Abstract
The evolution of mimicry is one of the most powerful examples of evolution driven by natural selection; however it is rare in non-insect taxa and thus is understudied. Ranitomeya imitator underwent a ‘mimetic radiation’ and now mimics three congeneric model species (R. fantastica, R. summersi, and two morphs of R. variabilis), creating geographically distinct populations of the species, including four allopatric mimetic morphs. These complexes are thought to represent a case of Müllerian mimicry, but no prior empirical studies on learned avoidance by predators support this claim. In this study we used young chickens (Gallus domesticus) as naïve predators to determine if a co-mimetic morph of R. imitator and R. variabilis contribute to reciprocal learned avoidance by predators—a key component of Müllerian mimicry. Chickens exposed to either stimulus species demonstrated reciprocal learned avoidance; thus our results indicate that this complex functions as a Müllerian mimicry system. This study provides novel empirical evidence supporting predictions of the Müllerian mimicry hypothesis in anurans. Our study shows no difference between learned avoidance in stimuli frogs and a ‘novel’ morph of R. imitator that differed in both color and pattern, indicating that learned avoidance by predators may be generalized in this system. Generalized learning provides a plausible mechanism for the maintenance of both polytypic mimicry and the maintenance of intrapopulation phenotypic heterogeneity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poison frogs, family Dendrobatidae, are known for their unpalatibility, toxicity, and bright aposematic coloration (Daly and Myers 1967; Saporito et al. 2007). Their toxicity results from the possession of skin alkaloids, which act as a deterrent to potential predators (Daly et al. 2005; Darst and Cummings 2006). Ranitomeya imitator, Schulte, (formerly Dendrobates imitator—see Grant et al. 2006 and Brown et al. 2011) is a poison frog found in lowland and montane forests of the Peruvian Amazon (Schulte 1986). This species mimics multiple sympatric species throughout its range (Symula et al. 2001; Yeager et al. 2012; Twomey et al. 2013). Phylogenetic analyses (Symula et al. 2001, 2003) indicate R. imitator has undergone a rapid mimetic radiation to adverge on the aposematic signals of sympatric congeners (Yeager et al. 2012, but see Chouteau et al. 2011 for discussion). This indicates that R. imitator is the mimic in this system, having evolved to resemble established populations of R. fantastica (Boulenger), R. summersi (Brown, Twomey, Pepper, and Rodriguez), and two morphs of R. variabilis (Zimmermann and Zimmermann), one formerly designated R. ventrimaculata—see Brown et al. (2011). Morphological advergence likely occurred in the context of experienced predators avoiding individual R. imitator that resembled local models (e.g. Ihalainen et al. 2008), leading to frequency-dependent selection for Müllerian mimicry (Sherratt 2008).
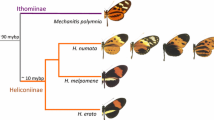
While numerous authors (Symula et al. 2001; Sherratt 2008; Brown et al. 2011; Yeager et al. 2012) have argued that R. imitator is a Müllerian mimic, key predictions of this hypothesis remain to be tested empirically. Müllerian mimicry is a phenomenon in which evolutionarily distinct species possessing secondary defenses evolve to appear morphologically similar and thus share the burden of predator learning. Predators need to ‘sample’ individuals in order to learn that they are toxic (Müller 1878, 1879) and, intuitively, this can have a detrimental effect on those individuals ‘sampled.’ Commonly cited and well-studied examples of Müllerian mimicry are the “mimicry rings” involving Heliconius butterfly communities, and these provide a close parallel to mimicry complexes in Ranitomeya (Joron and Mallet 1998; Mallet and Barton 1989; Mallet and Joron 1999). In these mimicry rings, novel or rare phenotypes are more likely to be attacked by predators if they are not recognized as toxic or unpalatable (Mallet and Joron 1998; Kapan 2001; Sherratt 2008). Thus predators are thought to select against polymorphism in Müllerian mimics (Speed 1993, 1999; Joron et al. 2001).
Many authors have suggested that avian predators are the primary force driving the evolution of color and pattern in dendrobatid frogs (e.g. Symula et al. 2001; Darst and Cummings 2006; Darst et al. 2006; Saporito et al. 2007; Noonan and Comeault 2009). Several factors support this: (1) avian peak activity coincides with peak poison frog activity (early morning and late afternoon), and daylight is likely an important component of the aposematic signal (Schulte 1986; Duellman and Trueb 1994: Poulin et al. 2001); (2) birds are common predators of frogs in the Neotropics (Stiles and Skutch 1989), although Poulin et al. (2001) found that toxic dendrobatids were conspicuously absent in samples of avian stomach contents; (3) birds are able to detect the conspicuous color signals of dendrobatid frogs (Siddiqi et al. 2004; Maan and Cummings 2012); (4) birds frequently attack clay models of dendrobatid frogs (Saporito et al. 2007; Noonan and Comeault 2009; Chouteau and Angers 2011); and (5) birds have been observed preying upon poison frogs (Master 1999; Alvarado et al. 2013). Further, (Maan and Cummings 2012) demonstrated that Oophaga (Dendrobates) pumilio signals its toxicity honestly from the perspective of avian predators; thus visual conspicuousness is correlated with increased toxicity. Collectively these observations indicate that potential avian predators are able to detect aposematic signals in dendrobatid frogs and may be the primary recipients of these signals, even if they are not the most common predators of poison frogs. Additionally, there is evidence that the combination of aposematic coloration and diurnal habits may be enough to deter many potential predators (Siddiqi et al. 2004; Brodie 1993)—indicating an effective aposematic signal.
