%, between-plate = 9%). We found mean RTL (T/S) (n = 76) was highly correlated regardless of method (>0.8), but the lowest coefficient of variation for RTL sample estimates was from comparative Cq (13%, as opposed to 20%), and we therefore proceeded using comparative Cq with all subsequent analyses. Samples within a batch were excluded or rerun if their efficiency fell 2.5% outside the mean. All batch mean efficiencies for telomere as well as single copy gene reactions ranged from 1.79 to 1.81 (as calculated within LinRegPCR from raw qPCR output), similar to Olsen et al. (2012); batches that exhibited means outside this range were rerun. Mean RTL for each sample were used in subsequent analyses.
Predictors of telomere length
We examined how potential variables could influence relative telomere length in black bears. Specifically, we considered two groups of variables: individual and environmental. Individual variables measured included age, sex, and body size. Teeth (first premolar) from each carcass were used to determine age by counting the cementum annuli (Matson’s Lab, Milltown, MT) with standard procedures (Willey 1974), and sex and body size (approximated by zygomatic width) were determined at time of sampling. Hunters provided GPS locations used to extract environmental characteristics (Fig. 1). We characterized environmental characteristics of bear locations via measures of both vegetative productivity and human development. Though bear home ranges can vary widely, Colorado bears typically range less than 50 km2 (Baldwin 2008; Baruch-Mordo et al. 2014). Thus, we buffered each bear harvest location by approximately 50 km2 (a radius of 4 km) to analyze environmental variables and elevation, which we calculated in ArcGIS (ESRI, v.10). We considered mean growing season Normalized Difference Vegetation Index (NDVI) in 2011, with higher NDVI values representing greater primary productivity, because NDVI has been used to predict habitat selection in brown bears (Wiegand et al. 2008). We took monthly composites (0.1 degrees) collected by Terra/MODIS (NASA Earth Observations) and averaged them across the growing season (April–October) in ArcGIS (ESRI, v.10). We extracted mean growing season NDVI of each buffered bear location using Geospatial Modelling Environment (GME, v. 0.7.2.1). Within each buffer, we considered human development indexed by housing density from 2010 Block Level Housing Density (Radeloff et al. 2010). We also considered latitude and longitude in UTMs of each bear location, and calculated elevation from the National Elevation Dataset (USGS 2009).
Analyses were conducted in R package v 3.1.1. We first explored relationships among covariates with Pearson correlations, and excluded highly correlated variables (≥0.6), or those that increased a variance inflation factor >2, from further analyses. In constructing our set of models, we tested for interactions between age and latitude, age and NDVI, and latitude and NDVI by comparing the additive and interactive models using ANOVAs. We then considered a suite of potential linear models with RTL as the response variable, and compared all possible combinations of individual and environmental covariates. All variables were fitted as continuous except sex, and housing density was log-transformed to meet assumptions of normality. We considered only individuals for which we had complete data on all variables (n = 195) and used Akaike’s Information Criterion to select the best models.
Genetic structure
Although a single panmictic population was determined using 8 hyper-variable microsatellites during a previous project conducted in Colorado (Alldredge et al. 2008), we also explored genetic structure within our sampled individuals. To that end, we genotyped a subset of bear samples, stratified by latitude (n = 100), at 4 previously described bear-specific microsatellite loci (G1A, G1D, G10C, G10L; Paetkau and Strobeck 1994). Unlabeled reverse primers and fluorescent-labeled forward primers were obtained from Integrated DNA Technologies or Life Technologies- Applied Biosystems. All reactions were carried out in singleplex according to protocols in Brown et al. (2009) before combined into panels and submitted for fragment analysis at UW Biotechnology using a 3730 × l DNA Analyzer (Applied Biosystems). Alleles were scored using GeneMapper v.4.1, and PCR was repeated for any sample or marker that produced an ambiguous genotype. We used program STRUCTURE v.2.3 to estimate whether there were genetically distinct populations in the samples across Colorado (Pritchard et al. 2000). We ran STRUCTURE for K = 1–10 populations and evaluated each value of K using the log-likelihood of the data given K[lnPr(X|K)]. We used 10,000 “burn-in” iterations followed by 50,000 iterations for analyses.
