Abstract
Reproductive barriers reduce gene flow between populations and maintain species identities. A diversity of barriers exist, acting before, during and after mating. To understand speciation and coexistence, these barriers need to be quantified and their potential interactions revealed. We use the hybridising field crickets Gryllus bimaculatus and G. campestris as a model to understand the full compliment and relative strength of reproductive barriers. We find that males of both species prefer conspecific females, but the effect is probably too weak to represent a barrier. In contrast, prezygotic barriers caused by females being more attracted to conspecific male song and preferentially mounting and mating with conspecifics are strong and asymmetric. Postzygotic barriers vary in direction; reductions in fecundity and egg viability create selection against hybridisation, but hybrids live longer than pure-bred individuals. Hybrid females show a strong preference for G. bimaculatus songs, which together with a complete lack of hybridisation by G. campestris females, suggests that asymmetric gene flow is likely. For comparison, we review reproductive barriers that have been identified between other Gryllids and conclude that multiple barriers are common. Different species pairs are separated by qualitatively different combinations of barriers, suggesting that reproductive isolation and even the process of speciation itself may vary widely even within closely related groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elucidating the factors isolating species and understanding how these reproductive barriers evolve has been a central aim of evolutionary biology. Identifying species isolating mechanisms is crucial to understand patterns of species diversity and coexistence. This is fundamental to our understanding of evolution as expressed by Dobzhansky (1937, p. 419); A thorough understanding of the nature and the functioning of isolating mechanisms is essential, because without it no trustworthy picture of the mechanism of evolution can be drawn.
To quantify the net isolation between closely related species, a comprehensive assessment of all likely barriers throughout the reproductive phase of an individual and ideally their fitness consequences for subsequent generations is required (Arnold and Hodges 1995; Mendelson and Shaw 2002; Birge et al. 2010; Lemmon and Lemmon 2010; Izzo and Gray 2011). Reproductive barriers are typically divided into prezygotic barriers (e.g., differences in mating behaviour, timing and location), and postzygotic barriers (e.g., genetic incompatibilities), which include fitness effects in future generations, such as reduced fitness of backcrosses (Wiley et al. 2009). Negative effects of hybridisation dominate the literature, perhaps partly because they are often strong and relatively easy to detect. However, positive effects of hybridisation are also known to occur (i.e., hybrid vigour (Arnold 1992, see Arnold and Hodges 1995 for review)). In fact, the selection pressure exerted by many of the mechanisms that commonly create reproductive barriers can in theory increase gene flow.
Despite the complexity and challenges of the task, comprehensively quantifying reproductive barriers within systems should be a focus of speciation research as indicated by the Marie Curie Speciation Research Network (2012); … we suggest that speciation research is now at a stage where systematic documentation of the contribution of different processes is more important than the collection and categorisation of isolated examples. Few comprehensive overviews have been published (Ramsey et al. 2003; Lemmon and Lemmon 2010). There are some exceptions, most notably in Pseudacris frogs (Lemmon 2009; Lemmon and Lemmon 2010), Ficedula flycatchers (Veen et al. 2001; Svedin et al. 2008; Sætre and Sæther 2010), fruit flies (Coyne and Orr 1989, 1997), damselflies (Sánchez-Guillén et al. 2012) and several cricket species (Jang et al. 2009; Maroja et al. 2009b, Larson et al. 2012).
The first aim of this study is to contribute a comprehensive experimental assessment of reproductive barriers between a little studied pair of sister species (Huang et al. 2000): G. bimaculatus (De Geer) and G. campestris (Linnaeus). Their distributions are largely allopatric, but overlap in south-eastern Spain (Pardo et al. 1993) and probably at similar latitudes in more easterly locations.
Prezygotic barriers in crickets might occur both during long-range and short-range mate choice. These two contexts are generally accepted as serving a different function, namely identifying a partner of the right species (long-range signals) and of a high quality (short-range signals) (Gray 2005). Quality refers here to any fitness consequences other than those arising through interspecific interactions, e.g. low viability of hybrids. We assessed these different functions using playback experiments and mating trials, respectively.
Multiple postzygotic barriers were examined by making crosses among species and hybrids. It is often assumed that if independently evolved genetic systems (here represented by the different species) hybridise, the resulting offspring are less fit due to incompatibilities of certain gene combinations (e.g., Bateson-Dobzhansky-Muller incompatibilities (Orr 1995)). We therefore predict that the fertility and viability of hybrids is reduced compared to the parental species.
Furthermore, we investigated sexual selection against hybrids. Earlier studies showed that male song traits and female preferences of hybrids are intermediate between the parental species (Hoy and Paul 1973; Hoy et al. 1977; Shaw 2000). Based on this limited evidence, we predict that in choice experiments there will be no difference in preference of female hybrids for either parental species. However, making predictions for hybrids’ traits is difficult as it is dependent on the (typically unknown) genetics of the traits and is influenced by the often increased variance of hybrids compared to the parental species.
The strength of each individual barrier to reproductive isolation (RI; Coyne and Orr 1989) between the two species was calculated. As earlier acting barriers have a stronger effect on gene flow compared to later acting ones, we calculated the strength of each barrier and assessed the absolute contribution (AC: Ramsey et al. 2003) of each barrier.
Once considerable knowledge of reproductive barriers between species belonging to the same clade is collated, a comparison of similarities of barriers among each individual species pair may be conducted. This may shed light on how common multiple barriers are and whether or not similar combinations of barriers cause interspecific reproductive isolation within a clade. Furthermore, the presence/absence of certain sets of reproductive barriers may provide insights into how these barriers evolved (i.e. speciation). For example, the process of reinforcement requires a cost to hybridisation to be present, which drives divergence of prezygotic barriers such as mate choice (e.g., Servedio and Noor 2003). In the second part of the paper we combine our findings with a comprehensive overview of published barriers between ‘true cricket’ species (Gryllidae family). We hypothesise that similarities in reproductive barriers across different species pairs suggests a shared speciation mechanism.
Materials and methods
Barriers between G. bimaculatus and G. campestris
Study species
Gryllus bimaculatus and G. campestris are sister species and represent the European clade of Gryllus genus (Family Gryllidae) (Huang et al. 2000). The distribution of G. campestris in Europe covers large parts of France and Spain and extends east along the same latitude. G. bimaculatus occupies a more southerly distribution, mainly restricted to the coastal region around the Mediterranean. The two species meet in south-eastern Spain (Gorochov and Llorente 2001; Pardo et al. 1993), and presumably in additional locations such as Italy and further East (Popov and Shuvalov 1977). Earlier descriptive studies showed that female G. bimaculatus hybridise readily with G. campestris but that the reverse pairing is extremely rare in captivity (Cousin 1933; von Hörmann-Heck 1955) or absent (Veen et al. 2011). In this study we used individuals collected from areas in Spain where only one of both species is living at present. G. campestris was not found within an area of about 40 km in diameter around the G. bimaculatus collection site near Valencia (N43 27.193 W5 50.407), and the closest population of G. bimaculatus is at least 60 km away from the G. campestris collection site near Oviedo (N39 35.936 W0 34.087), with the Cantabrian Mountains likely acting as a geographic barrier. Only reproductively mature males (i.e. with a spermatophore present) were used. We use the term ‘hybrid’ for offspring from a female G. bimaculatus × male G. campestris pairing, as this was the only type of hybrid produced during our experiments. Hybrid offspring from 10 unique pairs were obtained and each experiment consisted of a mixture of hybrids from at least five of these pairs.