Ranitomeya imitator is a hypothetical Müllerian mimic of multiple sympatric congeners (Symula et al. 2001; Ruxton et al. 2004; Sherratt 2008; Brown et al. 2011; Yeager et al. 2012). A recent field study by Chouteau and Angers (2011) examined this mimicry complex in situ using plasticine clay models, which they reciprocally transplanted between two sites containing mimetic populations of R. imitator and R. variabilis—a highland site with the spotted morph of both frogs and a lowland site with the striped morphs of both species. They demonstrated that local avian predators discriminate between local versus novel morphs and that local morphs experience a significantly lower rate of predation. This indicates that avian predators are a rapid, homogenizing selective force maintaining the geographical organization of coloration in these two species (Chouteau and Angers 2011). However, it does not indicate whether learned predator avoidance is reciprocal (driven by both species) or unidirectional (driven by one species).
The aim of our study was to examine a putative Müllerian mimicry system and determine if co-mimetic species (R. imitator and R. variabilis) contribute to reciprocal learned avoidance by predators. We used naïve chicks (Gallus domesticus) as model predators to test this hypothesis because birds are hypothesized to be the main drivers of color and pattern evolution in poison frogs (e.g. Symula et al. 2001; Darst and Cummings 2006; Darst et al. 2006; Saporito et al. 2007; Noonan and Comeault 2009; Maan and Cummings 2012), previous studies have used chicks effectively (e.g. Darst and Cummings 2006) and because Chouteau and Angers (2011) demonstrated that avian predators are able to differentiate between local and novel morphs in R. imitator/variabilis.
In addition to the prediction that both co-mimetic species should possess secondary defenses, the hypothesis of Müllerian mimicry predicts that co-mimetic species should reciprocally confer learned avoidance by predators. That is, predators should learn to avoid both species after interacting with either one. We aimed to test this prediction by presenting naïve chicks with one of two stimuli, either the spotted morph of R. variabilis or the corresponding mimetic spotted morph of R. imitator and giving them the opportunity to smell, taste, and prey upon wild-caught poison frogs in a series of learning trials. We compared their learned avoidance to baseline data collected prior to learning trials by recording the interaction time spent with these frogs in timed trials.
In addition to reciprocal learned avoidance, we tested whether learned avoidance is generalized or exact through the addition of a distinct but geographically proximate color pattern morph of R. imitator in our study. If predators exhibit exact learning, we would expect this to partially explain the evolution and maintenance of mimetic polytypism in R. imitator. Generalized learned avoidance may provide a mechanism for the maintenance of polymorphism within populations, and for the formation of hybrid zones between these morphs.
Materials and methods
To test whether conferred protection from predators is reciprocal versus unidirectional (and thereby test a key prediction of the hypothesis of Müllerian mimicry), we conducted predator-learning trials with chicks in one of two treatment groups (see Fig. 1), the model (i.e., the spotted morph of R. variabilis, n = 18) or the corresponding mimetic spotted morph of R. imitator (n = 17). Note that in Müllerian mimicry, the two mimetic species are generally referred to as co-mimics. However, in this case, previous research indicates that R. imitator has evolved to resemble other model species (Symula et al. 2001), rather than convergence on a shared morphology by both species. Hence we refer to R. imitator as the mimic, and the other species as models, in this paper. These experiments (experimental protocol approved via ECU AUP: #D225 and Ministry of Natural Resources (DGFFS) in Lima, Peru: Resolución Directoral No 033-2011-AG-DGFFS-DGEFFS) were conducted in the department of San Martin, Peru in 2011 and 2012. We used one to one and a half month old naïve chicks (Gallus domesticus) as naïve predators. Chickens were used because birds are known to differentiate colors (Siddiqi et al. 2004) and have been widely implicated as a selective force in amphibian aposematic systems (see above). Chicks were obtained from a local market in the city of Tarapoto. These chicks were bred, born and raised within the city limits, and did not have the opportunity to encounter live frogs (including poison frogs) during the brief span of time that passed before they were sold in the city market. The chicks were given water ad libitum, fed cracked corn twice daily, typically after trials, and housed in a 1 × 2 m wire cage. Housing cages were designed to resemble the experimental arena, a 1 m2 wooden enclosure with an earthen floor divided into four 50 cm2 quadrants via demarcations on the side of the experimental arena (see Fig. 2). All poison frogs were collected within 7-10 days of the initiation of experimental trials; toxins in dendrobatid frogs are sequestered from prey items (Daly et al. 1994) and are retained for extended periods in captivity (Myers and Daly 1976). Thus frogs presumably did not lose their toxicity during their brief captivity during this study. Individuals of the spotted morph (both R. imitator and R. variabilis) were collected from a forested plot in the mountains to the east of Tarapoto, San Martin and striped R. imitator were collected from one of two populations near Pongo de Caynarachi or further east. Chicks were fed palatable, cryptic control frogs (Leptodactylus sp.) prior to trials to ensure they recognized frogs as potential prey items.