Results
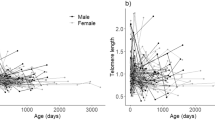
Colorado black bears, aged 1–21, exhibited wide variation in relative telomere lengths (RTL), ranging from 1.28 to 6.99, with a mean of 3.43. In determining our model set to explain RTL, we did not find any interaction between age and latitude (F 1,191 = 1.57, P = 0.21), age and NDVI (F 1,191 = 0.04, P = 0.85), or latitude and NDVI (F 1,191 = 0.43, P = 0.51), so we proceeded with a suite of additive models. Model selection comparing individual and environmental influences on telomere length revealed that environmental variables had the strongest relationship with telomere length. The top models all included latitude and NDVI as influential covariates (Table 1). Bears harvested in northern Colorado had shorter relative telomere lengths than those harvested in southern Colorado, and those in areas of higher vegetative productivity exhibited shorter telomeres than those in areas of lower vegetative productivity, regardless of individual characteristics.
Although age and sex were included as covariates in some of the top models, they were not significant, and less complex models performed better. In fact, we did not detect any relationship between individual characteristics and telomere length, including age, sex, and zygomatic width (Fig. 2a). In contrast, telomere length of black bears exhibited patterns with environmental variables—both latitude and NDVI, but neither elevation nor housing density (Fig. 2b). We also detected a significant positive correlation between latitude and NDVI (r = 0.22, P = 0.002), which, although small, could be driving some of the relationship between NDVI and RTL. Elevation was also positively correlated with NDVI (r = 0.43, P < 0.001) and negatively correlated with housing density (r = −0.42, P < 0.001), though we did not find a significant correlation between NDVI and housing density (r = −0.13, P = 0.06).
In addition, the genetic structure analysis for the subset of sampled bears indicates that the most likely number of genetic populations was K = 1, confirming little or no genetic structure for black bears throughout the state (see Table 2 for microsatellite descriptive statistics). This lack of structure along the latitudinal cline indicates a panmictic population, and suggests that underlying population genetic differences are unlikely to be a primary driver of this pattern in telomere lengths.
Discussion
Our results suggest that individual factors do not strongly influence genetic markers of biological aging in Colorado black bears. Instead, the emergent pattern we detected was latitudinal: latitude of bear harvest was negatively correlated with telomere length. We suggest that this pattern reflects differences in important environmental conditions that are overwhelming potential relationships of individual variables to biological aging.
Though initial research into telomeres suggested they shorten with cellular replication (and chronological age), an increasing number of studies have illustrated that telomere length is not always an effective marker of chronological age (Dunshea et al. 2011; Horn et al. 2010; Monaghan 2010b; Ujvari and Madsen 2009). In black bears, we found a slight, although non-significant, decline in telomere length with age. Cross-sectional studies such as this one may not detect an age effect because of selective disappearance of individuals with short telomeres from the population. Previous studies on wild mammals using cross-sectional data have found age-related declines in telomere length in martens (Pauli et al. 2011) and sea lions (Izzo et al. 2011) for example, but not in hyenas (Lewin et al. 2015). Longitudinal sampling may be required to illuminate age-specific declines, as found recently in badgers (Beirne et al. 2014). Even within longitudinal studies, however, much of the selective disappearance may occur earlier in life, rather than as adults (Fairlie et al. 2016). Thus, while we did not find an age-related decline in telomere length in black bears, we cannot rule out age-specific differences in telomere rates of change. We also found no sex-specific differences in telomere length in our population. In most mammals, females have higher telomerase activity thought to be due to estrogen (Leri et al. 2000), as well as lower adult mortality (Liker and Szekely 2005). Though sex can influence telomere dynamics, no single theory yet explains the complex relationship among telomere length, sex, and survival (Barrett and Richardson 2011). In black bears then, sex does not appear to be influential on telomere length.