Reproductive barriers
Pre- and postzygotic barriers are described relative to the hybridising pair. Thus, a reduced willingness of female hybrids to mate relative to the parental species acts at the same (prezygotic) stage of an individual’s life cycle but is a postzygotic barrier in the same way that reduced viability of hybrid offspring is postzygotic.
Obtaining a comprehensive measure of the reproductive barriers between two species involves quantifying fitness estimates over multiple generations. An overview of reproductive barriers studied, including the methods used, is presented in Fig. 1a (here we focus on the parental generation (comparing pure-species with mixed-species pairings) and the F1 hybrid generation (contrasted to pure-species parings) in order to obtain large enough sample sizes). Our experiments were conducted for consecutive parts of the life cycle of both parental species and hybrids, hence we conducted statistical tests comparing the different pairing types within each of the stages and present the results accordingly (e.g. mating success of hybrids is presented before the hatching success of hybrid eggs, but acts later as a reproductive barrier). The different components of the pre- and postzygotic barriers are combined and discussed in the appropriate order in the Discussion. We refer to any mechanism potentially influencing gene flow as a reproductive barrier, even if our result is that it enhances gene flow.
An overview of the methods used (in grey box) to assess the strength of different reproductive barriers between G. bimaculatus and G. campestris (a). The absolute contribution (AC) of each of the barriers to reproductive isolation (b). The AC values are calculated relative to the measurement of interest of pure species parings or pure species (which was lacking for hybrid calling song preference hence the exclusion). See main text for details
Measuring reproductive isolation
We calculate the contribution of each barrier to reproductive isolation using the methods as outlined in Ramsey et al. (2003), to which we refer the reader for details. In short, RI quantifies the relative strength of a barrier and typically varies between 0 (no barrier) and 1 (complete barrier). The general form is
where heterospecific is the measurement for the mixed-species pairing or hybrid and conspecific for the pure-species paring or pure species (Coyne and Orr 1989). The RI for each barrier is defined in the next sections. The absolute contribution (AC) represents the contribution of a barrier while accounting for the reduction due to earlier acting barriers (equation 1–4 in Ramsey et al. 2003). The RI of prezygotic barriers was assessed relative to the pure-species pairing of the focal sex (e.g. female in the long-range calling song tests) and maternal species for the postzygotic barriers where relevant.
Prezygotic barriers
We conducted two sets of mate choice experiments following the sequence of mating behaviours in the wild. In both species, females locate a male based on his courtship song (long-range) and after approaching and making physical contact, the pair engages in a sequence of courtship behaviours, which may lead to mating (short-range: Adamo and Hoy 1994).
Long-range mating behaviour
In the first test, a typical courtship song (a long-range signal) of both pure species was played-back to a G. bimaculatus, G. campestris or a hybrid female through speakers. For the playback we constructed ‘typical’ songs of males of both species using the mean number of pulses per chirp, the chirp length and time between chirps, all which have been found to affect mate choice (Thorson et al. 1982; Simmons 1988a, b; Doherty 1991; see Supporting information A for details and discussion).
The response of the cricket to the song played was measured using a trackball system. When responding phonotactically, the cricket turns towards the sound source (i.e., the speaker). In the trackball set-up however the cricket is fixed on top of a lightweight sphere, which turns in response to her activity. This movement was recorded by two optical sensors and stored every 0.5 s (see Supporting information B for a detailed description and advantages of the method). We focus on the steering behaviour of the crickets, rather than their forward motion, since the rotation of the sphere around its vertical axis represents an attempt by the female to turn towards or away from either sound source (Hedwig and Poulet 2005). We capture this behaviour by taking the mean of the recorded turning effort over the playback period (the recording frequency) (‘turning effort’ in degrees s−1) towards the active speaker (or the conspecific song in case of the two-choice trials, see below).
Each playback trial started with a period of acclimatisation by the target female to the sphere (1–5 min). The experiment started with a period of silence (60 s), followed by playback 1 from side A (50 s), silence (10 s), playback 2 from side B (50 s), silence (10 s), and playback 1 and 2 simultaneously, each played from one side (50 s). Each female was tested on two consecutive days. On the second day, the species order of playback (i.e., which species’ call was played first) was reversed to control for potential effects of whether or not a conspecific song was played first. The species and side of the first playback sequence was drawn randomly and each trial started with two no-choice tests and ended with a two-choice test. In each no-choice test, the songs of both species were used, played sequentially from different sides. It is valuable to use both experimental designs as they represent different empirical scenarios (Wagner 1998; Coyne and Orr 2004). The no-choice playback simulates a situation with only one type of male (either con- or heterospecific), which is an essential test as females have been found to respond to heterospecific calls in the absence of conspecifics (Jang and Gerhardt 2006a). The two-choice test provides information about situations where both con- and heterospecific males are present simultaneously.
Individuals that repeatedly ran backwards, pushed the sphere up and down or nibbled on the sphere after the initial acclimatisation period were excluded. Lab-reared 10 day old crickets of both species were used and all experiments took place at 28 °C ± 1 °C under red light. The peak sound level of both speakers was normalised to 84 ± 0.5 dB (re 20 μPa) using a Tenna 72-6635 sound pressure meter.
Statistical analyses of the no-choice and two-choice tests were performed separately using linear-mixed models (l mm: the nlme library in R (R Development Core Team 2011)). We used a mixed model with turning effort as response variable and ‘female ID’ (a unique number for each individual female) as random effect to control for multiple uses of some females. As explanatory variables we included: ‘female identity’ (G. bimaculatus, G. campestris and hybrid), ‘song type’ (conspecific or heterospecific song; G. bimaculatus was arbitrarily chosen to be named conspecific for hybrid females for ease of use) and their interaction. The location of the active speaker (‘side’; left or right) and the order of con- or heterospecific song playback (‘run’) were included to control for their potential effects on the response variable, and female identity × song type interaction was included to test for response differences between types of females. Subsequently, three pairwise comparisons were conducted using the same statistical approach to determine which female groups differed significantly from each other. The two-choice trials were analysed in a similar way, but without the explanatory variables run and song type, as both types of song were, by definition, played simultaneously and the response is measured with respect to the conspecific song (‘conspecific phonotaxis’) as described above.
A separate l mm was run for each female type and for no and two-choice tests to test whether the females showed positive conspecific phonotaxis, as indicated by a significant intercept term.
Explanatory variables were excluded if they did not significantly reduce model fit as tested using an anova or likelihood ratio test (Crawley 2007). We present the p values of the model selection results in the main text and a detailed summary in the Supporting information. All analyses in this paper were performed using R (R Development Core Team 2011).
The RI was only calculated for the no-choice test as it allows us to calculate the relative female response to hetero- versus conspecific males
Calculating an RI for hybrids is not trivial as gene flow is impeded if hybrids prefer to mate with other hybrids instead of backcrossing with either of the parental species, or are less inclined to mate in general (no data available).