Flowchart of methodological progression. Pre-learning trials paired a Leptodactylus sp. frog with 3 different frogs (spotted R. imitator, spotted R. variabilis, and striped R. imitator) and were each repeated thrice. Learning trials were conducted afterwards and chicks were exposed to either the spotted R. imitator or R. variabilis stimulus. Post-learning trials followed and were a repeat of the initial pre-learning trials
A pictoral depiction of the experimental arena. The arena was 1 × 1 m and had demarcations at the 50 cm marks to subdivide the arena into 4 quadrants. In this example the arena is set up for a pre-learning or post-learning trial with two frogs (R. variabilis top right and Leptodactylus sp. bottom left) under small glass cubes
Our experimental design had three main features: pre-learning trials, learning trials, and post-learning trials. Pre-learning trials allowed us to establish a baseline for the behavior of naïve chicks when interacting with poison frogs and control (palatable) frogs. In the context of testing predictions concerning predator learning in the context of Müllerian mimicry, these trials allowed us to compare the behavior of chicks before and after learning about the toxicity of the poison frogs. The learning trials provided the chicks with the opportunity to interact directly with the frogs, and hence to learn about the differences in toxicity between the poison and control frogs through direct sensory perception (e.g. taste, smell). In the context of testing predictions concerning Müllerian mimicry, these trials provide the learning opportunity that is critical in testing whether the chicks are able to associate color pattern with unpalatability, and learn to avoid the brightly-colored, toxic frogs after interacting with them directly. The post-learning trials test the hypothesis that chicks will actually learn to avoid one species after being exposed to (trained by) the other. This is a key prediction of the hypothesis of predator learning in the context of Müllerian mimicry. Figure 1 provides a visual summary of the experimental design used, to clarify the structure and flow of the experiments. These methods are similar to those used by Darst and Cummings (2006), with modifications for the specific circumstances of this experiment. Below we provide details on the experimental protocols used in each of the different types of trials.
During pre-learning trials we paired control frogs (Leptodactylus sp.) with one of three aposematic and presumably toxic frogs of the genus Ranitomeya: the spotted morph of R. variabilis (model), spotted morph of R. imitator (mimic), or the striped morph of R. imitator (representing a novel morph). The novel morph was included because previous theoretical work indicates that novel or rare phenotypes are more likely to be attacked if they are not recognized as toxic or unpalatable (Müller 1879; Guilford and Dawkins 1993; Sherratt 2008), and we were interested in determining whether learning is exact (i.e., just the color/pattern predators sample during learning) or generalized in this system. Every chick (n = 35) was tested for each of these three experimental pairings once per day for three consecutive days and the order of these trials was randomized. Frogs were randomly assigned to individual quadrants and placed under glass domes (8 × 8 × 3.5 cm) with white bottoms to enhance visibility to chickens and make both the cryptic and aposematic frogs of approximately equal visibility. During these trials, chickens were able to see individual frogs but were not able to touch, taste, or smell them through the glass cubes. Trials lasted for 2 min and were video recorded with a Sony DSC-W20 in 2011 and a Nikon D3100 in 2012. We recorded (1) the number of pecks directed at each frog, (2) number of separate attack events, and (3) interaction time, which we defined as time a chick spent in the quadrant with, and oriented towards, a frog.
Subsequently, we followed with a series of learning trials in which chicks were randomly assigned to two groups and presented with only one putatively toxic species (either spotted R. variabilis, n = 18, or the corresponding spotted morph of R. imitator, n = 17) in a glass dome with the top removed. Unlike the pre-learning trials (and post-learning trials, see below), chicks were able to touch, taste, and smell the frogs during these learning trials. Chicks were observed for 2 min or until an attempted predation event and subsequent loss of interest in or consumption of the presented frog. Each chick was offered the same individual learning stimulus (except in the event of death of the trial frog) for eight learning trials; trials were conducted twice daily. In order to reduce frog mortality, chickens that ate two learning stimulus frogs were considered “educated” and no longer underwent learning trials. These chickens subsequently underwent post-learning trials.