Instead, we found that telomere lengths were influenced by broad-scale environmental variables. The strongest correlation we found was that bears at higher latitudes have shorter telomere lengths than bears living in southern Colorado. To the best of our knowledge, no cross-sectional study of a wild population has demonstrated a similar latitudinal cline in telomere lengths. Such a pattern could be due to population genetic differences, with variations in starting telomere lengths, or to differing environmental conditions that alter telomere dynamics as bears age. Because starting telomere length can be partially heritable (Horn et al. 2011), we examined the Colorado bear population for genetic structure. Our findings confirmed previous work that the Colorado black bear population is genetically mixed (Alldredge et al. 2008). This lack of distinct subpopulations suggests that genetic isolation is not the driving force behind our observations of telomere length in bears. However, this does not rule out the possibility of some sort of genetic cline influencing the pattern of bear telomere lengths. Future studies examining telomere inheritance within a bear population could quantify the influence of heritability on telomere dynamics in bears.
Alternatively, environmental conditions may be driving the latitudinal pattern in telomere lengths. Northern Colorado is associated overall with higher elevations, cooler temperatures, and higher precipitation than southern Colorado, resulting in potential differences in bear habitat and food availability. We found that vegetative productivity, as predicted by NDVI, was negatively associated with telomere length, suggesting that bears in areas of greater natural food abundance had shorter telomere lengths and greater chronic stress. At first glance, this pattern seems counterintuitive, and could be driven in part by the small correlation between NDVI and latitude, with northern Colorado exhibiting higher values of NDVI. Latitude on its own explains more variation in telomere length than NDVI alone (Table 1). Additionally, NDVI may not adequately capture natural food availability, as it could miss important bear foods such as berries (Wiegand et al. 2008), or alternatively over-represent conifers. Moreover, we recently showed that diet of Colorado black bears varies directly with human influence (Kirby et al. 2016); bears consume more human-derived foods in areas of higher housing density, as well as forage at a higher trophic level. Though we found no relationship between telomeres and human development in this study, the relationship between food availability and telomere length is likely more complex than measuring only vegetative productivity.
If telomere length accurately reflects underlying stress, bears experiencing less stress throughout their lives should have longer telomeres. As food availability drives much of bear body condition and reproduction, as well as hibernation length, we suspect that these latitudinal patterns are linked to the complex influences of habitat quality. In particular, hibernation has been linked to increased annual survival and longevity across a diverse assemblage of species (Lyman et al. 1981; Melvin and Andrews 2009; Turbill et al. 2011; Wilkinson and South 2002), and has recently been shown to slow telomere attrition in rodents (Turbill et al. 2012, 2013). If large-bodied hibernators respond similarly to rodents, hibernation length should decelerate bear telomere attrition. Unfortunately, this study does not have a direct measure of hibernation length. However, as habitat quality and food availability determine denning chronology (Johnson and Pelton 1980), bears with better access to food tend to hibernate for shorter periods (Baldwin and Bender 2010; Bridges et al. 2004) and, consequently they might exhibit amplified cellular aging, despite possible trade-offs with enhanced body condition and fecundity.
Specifically, bears residing at higher latitudes in Colorado (and higher NDVI), likely have access to more food, and thus may hibernate for shorter periods of time, reflected in shorter telomeres and accelerated biological aging. A further complication to this story, however, is human activity—black bears that overwinter near urban areas can also exhibit shorter denning periods, presumably due to supplemental human food (Baldwin and Bender 2009; Beckmann and Berger 2003). As we did not find a significant relationship between human development and telomere length in this study, the relative contribution of overall food availability to bear stress and hibernation length remains unknown. Furthermore, bears hibernating in colder temperatures utilize more energy (Tøien et al. 2015). As northern Colorado tends to be colder than southern Colorado, altered hibernation dynamics could include increased energy usage and thus reduced ameliorating effects of hibernation on telomere attrition.
Our pattern-based cross-sectional analysis suggests that emergent environmental properties are driving telomere length in black bears. Though determining the mechanism behind biological aging from this data set is not possible, this latitudinal pattern is strongly suggestive that genetic markers of biological aging reflect extrinsic environmental conditions, rather than simply individual characteristics of black bears. We attribute these results to bear habitat parameters, likely food availability and hibernation. Further work should incorporate survival and telomere dynamics of individuals at a fine scale to investigate the particular influences of habitat conditions.