Short-range mating behaviour
To test for barriers when the crickets come into physical contact, a hybrid individual was placed in a circular plastic container (14.5 or 17 cm diameter) together with a G. bimaculatus of the opposite sex but separated by a partition for the first 60 s. After removal of the partition, time keeping started after first physical contact. Whether or not the male produced courtship song, and whether the female mounted the male and mated, were recorded continuously during a 10-min period. The results of these experiments were compared with data obtained from pure and mixed-species mate choice trials of all combinations between G. bimaculatus and G. campestris (Veen et al. 2011). The different courtship components are sequential; starting with the male producing courtship song, followed by mounting and mating, which are induced by the female. We only have information about all possible crosses for G. bimaculatus (of both sexes) and we therefore use this as our focal species.
We analysed the three sequential short-range mate choice steps (male song—female mounting—mating) separately. As the response variables are binary, we used a generalised-linear mixed model with a binomial distribution and logit link function (glmm; R lme4 library). An individual’s ID was added as a random factor to control for multiple use of the same individual. Only one random factor could be added (otherwise the size of the random effects vector exceeded the number of observations) which was chosen to be the ID of the sex that instigated the behavioural response (the male for the song, and female for all other behaviours). As an explanatory variable we included species identity (G. campestris/G. bimaculatus/hybrid). Model selection was based on anovas using the Chi2 distribution (Crawley 2007). The G. campestris used originate from wild-caught and first-generation lab-reared individuals. We grouped these together as a previous study showed that they behaved similarly (Veen et al. 2011). Due to the seasonal availability of G. campestris we could not perform crosses between the latter species and hybrids. See Veen et al. (2011) for a detailed dissection of pure- and mixed-species pairing behaviour.
RIs were calculated for each of the three components as follows:
The RI for hybrids was not computed as hybrids were not tested against both pure species and hence a proper reference to determine the direction of gene flow is lacking.
Postzygotic barriers
We compared the reproductive success (number of eggs laid and hatching success) of mixed-species parings with that of both pure-species pairings, and the longevity of hybrids with that of both pure species.
Longevity was estimated for both sexes of all three groups as the time between adult emergence and death. All crickets were checked every third day (with few exceptions). To estimate longevity we used only data from the captive bred G. bimaculatus individuals, and offspring of wild caught G. campestris and offspring of crosses between wild caught G. campestris and captive bred G. bimaculatus. The longevity data were not normally distributed and therefore we analysed them using a Kruskal–Wallis test. Pair-wise comparisons between the three groups were conducted using the kruskalmc function from the pgirmess package (Giraudoux 2008). We subsequently tested whether longevity differed between the sexes within each of the three species groups.
Sexes were pooled as longevities were very similar (although statistically significantly different for G. campestris).
The reproductive success of the different pairing types was assessed by counting the number of eggs laid and hatched. After a successful mating, the spermatophore was left attached to the female for 60 min for most trials to facilitate sperm transfer. To increase the sample size we included unpublished data from mating trials using the same G. bimaculatus stock where the spermatophore was left attached for 45 min. Sperm transfer rate declines over time and the majority of sperm in the ejaculate is present inside the female after 45 min (Simmons 1986). After removal of the spermatophore, the female was kept in a plastic container with a small petri-dish with moist sand to lay eggs in. After 48 h the eggs were extracted from the sand by rinsing the petri-dish with water, counted, and transferred onto a moist layer of cotton wool in a large petri-dish where they remained until hatching (Simmons 1988a, b). The emerged nymphs were counted and transferred to standard housing conditions. The egg pads were monitored for at least 1 week after first hatching. Approximately 10 % of the broods were lost due to fungal infections affecting the entire clutch. All procedures were conducted at 28 °C.
The differences in number of eggs laid for the different pairing types were analysed using a glm with quasipoisson error (as the data were overdispersed). Differences in hatching success between the paring types were analysed with a logistic regression (glm with binomial distribution). Only wild caught G. campestris females were used for this experiment. These were caught early in the season when very few matings would have occurred so most of them were likely to be virgins, but this cannot be certain for any individual.
Literature review
For our review of reproductive barriers in crickets we included all ‘true’ cricket species (Gryllidae): nine more Gryllus species, three Allonemobius species, three Laupala species and five Teleogryllus species. We conducted a literature search using the key words speciation, reproductive barrier, crickets and Gryllidae on the Web of Knowledge and Google Scholar alongside checking references in relevant papers and tracking subsequent citations of these papers. We focussed on barriers typically recorded under laboratory conditions. Different barriers, such as timing of reproduction might be present in nature but are beyond the scope of this review.
Results
Reproductive barriers between G. campestris and G. bimaculatus
Prezygotic barriers
The behaviour of female G. bimaculatus and hybrids on the sphere was similar to when they were observed in a large chamber. In contrast, more female G. campestris switched between running forward (typical for G. bimaculatus females) and running backwards and persistently trying to escape. Any trials with such erratic behaviour were excluded from the analysis. A total of 38 out of 41 G. bimaculatus females were tested successfully, 37 of which provided data for both trials (on consecutive days). For G. campestris, 20 out of 45 females were successfully tested, with 14 providing 2 trials. Out of the 35 hybrids females tested, 26 could be used, with 24 double trials.
Long-range mate choice
No-choice experiment
Females of the three species groups did not differ in their response towards a con- or heterospecific song type as indicated by the exclusion of the song type × female identity interaction and song type (p = 0.282 and p = 0.179 respectively, Fig. 2). Female identity, run and side remained in the final model (p = 0.003, p = 0.005, p < 0.001, respectively).
The phonotactic response measured as turning effort towards the sound source of female G. bimaculatus (‘bimac’), G. campestris (‘camp’) and hybrids (‘hybrid’) towards con- and heterospecific male calling songs. Each female was tested in a no-choice (a) and two-choice b set-up. We arbitrarily choose G. bimaculatus as conspecific for hybrid females. Note the different scales of the y-axis between a and b
The pair-wise comparisons between G. bimaculatus–G. campestris and G. bimaculatus—hybrid were very similar: for both, the song type × female identity interaction (p = 0.876 and p = 0.164, respectively) and song type (p = 0.799 and p = 0.143, respectively) were excluded and run, side and female species remained. For the G. campestris—hybrid comparison, the interaction was also non-significant (p = 0.097) as well as female species (p = 0.052), with song type just significant (p = 0.049).
In the next step we tested for the strength of phonotactic response of each female group by examining whether the intercept was significant in the final model. This revealed that only G. bimaculatus females showed significant phonotaxis (p intercept < 0.001), i.e., a turning effort significantly deviant from zero. The final G. bimaculatus model furthermore included side (p < 0.001). The G. campestris intercept approached significance (p intercept = 0.054) and the final model contained run (p = 0.013). The intercept of hybrid females was not significant (p intercept = 0.120) and the final model contained; run (p = 0.034), side (p < 0.001) and song type (p = 0.012). See Supporting information C for full statistical results.
Two-choice experiment
Female identity was not significant in the full model (p = 0.474), nor in any of the pair-wise comparisons (p > 0.202). Conspecific phonotaxis was significant in the G. bimaculatus–G. campestris and G. bimaculatus—hybrid comparison (both p < 0.001).
A preference for conspecific song is indicated by separate lmms for each female group, revealing inclusion of conspecific phonotaxis in the final model for female G. bimaculatus and hybrids (p < 0.001 and p = 0.019, respectively) but not G. campestris (p = 0.349). (Note that for hybrid females G. bimaculatus is ‘conspecific’). See Supporting information D for full statistical results.