Post-learning trials followed the completion of learning trials and were conducted in the same manner as pre-learning trials (chickens were able to see frogs through glass cubes but not touch, taste, or smell them). In addition to these trials, chicks were offered palatable control frogs prior to pre-learning trials, following learning trial number 4, and prior to post-learning trials. These interactions with palatable control frogs provided the opportunity to learn that control frogs were palatable, this is similar to natural systems in which predators are exposed to both toxic and palatable prey items. We then compared pre-learning baseline data to post-learning data by analyzing interaction time, number of pecks, and the number of separate attack events for each stimulus. Comparisons of pre-learning and post-learning behavior among chicks for both R. imitator and R. variabilis stimulus chicks were done using one-tailed, paired sample t-tests for the average ratio of time spent with each frog per trial (defined as time spent with the poison frog divided by total time spent with either frog in seconds). The analyses reported here from t tests are one-tailed because (1) if poison frogs were palatable this would be expressed through consumption of the majority of poison frogs and (2) we were only interested in testing the hypothesis of Müllerian mimicry (reciprocal learned avoidance), thus a null hypothesis of no learned avoidance is functionally equivalent to no effect (i.e., not Müllerian mimicry). Thus, analyses presented here are one-tailed. Analyses were corrected using a false discovery rate written into the syntax (code: input Test$ Raw_P @@; datalines; proc multtest inpvalues = low1 stepsid fdr bon;), which correct raw p values for the effects of multiple analyses in an attempt to reduce type I errors but are less conservative than other corrections (e.g. Bonferroni); all p values reported here are the corrected values. The use of this test has been recommended for ecological studies (Garcia 2003, 2004). We also analyzed the difference between pre- and post-learning using a one-way ANOVA between groups to analyze whether learning is exact (specific to the spotted morph) or generalized (learned avoidance protecting a wider array of phenotypes, in this study the ‘novel’ striped morph).
Ethical notes
This research was approved by East Carolina University (IACUC D225) as well as through the Ministry of Natural Resources (DGFFS) in Lima, Peru (Resolución Directoral No 033-2011-AG-DGFFS-DGEFFS). Additionally, this research closely follows the methodology of Darst and Cummings (2006), published in Nature. Live animals were required for the experiments due to the nature of the hypotheses being tested. No functional data on toxicity of these species existed at the time of this study and therefore no proxy for live, toxin-bearing frogs existed for use in predator learning trials. Frogs were housed 2–3 in glass or plastic vivariums with typical daylight cycles, available water, fed fruit flies daily, and appeared comfortable; frogs consistently called in the mornings and late afternoon, and engaged in courtship and breeding. Inside of the 2 min time period, the death of frogs was used as an endpoint in learning trials primarily for two reasons: (1) we were testing the hypothesis of learned avoidance by predators as a result of toxicity, making it imperative to avoid interruption by researchers, and (2) frog death typically occurred too rapidly for it to be prevented. No chickens exhibited signs of illness after consuming either control (Leptodactylus spp) frogs or poison frogs. Poison frogs which were grasped were generally dropped immediately and typically exhibited no signs of physical harm, however a few did exhibit small marks consistent with those visible on a small proportion of wild frogs. These frogs were treated with a small dab of Neosporin and showed no further sign of discomfort, injury, infection, or difficulty with locomotion or eating. The number of trials used was designed to achieve sufficient power for adequate statistical evaluation of the treatment effect, while not being excessive. Frogs were assigned to individual chickens and were not used for other trials. Two frogs of each type were kept on standby during trials, housed separately, and only used in the event a chicken consumed the frog.
Results
Given the opportunity to smell, taste, and prey on poison frogs, some chicks expressed innate neophobia and did not taste their stimulus species. However, all chicks expressed interest in the poison frogs and actively investigated them. In a typical sampling event, chicks grasped them in their bills and then immediately dropped them; they often expressed signs of distress and distaste such as bill wiping and eating dirt. Some chicks did consume poison frogs during learning trials (n = 3 for R. imitator and n = 1 for R. variabilis). There was no difference in chick mass between treatment group (t12 = 0.068, p = 0.947).
In analyses of interaction time between pre- and post-learning (Fig. 3 shows mean interaction times), chicks trained on R. variabilis learned to avoid their own species (t17 = 4.663, p < 0.01), the spotted morph of R. imitator (t17 = 2.704, p = 0.0094) and the novel striped morph of R. imitator as well (t17 = 2.544, p = 0.0105). Chickens trained on the R. imitator stimuli learned to avoid both the spotted and striped morph of R. imitator as well as the model R. variabilis (t16 = 1.730, p = 0.058; t16 = 1.705, p = 0.058; and t16 = 1.660, p = 0.058 respectively); this became more apparent when we removed outliers (see below). Additionally, we analyzed the data excluding the few chicks that ate frogs. This exclusion is justified because of the extreme size of these predators (on average >300 g) compared to the prey (typically <0.5 g), with the poison frogs comprising roughly 0.15 % of the chickens’ weight on average, and because chickens are much larger than the vast majority of potential avian predators in the wild. Of particular interest were chickens trained on the R. imitator stimuli, which showed an increase in learned avoidance (t13 = 2.822, p = 0.0117 and t13 = 2.391, p = 0.0206 for spotted R. imitator and R. variabilis respectively and t13 = 1.905, p = 0.0395 for the novel striped morph of R. imitator). We note that the results are very similar when analyzing with or without these individuals.
Pre- and post-learning means for chickens trained on R. imitator (n = 17) and R. variabilis (n = 18). From left to right these represent trials in which the control frogs were paired with R. variabilis, the spotted morph of R. imitator, and the striped (novel) morph of R. imitator. Interaction time is presented as a ratio of time with the poison frog to overall time with frogs. Error bars show standard error of the mean
Chicks did not avoid all frogs as a result of exposure to poison frogs and chickens continued to consume control frogs (Leptodactylus sp.) immediately. Further, total interaction time (in seconds) nearly doubled between pre- and post-learning trials. For the R. imitator stimulus chicks, total time spent with frogs increased between pre- and post-learning trials with R. variabilis and control frogs (t16 = −1.957, p = 0.068) and in trials with both spotted and striped R. imitator (t16 = −2.613, p = 0.019 and t16 = −2.583, p = 0.020 respectively). Total interaction time in R. variabilis stimulus chicks also increased, but not significantly (t17 = −1.268, p = 0.222 for R. variabilis, t17 = −2.015, p = 0.060 for spotted R. imitator and t17 = −1.790, p = 0.091 for striped R. imitator).