References
Alldredge MW (2008) Cougar demographics and human interactions along the urban-exurban Front Range of Colorado. Wildlife Research Report, Mammals Research, Colorado Parks and Wildlife
Angelier F, Vleck CM, Holberton RL, Marra PP (2013) Telomere length, non-breeding habitat and return rate in male American redstarts. Funct Ecol 27:342–350. doi:10.1111/1365-2435.12041
Aydos SE, Elhan AH, Tükün A (2005) Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet 272:113–116
Baldwin RA (2008) Population demographics, habitat utilization, critical habitats, and condition of black bears in Rocky Mountain National Park. Dissertation, New Mexico State University
Baldwin RA, Bender LC (2009) Survival and productivity of a low-density black bear population in Rocky Mountain National Park, Colorado. Human-Wildl Confl 3:271–281
Baldwin RA, Bender LC (2010) Denning chronology of black bears in Eastern Rocky Mountain National Park, Colorado. West N Am Nat 70:48–54. doi:10.3398/064.070.0106
Barrett ELB, Richardson DS (2011) Sex differences in telomeres and lifespan. Aging Cell 10:913–921. doi:10.1111/j.1474-9726.2011.00741.x
Baruch-Mordo S, Wilson KR, Lewis DL et al (2014) Stochasticity in natural forage production affects use of urban areas by black bears: implications to management of human-bear conflicts. Plos One 9:1–10. doi:10.1371/journal.pone.0085122
Beckmann JP, Berger J (2003) Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J Zool 261:207–212. doi:10.1017/S0952836903004126
Beirne C, Delahay R, Hares M et al (2014) Age-related declines and disease-associated variation in immune cell telomere length in a wild mammal. Plos One 9:e108964. doi:10.1371/journal.pone.0108964
Benetos A, Kimura M, Labat C et al (2011) A model of canine leukocyte telomere dynamics. Aging Cell 10:991–995
Bize P, Criscuolo F, Metcalfe NB et al (2009) Telomere dynamics rather than age predict life expectancy in the wild. Proc R Soc B Biol Sci 276:1679–1683. doi:10.1098/rspb.2008.1817
Bonier F, Martin PR, Moore IT, Wingfield JC (2009) Do baseline glucocorticoids predict fitness? Trends Ecol Evol 24:634–642. doi:10.1016/j.tree.2009.04.013
Bonneaud C, Mazuc J, Chastel O et al (2004) Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution 58:2823–2830. doi:10.1111/j.0014-3820.2004.tb01633.x
Bridges AS, Fox JA, Olfenbuttel C, Vaughan MR (2004) American black bear denning behavior: observations and applications using remote photography. Wildl Soc Bull 32:188–193. doi:10.2193/0091-7648(2004)32
Broussard D, Risch T, Dobson F, Murie J (2003) Senescence and age-related of female reproduction Columbian ground squirrels. J Anim Ecol 72:212–219
Brown SK, Hull JM, Updike DR et al (2009) Black bear population genetics in California: signatures of population structure, competitive release, and historical translocation. J Mammal 90:1066–1074
Bryant MJ, Reznick D (2004) Comparative studies of senescence in natural populations of guppies. Am Nat 163:55–68. doi:10.1086/380650
Buffenstein R, Edrey YH, Yang T, Mele J (2008) The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age 30:99–109. doi:10.1007/s11357-008-9058-z
Callicott RJ, Womack JE (2006) Real-time PCR assay for measurement of mouse telomeres. Comp Med 56:17–22
Cassidy A, De Vivo I, Liu Y et al (2010) Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr 91:1273–1280. doi:10.3945/ajcn.2009.28947.INTRODUCTION
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21. doi:10.1093/nar/gkn1027
Cichoń M, Sendecka J, Gustafsson L (2003) Age-related decline in humoral immune function in Collared Flycatchers. J Evol Biol 16:1205–1210. doi:10.1046/j.1420-9101.2003.00611.x