Short-range mate choice
For all three sequential mate choice steps we first discuss the three pairing types involving a female G. bimaculatus (open circles, connected by a line in Fig. 3) and then the three paring types involving a male G. bimaculatus (the column marked ‘bimac’ on the x-axis of Fig. 3). The outcome of the model comparisons is given in Table 1. Each individual is only used once for a particular pairing type and only once a day. A total of 127 mate choice experiments were performed (see Table 1 for sample sizes of the different pairing types). The total number of individuals, with number used multiple times in brackets, is as follows: 79 (7) male and 90 (6) female G. bimaculatus, 32 (0) and 12 (0) G. campestris, and 16 (0) and 25 (0) hybrids.
Mean responses with standard errors of males (initiation of courtship song (a)) and females (mounting (b) and mating (c)) to con- and heterospecific partners. Pairing types involving a female G. bimaculatus (open circles) are connected by a line. G. campestris is abbreviated as ‘camp’, G. bimaculatus to ‘bimac’ and hybrids to ‘hybrid’
Song
The different male groups responded differently towards female G. bimaculatus, as indicated by inclusion of species identity in the final model (Table 1a; Fig. 3a). Pair-wise comparisons showed that these differences are strong between all pairs, except the G. bimaculatus × G. campestris versus G. bimaculatus × hybrid pairing.
Similarly, G. bimaculatus males responded differently to females of the three groups (Table 1b; Fig. 3a). This can be attributed to the significant difference between G. bimaculatus × G. bimaculatus versus G. campestris × G. bimaculatus. These differences are however less pronounced compared to the responses of different male groups to female G. bimaculatus (Fig. 3a).
Mounting
Males of the three different groups differed significantly in how likely they were to be mounted by female G. bimaculatus (Table 1a; Fig. 3b). Pair-wise comparisons revealed that this is due to female G. bimaculatus mounting a conspecific significantly more often than they mount a G. campestris male. There was no significant difference in mounting between the G. bimaculatus × G. campestris versus G. bimaculatus × hybrid pairing comparison.
Females of the different groups responded differently to G. bimaculatus males (Table 1b; Fig. 3b). Furthermore, female G. bimaculatus and G. campestris differed significantly in their propensity to mount, with no mounting by female G. campestris (as reported by Veen et al. (2011)). G. campestris and hybrid females differed significantly.
Mating
The results of the full model are similar to mounting with the exception of G. bimaculatus × G. campestris pairings, as mounting results in a mating much less frequently for this group (Table 1a; Fig. 3c). The only significant pair-wise difference is between G. bimaculatus females × G. bimaculatus versus G. campestris males.
Similarly, the female groups differed in their response to male G. bimaculatus (Table 1b; Fig. 3c). Pair-wise differences were significantly different for G. bimaculatus versus G. campestris × male G. bimaculatus and female G. campestris versus hybrid × male G. bimaculatus.
Postzygotic barriers
Longevity
The three species groups differed significantly in longevity, with hybrids living the longest, G. campestris the shortest and G. bimaculatus intermediate (Fig. 4; Table 2a). Longevity differed significantly between all three pair-wise species comparisons. Post hoc Kruskal–Wallis comparisons for each species group to test for differences between the sexes revealed a sex difference in longevity for G. campestris but not for G. bimaculatus or hybrids (Table 2b).
Number of eggs
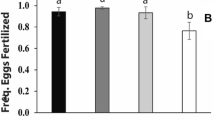
The number of eggs laid differed significantly between the three pairing types as indicated by the inclusion of species group in the final model (p < 0.001). The pair-wise comparison between the two pure species pairings (G. bimaculatus and G. campestris) and pure G. campestris pairs versus female G. bimaculatus × male G. campestris significantly differed (both p < 0.001) with a much higher number of eggs for G. campestris females (Fig. 5). Pure G. bimaculatus laid more eggs (Fig. 5), but not significantly so compared to female G. bimaculatus × male G. campestris pairs (p = 0.068). See Supporting information F for full statistical details.
Hatching success
Species group was retained in the full model (p < 0.001) indicating a significant difference between the three pairing types. Pair-wise comparisons showed a significantly lower hatching success of both pure G. campestris pairs and female G. bimaculatus × male G. campestris pairs compared with pure G. bimaculatus pairs (see Fig. 6). Hatching success of female G. bimaculatus × male G. campestris was significantly reduced compared to pure G. campestris pairs. (All pair-wise comparisons p < 0.001, see Supporting information G for full statistical results.)
Combined effect of reproductive barriers
The absolute contribution of the different barriers described above is depicted in Fig. 1b. It is clear that prezygotic barriers are very strong for both mixed-species pairing types, but especially those involving a female G. campestris. Postzygotic barriers affected reproductive isolation only very weakly.
Reproductive barriers in Gryllidae
Between species pairs, there were an average of 2.88 (se = 0.44. median = 2) prezygotic barriers and 2.29 (0.46, 2) postzygotic barriers identified. Not all barriers between two species have been assessed for both types of mixed-species pairing types (Fig. 7).
An overview of the reproductive barriers documented between species belonging to the true crickets (Gryllidae). For each species pair, the two different mixed-species pairing types were compared to pure species pairing. Only the female species of these pairs is presented to keep the figure concise, and a line to aid comparison separates the different pairs. In case of uncertainty, e.g., due to an inconclusive study or multiple studies with conflicting result, the thickness of the bar is reduced in size. Most traits studied in the category ‘other’ are presumed to have an effect (e.g., differences in cuticular hydrocarbons were found between the species and these were used for mate choice) but needs experimental verification. See supporting information H for a detailed description of these barriers. *Includes survival of nymphs, **If intermediate between parents it is assumed to contribute to a barrier but as ‘less certain’. References: 1 (Gregory and Howard 1993), 2 (Howard 1986), 3 Howard et al. (1998), 4 Howard and Gregory (1993), 5 Howard et al. (1993), 6 Birge et al. (2010), 7 Ross et al. (2008), 8 Veech et al. (1996), 9 Howard and Waring (1991), 10 Gregory and Howard (1994), 11 Traylor et al. (2008), 12 Marshall (2004), 13 Marshall (2007), 14 Mendelson and Shaw (2005), 15 Shaw (2000), 16 Mendelson and Shaw (2002), 17 Mullen et al. (2007), 18 Mendelson and Shaw (2006), 19 Honda-Sumi (2005), 20 Ohmachi and Masaki (1964), 21 Hill et al. (1972), 22 Hennig and Weber (1997), 23 Pollack (1982), 24 Lim (1970), 25 Hoy et al. (1977), 26 Hoy and Paul (1973) 27 Harrison and Bogdanowicz (1997), 28 Larson et al. (2011), 29 Harrison (1983), 30 Harrison (1985), 31 Harrison and Arnold (1982), 32 Doherty and Storz (1992), 33 Ross and Harrison (2002), 34 Maroja et al. (2009b) 35 Maroja et al. (2009a), 36 Izzo and Gray (2011), 37 Gray and Cade (2000), 38 Izzo and Gray (2004), 39 Gray (2005), 40 Fitzpatrick and Gray (2001), 41 Smith and Cade (1987), 42 Cade and Tyshenko (1990), 43 Jang and Gerhardt (2006a), 44 Jang and Gerhardt (2006b), 45 Jang and Gerhardt (2007), 46 Jang et al. (2009), 47 Leonard and Hedrick (2009), 48 Cousin (1933), 49 von Hörmann-Heck (1955), 50 Veen et al. (2011), 51 Tyler et al. (in press)
Three out of the 16 species pairs showed asymmetric prezygotic barriers. This was 4 out of 13 for the postzygotic barriers (the G. bimaculatus and G. campestris pair was excluded, as postzygotic barrier differences cannot be assessed due to complete asymmetric prezygotic isolation).