Additionally, we compared the interaction time of chickens during the pre-learning baseline time using 2-tailed t tests to the expected interaction time of 50 % with each frog given that these chickens had no experience with poison frogs. We found that chickens that were to-be-trained on R. variabilis showed no difference in interaction time from what we expected. However, chickens that were to-be-trained on R. imitator showed a highly significant difference from what we would expect. These chicks spent significantly less time with both spotted types, R. variabilis (t16 = −2.990, p = 0.0113), the spotted morph of R. imitator (t16 = −3.714, p = 0.0033), and there was a similar trend with the striped morph of R. imitator (t16 = −1.725, p = 0.1040). Although interaction time in our baseline (pre-learning) data for chicks to-be-trained on the R. variabilis stimulus did not differ from this expected value, chicks to-be-trained on R. imitator spent significantly less time with poison frogs than expected. In essence, our randomly assigned learning stimulus groups (R. imitator versus R. variabilis trained chicks) differed slightly in their pre-learning interaction time for unknown reasons.
We also compared pecks and independent attack events to an expected ratio of 50 % in 2-tailed t tests. In these analyses, chickens to-be-trained on both stimuli (R. variabilis or R. imitator) directed many more pecks and independent attack events towards Leptodactylus control frogs than expected (t17 < −7, p < 0.001 and t16 < −4, p < 0.001 pecks and independent attack events respectively), for all three treatment types (control frog with R. variabilis, spotted morph of R. imitator, and striped morph of R. imitator). These data indicate that chicks trained on both stimuli directed significantly more pecks and independent attack events towards control frogs than expected. This demonstrates statistically significant innate neophobia of aposematically colored poison frogs.
We also ran one-way ANOVAs on the differences between pre- and post-learning interaction times between R. imitator and R. variabilis stimulus chicks. Differences were not significant (p > 0.05 in all cases, except for the R. variabilis type pre-learning trials and the spotted R. imitator type pre-learning trials (F1,33 = 4.917 p = 0.034 and F1,33 = 3.537 p = 0.069 respectively)), indicating that there was no difference between treatments in the learned avoidance trials. This provides further evidence for reciprocal learned avoidance by predators. Furthermore, these data indicate that predators did not discriminate between the ‘local’ spotted morph which they were trained on and a ‘novel’ striped morph; this indicates that learning is ‘generalized’ and not ‘exact’ in this system.
Additionally, we analyzed the number of pecks and number of independent attack events between pre- and post-learning using paired t-tests. None of these data were statistically significant for either R. imitator or R. variabilis. Although these data do not support our prediction that predator learned avoidance would decrease the number of pecks and attacks directed at poison frogs, this is likely an effect of our baseline data being so heavily skewed away from the poison frogs (p < 0.001 for both R. imitator and R. variabilis for all three treatment types), which is consistent with innate neophobia.
Discussion
Müllerian mimicry has been proposed for a number of anuran systems, including Ranitomeya imitator (Symula et al. 2001; Sherratt 2008; Brown et al. 2011), other complexes of Ranitomeya (Brown et al. 2011), mantellids (Schaefer et al. 2002), and between Amereega picta and Leptodactylus lineatus (Prates et al. 2012). However, no study to date has demonstrated reciprocal learned avoidance by predators of a shared morph within anura—a key component of Müllerian mimicry. Our data indicate reciprocal learned avoidance by predators between co-mimetic species (R. variabilis and R. imitator) in this system, providing the first experimental evidence in support of a major prediction concerning predator learning in the context of Müllerian mimicry.
Chickens expressed innate neophobia of the spotted stimuli frogs (R. variabilis and R. imitator), and were more likely to attempt to attack cryptic control frogs than aposematically colored frogs when given the choice. This provides documentation of this phenomenon in birds with respect to potential anuran prey, just as prior studies have demonstrated innate neophobia by potential avian predators in response to potential prey from other taxa (e.g. snakes-Smith 1975; and birds-Marples et al. 1998).
Innate neophobia may be important in the maintenance of aposematic signals, favoring aposematic species in two ways: (1) by decreasing overall attack rates, and (2) eliciting hesitation to attack (often seen in our study), which may provide an aposematic individual time to escape. These are especially important because predator communities are continually changing due to recruitment, immigration, emigration, etc. Recruited, naïve individuals that display conservative behavior or innate neophobia are less likely to attack aposematic prey items (or more likely to allow aposematic prey to escape), and juveniles are more wary than adults with aposematic prey (Marples et al. 1998; Lindström et al. 1999). Attacks, when they occur, if not a direct cause of mortality may have substantial implications for lifetime fitness. A small proportion of individuals in the wild have lost digits/limbs or have visible scarring (AS pers. obs.) and attacks/injuries may lead to infection. Further, attacks may lead to a decrease in fitness through physical injury (e.g. loss of digits or limbs) or reduced sexual fitness due to the effects of scarring on mate choice or changes in behavior to decrease the risk of further attack. Additionally, attacks may lead to a decrease in toxicity of the aposematic individual as a result of selective secretion of toxins. As a result, predator neophobia favors the maintenance of aposematically colored prey.