Costello C, Jones D, Inman R et al (2003) Relationship of variable mast production to American black bear reproductive parameters in New Mexico. Ursus 14:1–16
Daniala L, Benetos A, Susser E, Kark JD et al (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 4:1597
Dunshea G, Duffield D, Gales N et al (2011) Telomeres as age markers in vertebrate molecular ecology. Mol Ecol Resour 11:225–235. doi:10.1111/j.1755-0998.2010.02976.x
Epel ES, Blackburn EH, Lin J et al (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101:17312–17315. doi:10.1073/pnas.0407162101
Fairlie J, Holland R, Pilkington JG et al (2016) Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15:140–148
Fedorov VB, Goropashnaya AV, Tøien O et al (2009) Elevated expression of protein biosynthesis genes in liver and muscle of hibernating black bears (Ursus americanus). Physiol Genomics 37:108–118. doi:10.1152/physiolgenomics.90398.2008
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Gilbert ER, Wong EA, Vaughan M, Webb KE (2007) Distribution and abundance of nutrient transporter mRNA in the intestinal tract of the black bear, Ursus americanus. Comp Biochem Physiol Part B 146:35–41
Gomes NMV, Ryder OA, Houck ML et al (2011) Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 10:761–768. doi:10.1111/j.1474-9726.2011.00718.x
Haussmann MF, Winkler DW, O’Reilly KM et al (2003) Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc R Soc B Biol Sci 270:1387–1392. doi:10.1098/rspb.2003.2385
Hilderbrand GV, Schwartz CC, Robbins CT et al (1999) The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can J Zool 77:132–138. doi:10.1139/cjz-77-1-132
Horn T, Robertson BC, Gemmell NJ (2010) The use of telomere length in ecology and evolutionary biology. Heredity 105:497–506. doi:10.1038/hdy.2010.113
Horn T, Robertson BC, Will M et al (2011) Inheritance of telomere length in a bird. Plos One 6:e17199. doi:10.1371/journal.pone.0017199
Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ (2008) Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 44:235–246. doi:10.1016/j.freeradbiomed.2007.10.001
Izzo C, Hamer DJ, Bertozzi T et al (2011) Telomere length and age in pinnipeds: the endangered Australian sea lion as a case study. Mar Mamm Sci 27:841–851
Jacoby ME, Hilderbrand GV, Servheen C et al (1999) Trophic relations of brown and black bears in several western North American ecosystems. J Wildl Manag 63:921–929
Johnson KG, Pelton MR (1980) Environmental relationships and the denning period of black bears in Tennessee. J Mammal 61:653–660
Kirby R, Alldredge MW, Pauli JN (2016) The diet of black bears tracks the human footprint across a rapidly developing landscape. Biol Cons 200:51–59
Kotrschal A, Ilmonen P, Penn DJ (2007) Stress impacts telomere dynamics. Biol Lett 3:128–130. doi:10.1098/rsbl.2006.0594
Laske TG, Garshelis DL, Iaizzo PA (2011) Monitoring the wild black bear’s reaction to human and environmental stressors. BMC Physiol 11:13. doi:10.1186/1472-6793-11-13
Leri A, Malhotra A, Liew C et al (2000) Telomerase activity in rat cardiac myocytes is age and gender dependent. J Mol Cell Cardiol 32:385–390
Lewin N, Treidel LA, Holekamp KE et al (2015) Socioecological variables predict telomere length in wild spotted hyenas. Biol Lett 11:20140991
Liker A, Szekely T (2005) Mortality costs of sexual selection and parental care in natural populations of birds. Evolution 59:890–897
Lyman CP, Brien RCO, Greene GC, Papafrangos ED (1981) Hibernation and longevity in the Turkish hamster Mesocricetus brandi. Science 212:668–670
Mackenzie DK, Bussière LF, Tinsley MC (2011) Senescence of the cellular immune response in Drosophila melanogaster. Exp Gerontol 46:853–859. doi:10.1016/j.exger.2011.07.004