Discussion
We found multiple pre- and postzygotic reproductive barriers between G. bimaculatus and G. campestris and show that this is widespread among Gryllidae. The prevalence of prezygotic barriers suggest an important role for sexual selection in the maintenance of species, but its role in speciation is much more controversial (Ritchie 2007). We discuss the potential effects of multiple barriers and asymmetric hybridization, as recorded in crickets, on the evolution of reproductive barriers.
The first barrier is a weak prezygotic barrier during the first (long-range) mate attraction phase. This data should be treated with care as G. campestris females behaved erratically on the sphere. This may be because, unlike G. bimaculatus, this species is very active during the day and uses a burrow to escape from predators (Rodríguez-Muñoz et al. 2010; Rodríguez-Muñoz et al. 2011). If indeed present, the lack of species discrimination in G. campestris females is surprising given the females’ absolute preference function (but see Thorson et al. 1982 for similar results). Female G. bimaculatus only preferred conspecific song in the two-choice test, which may affect mate choice in nature if species densities influence mate choice patterns. When confronted with only heterospecifics, G. bimaculatus females are likely to hybridise, but this will happen less frequently if conspecifics are around. Experimental work has shown effects of prior experience on con- and heterospecific mate choice (Izzo and Gray 2011). Under natural conditions, however, these effects are unclear.
Our results do not strongly support the notion that calling and courtship song differ in their effects on species isolation (Gray 2005) as we find that both song types influence reproductive isolation but only weakly. Instead, short-range mate choice appears to be a very important prezygotic barrier in this species pair. This appears to be common among the Gryllidae and leads us to conclude that there is only weak support at best for the idea that long-distance signals are more important in species recognition. Furthermore, a phylogenetic analysis of acoustic communication failed to find a univocal pattern of song divergence explaining speciation in the Gryllus genus (Desutter-Grandcolas and Robillard 2003), indicating a more complex scenario of character divergence underlying speciation.
More generally, the distinction between ‘species recognition’ signals and those used for ‘classic’ (intraspecific) sexual selection, as suggested for crickets, has been challenged (Sullivan 2009; Mendelson and Shaw 2012). In various organisms, such as Tungára frogs, sexual signals appear to be part of a continuum instead of resulting from distinct selection processes (Ryan and Rand 1993; Phelps et al. 2006). The mating pattern emerging from sexual selection (this includes species recognition) is driven by differential fitness return of the mating partner(s), which can be due to ‘intraspecific’ qualities such as parental effort, or genetic incompatibilities between individuals belonging to different populations. These selection forces act simultaneously and may interact. Signals have been found to combine a function in obtaining intraspecific mate choice benefits and enhance species-assortative mating in insects (Higgie and Blows 2007; Svensson et al. 2007), fish (e.g., Barlow and Siri 1997; Hankison and Morris 2002; van der Sluijs et al. 2008) and birds (e.g., Pryke and Andersson 2008). The continuum of signals hypothesis can explain a wide variety of mating patterns and signal properties (such as static and dynamic: Gerhardt 1991) and may provide a useful framework to study the evolution of sexual signals during speciation.
The first important postzygotic barrier is reduced hatching success of mixed-species pairs, which could be caused by problems during egg and/or embryo development. In contrast to many studies finding a reduced viability of hybrids across many taxa, hybrids in our study lived longer than the two pure species. This stresses the importance of quantifying multiple barriers to estimate gene flow between two species. In our case gene flow will probably not be enhanced much as hybrid longevity is a late acting barrier.
Sexual selection as a postzygotic barrier
Sexual selection is a contentious issue in speciation (reviewed in Ritchie 2007), but its contribution as a postzygotic barrier through hybrids is little studied (Nosil et al. 2005). We find several lines of evidence that suggest this may be important. We showed that male hybrids have a reduced rate of initiating courtship song. Furthermore, G. bimaculatus females mounted and mated with hybrids less frequently than conspecific males, but more frequently than G. campestris. Both processes are predicted to reduce gene flow between the two pure species. Gene flow could be further reduced by mate choice expressed by female hybrids, which was always intermediate to those of the pure species.
In contrast to our prediction, hybrid females showed a clear calling-song preference for G. bimaculatus (contra Hoy and Paul 1973), which was even stronger than female G. bimaculatus have for conspecific song. One possible explanation, that hybrids have an open-ended preference function, i.e. there is strong directional preference (e.g., for faster pulse rates) is unlikely as both parental species appear to have a unimodal (‘absolute’) preference (Popov and Shuvalov 1977; Thorson et al. 1982). The reduced attractiveness of male hybrid calling song is predicted to reduce gene flow, provided that female choice is not constrained. Theoretical models (Servedio 2004) together with studies of e.g., fish (Vamosi and Schluter 1999), insects (Naisbit et al. 2001, Bridle et al. 2006) and birds (Svedin et al. 2008) and our results indicate that sexual selection on hybrids may be widespread and should not be ignored in studies of speciation.
Combination of reproductive barriers and speciation pathways
One of the interests of studying reproductive barriers in detail, and in multiple closely related species groups, is to identify similarities in reproductive barriers among species pairs. This may in turn reveal patterns matching those predicted by different speciation pathways. In the Gryllidae, patterns of barriers match three pathways: speciation through sexual selection, reinforcement, and evolution of conspecific sperm precedence.
Speciation through sexual selection requires the presence of strong prezygotic barriers and no postzygotic barriers to rule out reinforcement. The reproductive barriers between G. rubens (Scudder) and G. texensis (Cade and Otte) are concentrated during the prezygotic phase, both during long and short-range mate choice (Gray and Cade 2000; Izzo and Gray 2004; Gray 2005). Contradictory results have been found with respect to postzygotic barriers, indicating no barrier, selection against hybrids and selection favouring hybrids (Smith and Cade 1987; Cade and Tyshenko 1990). The importance of sexual selection is enhanced by the absence of character displacement in sympatry, a typical pattern for reinforcement. Similarly, Laupala crickets show distinct differences in song, but are otherwise very similar in morphology and ecology (Mendelson and Shaw 2005), suggesting a crucial role for sexual selection in speciation.
The importance of sexual selection in maintaining species integrity is supported by the frequency of prezygotic barriers found in nature. Its relative importance during speciation is much harder to determine (Ritchie 2007).
Reinforcement is an example where mate choice is important, but interacts with other barriers, such as reduced hybrid viability. Patterns consistent with such an adaptive feedback between pre- and postzygotic barriers has, as far as we are aware, only been found in G. fultoni (Alexander) and G. vernalis (Blatchley) (Jang and Gerhardt 2006b, 2007; Jang et al. 2009). The prerequisites for reinforcement are present for G. bimaculatus, (and potentially also in G. campestris if courting heterospecifics is costly) and T. emma (Ohmachi and Matsuura) and T. taiwanemma (Ohmachi and Matsuura), but conclusive evidence for reinforcement is lacking.