When chicks sampled poison frogs, most dropped them immediately and lost interest. Some chicks exhibited distress signals such as bill wiping, indicating that these frogs have a noxious taste. Although a few chickens did consume poison frogs (three R. imitator stimulus chicks and one R. variabilis stimulus chick), we note that the birds used in this study (average weight = 305 g) likely weigh much more than the vast majority of potential avian predators that encounter these poison frogs in the wild. Due to the minute size of these frogs (typically under 0.5 g), toxin dilution could reduce the effectiveness of chemical defenses with heavier predators. Indeed, Exernová et al. (2008) noted that larger bird sizes increase a predator’s ability to handle the chemical defenses of heteropteran insects (see also Veselý et al. 2006). As a result the prey-to-predator weight ratio could be an important consideration in studies exploring the mimicry spectrum, and as such larger species (tinamous, chachalacas, guans, etc.) may play an important predatory role in this system. Although chicks that consumed poison frogs showed no obvious signs of poisoning afterwards, there could be a delayed effect on fitness or mortality for predators that consume poison frogs. A delayed effect is seen in native fence lizards (Sceloporus undulatus) that consume Solenopsis invicta, an introduced, toxic red fire ant which lends support to this hypothesis (Langkilde and Freidenfelds 2010).
Despite this, chickens trained on both stimuli learned to avoid poison frogs and support the hypothesis of Müllerian mimicry, in both analyses with and without outlier chicks that ate poison frogs. We excluded some outliers because our chicks have much higher mass than most potential avian predators of frogs, and larger predators have been shown to function differently as predators (Veselý et al. 2006; Exernová et al. 2008). Further, the difference in our baseline data of chicks to-be-trained on R. imitator and R. variabilis should be taken into account in our final results. Ranitomeya imitator chicks all interacted with poison frogs significantly less than the expected ratio of 50 % of the time (p < 0.05 in all treatments) in pre-learning trials whereas R. variabilis chicks did not (p ≫ 0.05 in all treatments). This likely has a dampening effect on our ability to detect learned avoidance in R. imitator stimuli chicks and explains at least some of the discrepancy between learned avoidance results between R. imitator and R. variabilis chicks. The fact that we see reciprocal learned avoidance in this study despite the dampening effect of differential baseline (pre-learning) data strongly supports the hypothesis of unpalatability and is consistent with Müllerian mimicry.
We used learned avoidance by predators as a proxy for toxicity. Although the difference in interaction time between learning stimulus species is not statistically significant (likely due to extremely small sample size), more chickens consumed R. imitator than R. variabilis (n = 3 and n = 1 respectively). Thus there may be slight differences in toxicity and R. variabilis may be the more toxic species. Future work should investigate the chemical suites that these frogs possess.
Polytypism in a Müllerian mimic is theoretically detrimental because predators are thought to learn to avoid individuals of the same morphological appearance, i.e., exact learning (Sherratt 2008). However, we found no difference in learned avoidance between ‘local’ spotted R. imitator and R. variabilis and ‘novel’ striped R. imitator. These results indicate that our predators did not discriminate between their learning stimuli (the spotted morph) and the ‘novel’ striped morph (i.e., they displayed generalized learning).
These data are contrary to how Müllerian systems are assumed to work—with rapid negative selection acting against novel or rare phenotypes (Benson 1972; Mallet and Barton 1989; Kapan et al. 2001; Pinheiro 2003; Ihalainen et al. 2008; Sherratt 2008; but see Ihalainen et al. 2006 in support of our findings). Furthermore, in situ studies of predation using clay models of poison frogs (including the spotted morph of R. imitator/variabilis) have demonstrated that novel phenotypes are attacked more frequently by avian predators and experience negative selection, thus maintaining the common shared aposematic signal (Saporito et al. 2007; Noonan and Comeault 2009; Chouteau and Angers 2011). This is important for the evolution and maintenance of mimicry complexes as experienced predators will continually be a source of purifying selection against rare or novel morphs and push populations towards phenotypic homogeneity (Saporito et al. 2007; Noonan and Comeault 2009; Chouteau and Angers 2011).