Melvin RG, Andrews MT (2009) Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol Metab 20:490–498. doi:10.1016/j.tem.2009.09.005
Meyne J, Ratiliff RL, Moyzis RK (1989) Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA 86:7049–7053
Mizutani Y, Tomita N, Niizuma Y, Yoda K (2013) Environmental perturbations influence telomere dynamics in long-lived birds in their natural habitat. Biol Lett 9:20130511. doi:10.1098/rsbl.2013.0511
Monaghan P (2010a) Telomeres and life histories: the long and the short of it. Ann N Y Acad Sci 1206:130–142. doi:10.1111/j.1749-6632.2010.05705.x
Monaghan P (2010b) Crossing the great divide: telomeres and ecology. Heredity 105:574–575. doi:10.1038/hdy.2010.120
Monaghan P, Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21:47–53. doi:10.1016/j.tree.2005.11.007
Nakagawa S, Gemmell NJ, Burke T (2004) Measuring vertebrate telomeres: applications and limitations. Mol Ecol 13:2523–2533. doi:10.1111/j.1365-294X.2004.02291.x
Noyce K, Garshelis D (1994) Body size and blood characteristics as indicators of condition and reproductive performance in black bears. Int Conf Bear Res Manag 9:481–496
Nussey DH, Froy H, Lemaitre J-F et al (2013) Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225. doi:10.1016/j.arr.2012.07.004
Olsen MT, Bérubé M, Robbins J, Palsbøll PJ (2012) Empirical evaluation of humpback whale telomere length estimates; quality control and factors causing variability in the singleplex and multiplex qPCR methods. BMC Genet 13:77. doi:10.1186/1471-2156-13-77
Olsson M, Pauliny A, Wapstra E et al (2011) Sexual differences in telomere selection in the wild. Mol Ecol 20:2085–2099. doi:10.1111/j.1365-294X.2011.05085.x
Paetkau D, Strobeck C (1994) Microsatellite analysis of genetic variation in black bear populations. Mol Ecol 3:489–495
Patel R, McIntosh L, McLaughlin J et al (2002) Disruptive effects of glucocorticoids on glutathione peroxidase biochemistry in hippocampal cultures. J Neurochem 82:118–125
Pauli JN, Whiteman JP, Marcot BG et al (2011) DNA-based approach to aging martens (Martes americana and M. caurina). J Mammal 92:500–510. doi:10.1644/10-MAMM-A-252.1
Pauliny A, Wagner RH, Augustin J et al (2006) Age-independent telomere length predicts fitness in two bird species. Mol Ecol 15:1681–1687. doi:10.1111/j.1365-294X.2006.02862.x
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multi-locus genotype data. Genetics 255:945–959
Punzo F, Chavez S (2003) Effect of aging on spatial learning and running speed in the shrew (Cryptotis parva). J Mammal 84:1112–1120. doi:10.1644/BWG-106
Radeloff VC, Stewart SI, Hawbaker TJ et al (2010) Housing growth in and near United States protected areas limits their conservation value. Proc Natl Acad Sci 107:940–945. doi:10.1073/pnas.0911131107
Ringsby TH, Jensen H, Parn H et al (2015) On being the right size: increased body size is associated with reduced telomere length under natural conditions. Proc R Soc B Biol Sci 282:20152331
Robbins CT, Schwartz CC, Felicetti LA (2004) Nutritional ecology of ursids: a review of newer methods and management implications. Ursus 15:161–171
Robinson MR, Mar KU, Lummaa V (2012) Senescence and age-specific trade-offs between reproduction and survival in female Asian elephants. Ecol Lett 15:260–266. doi:10.1111/j.1461-0248.2011.01735.x
Rogers L (1987) Effects of food supply and kinship on social behavior, movements, and population growth of black bears in northeastern Minnesota. Wildl Monogr 97:3–72
Ruijter JM, Ramakers C, Hoogaars WMH et al (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. doi:10.1093/nar/gkp045
Schwartz CC, Keating KA, Reynolds HV et al (2003) Reproductive maturation and senescence in the female brown bear. Ursus 14:109–119