Lastly, conspecific sperm precedence has been found to limit gene flow between Allonemobius fasciatus (De Geer) and A. socius (Scudder)(Gregory and Howard 1994; Howard et al. 1998) along with several postzygotic barriers (Birge et al. 2010). Despite intensive work, no other prezygotic barriers of significance have been found between these two species (Veech et al. 1996; Birge et al. 2010). Conspecific sperm precedence might evolve quickly (Howard and Gregory 1993) and may play an important role as an emerging reproductive barrier during speciation (Howard 1999). Conspecific sperm precedence has been found in both (Allonemobius) species pairs and G. bimaculatus (Tyler et al. in press). In the latter species, multiple prezygotic barriers have been described, indicating that it is hard to disentangle the importance of one mechanism during speciation, nor is there conclusive evidence that it evolved as an adaptive response to increase reproductive isolation.
Using an overview of the presence/absence of reproductive barriers to infer speciation pathways has several caveats. Differences in research interests may bias the barriers studied by different scientists and hence influence which type of barriers have been described. Although the species pairs discussed above are among the best studied, no assessment of reproductive barriers can be completely comprehensive, as a multitude of mutually compatible, ecological, behavioural, and genetic factors have been found to act as reproductive barriers and hence to influence isolation (Coyne and Orr 2004). A case in point is provided by the G. pennsylvanicus (Burmeister) and G. firmus (Scudder) species pair, which are genetically distinct, exhibit sharp clines of morphological and molecular traits across patch boundaries, and only few hybrids are found in the wild (Ross and Harrison 2002). Laboratory studies only found weak prezygotic and weak asymmetric postzygotic barriers (Harrison 1983; Maroja et al. 2009b). Obviously important barrier(s) have not yet been described, and temporal differences in reproduction may play a role (Harrison 1985). This stresses the importance of measuring barriers in the wild as selection may differ from the laboratory (Coyne and Orr 1989). Furthermore, inferring the order and importance of reproductive barriers is very difficult (Schluter 2001; Coyne and Orr 2004) and interactions will affect the selection pressures (e.g., increased prezygotic isolation may decrease selection on postzygotic barriers). We assume that the absence of a barrier at present means it has never been important in speciation and, by looking at qualitative patterns only, that the selection coefficients are similar. These assumptions are hard to test. Figure 7 indicates however that many barriers in the Gryllidae remain untested. Filling these gaps is required before more general conclusions about speciation can be drawn.
Multiple (interacting) barriers
Multiple reproductive barriers have been found to contribute to reproductive isolation in well studied system such as the Ficedula flycatchers, Pseudacris frogs, fruit flies and damselflies, and our study provides one more example. This empirical pattern is in line with theoretical models showing that multiple sexual signals such as song, pheromones and courtship rituals, facilitate speciation (Proulx and Servedio 2009; Doebeli and Ispolatov 2010). The contribution of a potential set of barriers to reproductive isolation varies greatly among species, ranging from a few barriers of large effect to multiple with weak effect, and the barriers may furthermore differ between sexes (Svensson et al. 2007). The prevalence of multiple barriers and their complexity stresses the need to understand the interactions between reproductive barriers. Reinforcement is an example of an interaction between pre- and postzygotic barriers. Such interactions may also take place within the same behavioural sequence, e.g., the complete barrier due to no mounting by G. campestris females is predicted to result in strongly reduced (or no) selection on reducing sperm transfer (Fig. 1b). Assessing multiple barriers and the traits involved will be a first step. In order to understand the evolutionary dynamics of reproductive barriers, the genetic underpinning of these traits should be uncovered (Marie Curie SPECIATION Network 2012).
Asymmetric reproductive barriers
In a substantial percentage of cricket species pairs the reproductive barriers were found to be asymmetric (19 and 31 % of the pre- and postzygotic barriers, respectively). This is lower than the 63 % inferred from unidirectional introgression of mitochondrial DNA across a diverse group of animals (Wirtz 1999), but supports the idea that asymmetric barriers are widespread (e.g. Sánchez-Guillén et al. 2012; Coyne and Orr 2004). There are several mutually non-exclusive hypotheses to explain premating asymmetries. Kaneshiro (1976) proposed that sexual signals were lost during subsequent founder events and females of the derived forms are less discriminating as found in Drosophila species colonizing Hawaii. Recent work on G. rubens is suggestive of the Kaneshiro effect (Gray 2011). Another explanation is based on the ‘continuum of signals’ idea discussed above. If species come into secondary contact after a period of geographic isolation, sexual signals used for intraspecific mate choice may experience selection to enhance assortative mating with individuals from their own population to avoid costly hybridization (Pfenning 1998). Empirical evidence for this idea comes from spadefoot toads (Pfennig 2000), fruit flies (Hine et al. 2002; Higgie and Blows 2007, 2008) and damselflies (Svensson et al. 2007). Detailed information with respect to the selection underlying signal evolution is needed to test this hypothesis, but is hard to obtain. Interestingly, a different way to avoid this conflict is through multiple ornaments. In the pygmy swordtail, experimental manipulation of different sexual signals led to very different mate choice patterns, including a preference for heterospecific males (Hankison and Morris 2002) showing the importance (and complexity) of multiple signals in mate choice. Lastly, adaptation of the sensory system to the local environment may lead to coevolved signal-preference systems (sensory drive: Endler 1992). This may facilitate speciation (Boughman 2002), but can also constrain directions in which the sensory system can evolve causing certain signals to be preferred, resulting in asymmetric mate choice patterns.
Regardless of the underlying mechanism, asymmetric barriers are likely to result in different selection pressures acting on the species and may thus lead to differences in adaptive response (e.g., magnitude of character displacement). The magnitude and direction of selection may vary considerably among taxa and the different evolutionary consequences this may cause deserve further attention.
Conclusion
Multiple pre- and postzygotic barriers are common in Gryllids and these barriers may differ in strength and direction (some increasing gene flow), and vary within and among species pairs. Progress towards understanding the importance of different barriers and their evolution can be made by focusing on the details of interactions between barriers within species pairs, and filling in the gaps in our knowledge of reproductive barriers in clades of species. This increases our understanding of the feedback between micro- (e.g., local adaptation) and macroevolutionary processes (e.g., adaptive radiations), which will aid progress towards formulating a general theory of speciation (Weissing et al. 2011). The evidence for multiple speciation pathways underlying the divergence of Gryllidae indicates that this is likely to be a challenging task.