Our results with respect to generalized learning may be an artifact of experimental design and may result from exposure to both the learning stimuli morph (spotted) and the novel phenotype (striped) during pre-learning trials. Alternatively, these data may indicate how populations with great phenotypic variation persist (for example, clines where R. imitator transitions between mimetic morphs and exhibits great phenotypic variation) when theory holds that both intrapopulation phenotypic variation and mimetic polymorphism should be rare (Speed 1993; Mallet and Joron 1999). If predators are exposed to individuals that vary significantly in appearance but also share similar traits (e.g. color, pattern elements, or perhaps just an appearance of aposematism) they may attribute unpalatability to the entire spectrum of individuals to which they are exposed. Predator learning is often rapid (Kapan 2001; Rowland et al. 2007; Chouteau and Angers 2011), and this may contribute to generalized learning in instances where predators are exposed to varying aposematic signals. Individuals may become protected by learned avoidance if their coloration or general appearance is perceived as being ‘close enough’ by predators. This may partially explain the continued existence of polytypism, intrapopulation polymorphism, and the clines between distinct morphological populations and the shifting hybrid zones seen in the Heliconius systems parallel to these frogs (Blum 2002).
To our knowledge, our data represent the first experimental evidence for reciprocal learned avoidance by predators in the context of Müllerian mimicry in an anuran system, as well as the first example of innate neophobia of an anuran by an avian predator. Additionally we note generalized learning in this system, which presents a plausible mechanism for the maintenance of mimetic polytypism, intrapopulation polymorphism, and transition zone stability. This system presents further opportunities to study advergence as well as the evolution and maintenance of Müllerian mimicry in a vertebrate system that closely resembles Heliconius butterflies. Given the inherent variation among populations of these frogs, this system promises to provide interesting insights into mimicry in the future.
References
Alvarado JB, Alvarez A, Saporito RA (2013) Oophaga pumilio (strawberry poison frog). Predation. Herpetol Rev 44(2):298
Benson WW (1972) Natural selection for Müllerian mimicry in Heliconius erato in Costa Rica. Science 176:936–938
Blum MJ (2002) Rapid movement of a Heliconius hybrid zone: evidence for phase III of Wright’s shifting balance theory? Evolution 56(10):1992–1998
Brodie EDI (1993) Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution 47(1):227–235
Brown JL, Twomey E, Amézquita A, Barbosa de Souza M, Caldwell JP, Lötters S, von May R, Melo-Sampaio PR, Mejía-Vargas D, Perez-Peña P, Pepper M Poelman EH, Sanchez-Rodriguez M, Summers K (2011) A taxonomic revision of the neotropical poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083:1–120
Chouteau M, Angers B (2011) The role of predators in maintaining the geographic organization of aposematic signals. Am Nat 178(6):810–817
Chouteau M, Summers K, Morales V, Angers B (2011) Advergence in Müllerian mimicry: the case of the poison dart frogs of Northern Peru revisited. Biol Lett 7:796–800
Daly JW, Myers CW (1967) Toxicity of Panamanian poison frogs (Dendrobates): some biological and chemical aspects. Science 156(3777):970–973
Daly JW, Secunda SI, Garraffo HM, Spande TF, Wisnieski A, Cover JF Jr (1994) An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon 32(6):657–663
Daly JW, Spande TF, Garraffo HM (2005) Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds. J Nat Prod 68(10):1556–1575
Darst CR, Cummings ME (2006) Predator learning favours mimicry of a less-toxic model in poison frogs. Nature 440(9):208–211
Darst CR, Cummings ME, Cannatella DC (2006) A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proc Natl Acad Sci USA 103(15):5852–5857
Duellman WE, Trueb L (1994) Biology of amphibians. John Hopkins University Press, Baltimore, MD, p 670
Exnerová A, Svádová K, Fousová P, Fučíková E, Ježová D, Niederlová A, Kopečková M, Štys P (2008) European birds and aposematic Heteroptera: review of comparative experiments. Bull Insectol 61(1):163–165
García LV (2003) Controlling the false discovery rate in ecological research. Trends Ecol Evol 18:553–554
García LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105(3):657–663
Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Mus Nat Hist 299:1–262
Guilford T, Dawkins MS (1993) Receiver psychology and the design of animal signals. Trends Neurosci 16(11):430–436
Ihalainen E, Lindström L, Mappes J (2006) Investigating Müllerian mimicry: predator learning and variation in prey defences. J Evol Biol 20(2):780–791
Ihalainen E, Lindström L, Mappes J, Puolakkainen S (2008) Can experienced birds select for Müllerian mimicry? Behav Ecol 19:362–368
Joron M, Mallet JLB (1998) Diversity in mimicry: paradox or paradigm? Trends Ecol Evol 13(11):461–466
Joron M, Wynne IR, Lamas G, Mallet JLB (2001) Variable selection and the coexistence of multiple mimetic forms of the butterfly Heliconius numata. Evol Ecol 13:721–754
Kapan DD (2001) Three-butterfly system provides a field test of Müllerian mimicry. Lett Nat 409:338–340