Scott NM, Haussmann MF, Elsey RM et al (2006) Telomere length shortens with body length in Alligator mississippiensis. Southeast Nat 5:685–692. doi:10.1656/1528-7092(2006)5[685:TLSWBL]2.0.CO;2
Seluanov A, Chen Z, Hine C (2007) Telomerase activity coevolves with body mass, not lifespan. Aging Cell 6:45–52. doi:10.1111/j.1474-9726.2006.00262.x
Shi Q, Hubbard GB, Kushwaha RS et al (2007) Endothelial senescence after high-cholesterol, high-fat diet challenge in baboons. Am J Physiol Hear Circ Physiol 292:H2913–H2920. doi:10.1152/ajpheart.01405.2006
Smith DL, Mattison JA, Desmond RA et al (2011a) Telomere dynamics in rhesus monkeys: no apparent effect of caloric restriction. J Gerontol A Biol Sci Med Sci 66:1163–1168
Smith S, Turbill C, Penn DJ (2011b) Chasing telomeres, not red herrings, in evolutionary ecology. Heredity 107:372–373
Stevenson RD, Woods WA (2006) Condition indices for conservation: new uses for evolving tools. Integr Comp Biol 46:1169–1190. doi:10.1093/icb/icl052
Tarlow EM, Blumstein DT (2007) Evaluating methods to quantify anthropogenic stressors on wild animals. Appl Anim Behav Sci 102:429–451. doi:10.1016/j.applanim.2006.05.040
Tøien Ø, Blake J, Edgar DM et al (2011) Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331:906–909. doi:10.1126/science.1199435
Tøien Ø, Blake J, Barnes BM (2015) Thermoregulation and energetics in hibernating black bears: metabolic rate and the mystery of multi-day body temperature cycle. J Comp Physiol B 185:447. doi:10.1007/s00360-015-0891-y
Turbill C, Bieber C, Ruf T (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc B Biol Sci 278:3355–3363. doi:10.1098/rspb.2011.0190
Turbill C, Smith S, Deimel C, Ruf T (2012) Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol Lett 8:304–307. doi:10.1098/rsbl.2011.0758
Turbill C, Ruf T, Smith S, Bieber C (2013) Seasonal variation in telomere length of a hibernating rodent. Biol Lett 9:20121095
Ujvari B, Madsen T (2009) Short telomeres in hatchling snakes: erythrocyte telomere dynamics and longevity in tropical pythons. Plos One 4:e7493
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Watson JD (1972) Origin of concatameric T4 DNA. Nature 239:197–201
Wiegand T, Naves J, Garbulsky MF, Fernández N (2008) Animal habitat quality and ecosystem functioning: exploring seasonal patterns using NDVI. Ecol Monogr 78:87–103. doi:10.1890/06-1870.1
Wilkinson GS, South JM (2002) Life history, ecology and longevity in bats. Aging Cell 1:124–131
Willey C (1974) Aging black bears from first premolar tooth sections. J Wildl Manag 38:97–100
Young RC, Kitaysky AS, Haussmann MF et al (2013) Age, sex, and telomere dynamics in a long-lived seabird with male-biased parental care. Plos One 8:e74931. doi:10.1371/journal.pone.0074931
Young RC, Kitaysky AS, Barger CP et al (2015) Telomere length is a strong predictor of foraging behavior in a long-lived seabird. Ecosphere 6:1–26
Yu L, Li Q, Ryder OA, Zhang Y (2004) Phylogeny of the bears (Ursidae) based on nuclear and mitochondrial genes. Mol Phylogenet Evol 32:480–494. doi:10.1016/j.ympev.2004.02.015
Acknowledgements
Funding for this project was provided by Colorado Parks and Wildlife and an American Society of Mammalogists Grant-in-Aid. We thank all of the Colorado wildlife managers and bear hunters for generously collecting and providing samples. We are also grateful to Cristina Vaughan, Garrett Johnson, and Samantha Paddock for laboratory help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirby, R., Alldredge, M.W. & Pauli, J.N. Environmental, not individual, factors drive markers of biological aging in black bears. Evol Ecol 31, 571–584 (2017). https://doi.org/10.1007/s10682-017-9885-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-017-9885-4