References
Adamo SA, Hoy RR (1994) Mating-behavior of the field cricket Gryllus bimaculatus and its dependence on social and environmental cues. Anim Behav 47:857–868
Arnold ML (1992) Natural hybridization as an evolutionary process. Annu Rev Ecol Syst 23:237–261
Arnold ML, Hodges SA (1995) Are natural hybrids fit or unfit relative to their parents? Trends Ecol Evol 10:67–71
Barlow GW, Siri P (1997) Does sexual selection account for the conspicuous head dimorphism in the Midas cichlid? Anim Behav 53:573–584
Birge LM, Hughes AL, Marshall JL, Howard DJ (2010) Mating behavior differences and the cost of mating in Allonemobius fasciatus and A. socius. J Insect Behav 23:268–289
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17:571–577
Bridle JR, Saldamando CI, Koning W, Butlin RK (2006) Assortative preferences and discrimination by females against hybrid male song in the grasshoppers Chorthippus brunneus and Chorthippus jacobsi (Orthoptera: Acridiae). J Evol Biol 19:1248–1256
Cade WH, Tyshenko MG (1990) Geographic variation in hybrid fertility in the field crickets Gryllus integer, G. rubens and Gryllus sp. Can J Zool 68:2697–2700
Cousin G (1933) Sur l’hybridation de deux espèces de Gryllidae. (Acheta campestris et bimaculata). Bull Soc Ent Fr 12:189–193
Coyne JA, Orr HA (1989) Patterns of speciation in Drosophila. Evolution 43:362–381
Coyne JA, Orr HA (1997) Patterns of speciation in Drosophila revisited. Evolution 51:295–303
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates Inc, Sunderland
Crawley MJ (2007) The R book. Wiley, Chichester
Desutter-Grandcolas L, Robillard T (2003) Phylogeny and the evolution of calling songs in Gryllus (Insecta, Orthoptera, Gryllidae). Zool Scr 32:173–183
Dobzhansky T (1937) Genetic nature of species differences. Am Nat 71:404–420
Doebeli M, Ispolatov I (2010) Complexity and diversity. Science 328:494–497
Doherty JA (1991) Song recognition and localization in the phonotaxis behavior of the field cricket, Gryllus bimaculatus (Orthoptera, Gryllidae). J Comp Physiol A 168:213–222
Doherty JA, Storz MM (1992) Calling song and selective phonotaxis in the field crickets, Gryllus firmus and G. pennsylvanicus (Orthoptera, Gryllidae). J Insect Behav 5:555–569
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153
Fitzpatrick MJ, Gray DA (2001) Divergence between the courtship songs of the field crickets Gryllus texensis and Gryllus rubens (Orthoptera, Gryllidae). Ethology 107:1075–1085
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Giraudoux P (2008) Pgirmess: data analysis in ecology. R package version 1.3.7. http://perso.orange.fr/giraudoux/SiteGiraudoux.html
Gorochov AV, Llorente V (2001) Estudio taxonómico preliminary de los grylloidea de España (Insecta, Orthoptera). Graellsia 57:95–139
Gray DA (2005) Does courtship behavior contribute to species-level reproductive isolation in field crickets? Behav Ecol 16:201–206
Gray DA (2011) Speciation, divergence, and the origin of Gryllus rubens: behavior, morphology, and molecules. Insects 2:195–209
Gray DA, Cade WH (2000) Sexual selection and speciation in field crickets. Proc Natl Acad Sci USA 97:14449–14454
Gregory PG, Howard DJ (1993) Laboratory hybridization studies of Allonemobius fasciatus and A. socius (Orthoptera, Gryllidae). Ann Entomol Soc Am 86:694–701
Gregory PG, Howard DJ (1994) A postinsemination barrier to fertilization isolates 2 closely related ground crickets. Evolution 48:705–710
Hankison SJ, Morris MR (2002) Sexual selection and species recognition in the pygmy swordtail, Xiphophorus pygmaeus: conflicting preferences. Behav Ecol Sociobiol 51:140–145
Harrison RG (1983) Barriers to gene exchange between closely related cricket species. 1. Laboratory hybridization studies. Evolution 37:245–251
Harrison RG (1985) Barriers to gene exchange between closely related cricket species. 2. Life-cycle variation and temporal isolation. Evolution 39:244–259
Harrison RG, Arnold J (1982) A narrow hybrid zone between closely related cricket species. Evolution 36:535–552
Harrison RG, Bogdanowicz SM (1997) Patterns of variation and linkage disequilibrium in a field cricket hybrid zone. Evolution 51:493–505
Hedwig B, Poulet JEA (2005) Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J Exp Biol 208:915–927
Hennig RM, Weber T (1997) Filtering of temporal parameters of the calling song by cricket females of two closely related species: a behavioral analysis. J Comp Physiol A 180:621–630
Higgie M, Blows MW (2007) Are traits that experience reinforcement also under sexual selection? Am Nat 170:409–420
Higgie M, Blows MW (2008) The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62:1192–1203
Hill KG, Loftus-Hills JJ, Gartside DF (1972) Premating isolation between the Australian field crickets Teleogryllus commodus and T. oceanicus (Orthoptera: Gryllidae). Aust J Zool 20:153–163
Hine E, Lachish S, Higgie M, Blows MW (2002) Positive genetic correlation between female preference and offspring fitness. Proc R Soc Lond B Biol Sci 269:2215–2219
Honda-Sumi E (2005) Difference in calling song of three field crickets of the genus Teleogryllus: the role in premating isolation. Anim Behav 69:881–889
Howard DJ (1986) A zone of overlap and hybridization between two ground cricket species. Evolution 40:34–43
Howard DJ (1999) Conspecific sperm and pollen precedence and speciation. Annu Rev Ecol Syst 30:109–132
Howard DJ, Gregory PG (1993) Post-insemination signaling systems and reinforcement. Philos Trans R Soc Lond Ser B Biol Sci 340:231–236
Howard DJ, Waring GL (1991) Topographic diversity, zone width, and the strength of reproductive isolation in a zone of overlap and hybridization. Evolution 45:1120–1135
Howard DJ, Waring GL, Tibbets CA, Gregory PG (1993) Survival of hybrids in a mosaic hybrid zone. Evolution 47:789–800
Howard DJ, Gregory PG, Chu JM, Cain ML (1998) Conspecific sperm precedence is an effective barrier to hybridization between closely related species. Evolution 52:511–516
Hoy RR, Paul RC (1973) Genetic-control of song specificity in crickets. Science 180:82–83
Hoy RR, Hahn J, Paul RC (1977) Hybrid cricket auditory behavior—evidence for genetic coupling in animal communication. Science 195:82–84
Huang Y, Ortí G, Sutherlin M, Duhachek A, Zera A (2000) Phylogenetic relationships of the North American field crickets inferred from mitochondrial DNA data. Molec Phylog Evol 17:48–57
Izzo AS, Gray DA (2004) Cricket song in sympatry: species specificity of song without reproductive character displacement in Gryllus rubens. Ann Entomol Soc Am 97:831–837
Izzo AS, Gray DA (2011) Heterospecific courtship and sequential mate choice in sister species of field crickets. Anim Behav 81:259–264
Jang Y, Gerhardt HC (2006a) Divergence in female calling song discrimination between sympatric and allopatric populations of the southern wood cricket Gryllus fultoni (Orthoptera: Gryllidae). Behav Ecol Sociobiol 60:150–158
Jang Y, Gerhardt HC (2006b) Divergence in the calling songs between sympatric and allopatric populations of the southern wood cricket Gryllus fultoni (Orthoptera: Gryllidae). J Evol Biol 19:459–472
Jang Y, Gerhardt HC (2007) Temperature effects on the temporal properties of calling songs in the crickets Gryllus fultoni and G. vernalis: Implications for reproductive isolation in sympatric populations. J Insect Behav 20:33–52