Langkilde T, Freidenfelds NA (2010) Consequences of envenomation: red imported fire ants have delayed effects on survival but not growth of native fence lizards. Wildl Res 37:566–573
Lindström L, Alatalo RV, Lyytinen A, Mappes J (1999) Strong antiapostatic selection against novel rare aposematic prey. Proc Natl Acad Sci USA 98:9181–9184
Maan ME, Cummings ME (2012) Poison frog colours are honest signals of toxicity, particularly for bird predators. Am Nat 179(1):E1–E14
Mallet JLB, Barton NH (1989) Strong natural selection in a warning-colour hybrid zone. Evolution 43(2):421–431
Mallet JLB, Joron M (1999) Evolution of diversity in warning colour and mimicry: polymorphisms, shifting, balance, and speciation. Annu Rev Ecol Syst 30:201–233
Marples NM, Roper TJ, Harper DGC (1998) Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83(1):161–165
Master TL (1999) Predation by rufous motmot on black-and-green poison dart frog. Wilson Bull 111(3):439–440
Müller F (1878) Über die Vortheile der Mimicry bei Schmetterlingen. Zoologischer Anzeiger 1:54–55
Müller F (1879) Ituna and Thyridia: a remarkable case of mimicry in butterflies. Trans Entomol Soc Lond 1879:20–29
Myers CW, Daly JW (1976) Preliminary evaluation of skin toxins and vocalizations in taxonomic and evolutionary studies of poison-dart frogs (Dendrobatidae). Bull Am Mus Nat Hist 157(3):1–173
Noonan BP, Comeault AA (2009) The role of predator selection on polymorphic aposematic poison-frogs. Biol Lett 5(1):51–54
Pinheiro CEG (2003) Does Müllerian mimicry work in nature? Experiments with butterflies and birds (Tyrannidae). Biotropica 35(3):356–364
Poulin B, Lefebvre G, Ibanez R, Jaramillo C, Hernandez C, Rand AS (2001) Avian predation upon lizards and frogs in a neotropical forest understorey. J Trop Ecol 17:21–40
Prates I, Antoniazzi MM, Sciani JM, Pimenta DC, Toledo LF, Haddad CFB, Jared C (2012) Skin glands, poison and mimicry in dendrobatid and leptodactylid amphibians. J Morphol 273(3):279–290
Rowland HM, Ihalainen E, Lindström L, Mappes J, Speed MP (2007) Co-mimics have a mutualistic relationship despite unequal defences. Nature 448:62–67
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford University Press, New York, p 260
Saporito RA, Zuercher R, Roberts M, Gerrow KG, Donnelly MA (2007) Experimental evidence for aposematism in the dendrobatid poison frog Oophaga pumilio. Copeia 4:1006–1011
Schaefer H-C, Vences M, Veith M (2002) Molecular phylogeny of Malagasy poison frogs, genus Mantella (Anura: Mantellidae): homoplastic evolution of colour pattern in aposematic amphibians. Org Divers Evol 2:97–105
Schulte R (1986) Eine neue Dendrobates—art aus ostperu (Amphibia: Salienta: Dendrobatidae). Sauria 8:11–20
Sherratt TN (2008) The evolution of Müllerian mimicry. Naturwissenschaften 95:681–695
Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K (2004) Interspecific and intraspecific views of colour signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol 207:2471–2485
Smith SM (1975) Innate recognition of coral snake pattern by a possible avian predator. Science 187(4178):759–760
Speed MP (1993) Muellerian mimicry and the psychology of predation. Anim Behav 45:571–580
Speed MP (1999) Batestian, quasi-batesian or Müllerian mimicry? Theory and data in mimicry research. Evol Ecol 13:755–776
Stiles FG, Skutch AF (1989) A guide to the birds of Costa Rica. Cornell University Press, Ithaca, NY, p 656
Symula R, Schulte R, Summers K (2001) Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hypothesis. Proc R Soc Biol Sci 268:2415–2421
Symula R, Schulte R, Summers K (2003) Molecular systematics and phylogeography of Amazonian poison frogs of the genus Dendrobates. Mol Phylogenet Evol 26:452–475
Twomey E, Yeager J, Brown JL, Morales V, Cummings ME, Summers K (2013) Phenotypic and genetic divergence among poison frog populations in a mimetic radiation. PLoS ONE 8(2):e55443 Ed. Pawel Michalak
Veselý P, Veselá S, Fuchs R, Zrzavý J (2006) Are gregarious red-black shieldbugs, Graphosoma lineatum (Hempitera: Pentatomidae), really aposematic? An experimental approach. Evol Ecol Res 8:881–890
Yeager J, Brown JL, Morales V, Cummings ME, Summers K (2012) Testing for selection on colour and pattern in a mimetic radiation. Curr Zool 58(4):667–675
Acknowledgments
We would like to thank Molly Albecker, Trip Lamb, and Evan Twomey for their input on the manuscript. We would also like to acknowledge Lisa Schulte, Manuel Panaijo, Manuel Panaijo, and Caesar Lopez for assistance in the field and Evan Twomey for his willingness to share some of his hard-earned knowledge of localities. Financial assistance was provided by a grant to KS by National Geographic Society (8751-10) as well as partial assistance to AS by East Carolina University in the form of a Next Step Scholarship. Research permits were obtained through DGFFS in Lima, Peru (Resolución Directoral No 033-2011-AG-DGFFS-DGEFFS) and animal use permits (ECU IACUC D225)
Conflict of interest
We hereby confirm that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stuckert, A.M.M., Venegas, P.J. & Summers, K. Experimental evidence for predator learning and Müllerian mimicry in Peruvian poison frogs (Ranitomeya, Dendrobatidae). Evol Ecol 28, 413–426 (2014). https://doi.org/10.1007/s10682-013-9685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-013-9685-4