Jang Y, Won YJ, Choe JC (2009) Convergent and divergent patterns of morphological differentiation provide more evidence for reproductive character displacement in a wood cricket Gryllus fultoni (Orthoptera: Gryllidae). BMC Evol Biol 9:11
Kaneshiro KY (1976) Ethological isolation and phylogeny in the Planitibia subgroup of Hawaiian Drosophila. Evolution 30:740–745
Larson EL, Hume GL, Andrés JA, Harrison RG (2012) Post-mating prezygotic barriers to gene exchange between hybridizing field crickets. J Evol Biol 25:174–186
Lemmon EM (2009) Diversification of conspecific signals in sympatry: geographic overlap drives multidimensional reproductive character displacement in frogs. Evolution 63:1155–1170
Lemmon EM, Lemmon AR (2010) Reinforcement in chorus frogs: lifetime fitness estimates including intrinsic natural selection and sexual selection against hybrids. Evolution 64:1748–1761
Leonard AS, Hedrick AV (2009) Single versus multiple cues in mate discrimination by males and females. Anim Behav 77:151–159
Lim H-C (1970) Further cytological studies of Antipodean Teleogryllus species and their hybrids (Orthoptera: Gryllidae). Can J Zool 48:523–527
Marie Curie SPECIATION Network (2012) What do we need to know about speciation? Trends Ecol Evol 27:27–39
Maroja LS, Andrés JA, Harrison RG (2009a) Genealogical discordance and patterns of introgression and selection across a cricket hybrid zone. Evolution 63:2999–3015
Maroja LS, Andrés JA, Walters JR, Harrison RG (2009b) Multiple barriers to gene exchange in a field cricket hybrid zone. Biol J Linnean Soc 97:390–402
Marshall JL (2004) The Allonemobius-Wolbachia host-endosymbiont system: evidence for rapid speciation and against reproductive isolation driven by cytoplasmic incompatibility. Evolution 58:2409–2425
Marshall JL (2007) Rapid evolution of spermathecal duct length in the Allonemobius socius complex of crickets: species, population and Wolbachia effects. PLoS ONE 2:1–7
Mendelson TC, Shaw KL (2002) Genetic and behavioral components of the cryptic species boundary between Laupala cerasina and L. kohalensis (Orthoptera : Gryllidae). Genetica 116:301–310
Mendelson TC, Shaw KL (2005) Sexual behaviour: rapid speciation in an arthropod. Nature 433:375–376
Mendelson TC, Shaw KL (2012) The (mis)concept of species recognition. Trends Ecol Evol 27:241–427
Mullen SP, Mendelson TC, Schal C, Shaw KL (2007) Rapid evolution of cuticular hydrocarbons in a species radiation of acoustically diverse Hawaiian crickets (Gryllidae : Trigonidiinae : Laupala). Evolution 61:223–231
Naisbit RE, Jiggins CD, Mallet J (2001) Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc R Soc B Biol Sci 268:1849–1854
Nosil P, Vines TH, Funk DJ (2005) Perspective: reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59:705–719
Ohmachi F, Masaki S (1964) Interspecific crossing and development of hybrids between the Japanese species of Teleogryllus (Orthoptera: Gryllidae). Evolution 18:405–416
Orr HA (1995) The population genetics of speciation—the evolution of hybrid incompatibilities. Genetics 139:1805–1813
Pardo JE, Gómez R, del Cerro A (1993) Orthopteroidea de los sistemas montañosos de Castilla-La Mancha (España). II Ensifera. Zoologica Baetica 4:113–148
Pfennig KS (2000) Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behav Ecol 11:220–227
Pfenning KS (1998) The evolution of mate choice and the potential for conflict beween species recognition and mate-quality recognition. Proc R Soc B Biol Sci 265:1743–1748
Phelps SM, Rand AS, Ryan MJ (2006) A cognitive framework for mate choice and species recognition. Am Nat 167:28–42
Pollack GS (1982) Sexual differences in cricket calling song recognition. J Comp Physiol A 146:217–221
Popov AV, Shuvalov VF (1977) Phonotactic behavior of crickets. J Comp Physiol A 119:111–126
Proulx SR, Servedio MR (2009) Dissecting selection on female mating preferences during secondary contact. Evolution 63:2031–2046
Pryke SR, Andersson S (2008) Female preferences for long tails constrained by species recognition in short-tailed red bishops. Behav Ecol 19:1116–1121
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramsey J, Bradshaw HD, Schemske DW (2003) Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57:1520–1534
Ritchie MG (2007) Sexual selection and speciation. Annu Rev Ecol Evol Syst 38:79–102
Rodríguez-Muñoz R, Bretman A, Slate J, Walling CA, Tregenza T (2010) Natural and sexual selection in a wild insect population. Science 328:1269–1272
Rodríguez-Muñoz R, Bretman A, Tregenza T (2011) Guarding males protect females from predation in a wild insect. Curr Biol 21:1716–1719
Ross CL, Harrison RG (2002) A fine-scale spatial analysis of the mosaic hybrid zone between Gryllus firmus and Gryllus pennsylvanicus. Evolution 56:2296–2312
Ross CL, Benedix JH, Garcia C, Lambeth K, Perry R, Selwyn V, Howard DJ (2008) Scale-independent criteria and scale-dependent agents determining the structure of a ground cricket mosaic hybrid zone (Allonemobius socius—Allonemobius fasciatus). Biol J Linnean Soc 94:777–796
Ryan MJ, Rand AS (1993) Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47:647–657
Sætre G-P, Sæther SA (2010) Ecology and genetics of speciation in Ficedula flycatchers. Mol Ecol 19:1091–1106
Sánchez-Guillén RA, Wullenreuther M, Rivera AC (2012) Strong asymmetry in the relative strengths of prezygotic and postzygotic barriers between two damselfly sister species. Evolution 66:690–707
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Servedio MR (2004) The evolution of premating isolation: local adaptation and natural and sexual selection against hybrids. Evolution 58:913–924
Servedio MR, Noor MAF (2003) The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol Syst 34:339–364
Shaw KL (2000) Interspecific genetics of mate recognition: inheritance of female acoustic preference in Hawaiian crickets. Evolution 54:1303–1312
Simmons LW (1986) Female choice in the field cricket, Gryllus bimaculatus (De Geer). Anim Behav 34:1463–1470
Simmons LW (1988a) The contribution of multiple mating and spermatophore consumption to the lifetime reproductive success of female field crickets (Gryllus bimaculatus). Ecol Entomol 13:57–69
Simmons LW (1988b) The calling song of the field cricket, Gryllus bimaculatus (De Geer): constraints on transmission and its role in intermale competition and female choice. Anim Behav 36:380–394
Smith CJ, Cade WH (1987) Relative fertility in hybridization experiments using 3 song types of the field crickets Gryllus integer and Gryllus rubens. Can J Zool 65:2390–2394
Sullivan BK (2009) Mate recognition, species boundaries and the fallacy of “species recognition”. Open J Zool 2:86–90
Svedin N, Wiley C, Veen T, Gustafsson L, Qvarnström A (2008) Natural and sexual selection against hybrid flycatchers. Proc R Soc B Biol Sci 275:735–744
Svensson EI, Karlsson K, Friberg M, Eroukhmanoff F (2007) Gender differences in species recognition and the evolution of asymmetric sexual selection. Curr Biol 17:1–5
Thorson J, Weber T, Huber F (1982) Auditory behavior of the cricket. II. Simplicity of calling-song recognition in Gryllus and anomalous phonotaxis at abnormal carrier frequencies. J Comp Physiol A 146:361–378
Traylor T, Birand AC, Marshall JL, Howard DJ (2008) A zone of overlap and hybridization between Allonemobius socius and a new Allonemobius sp. Ann Entomol Soc Am 101:30–39
Tyler F, Harrison X, Bretman A, Veen T, Rodríguez-Muñoz R, Tregenza T (in press) Multiple post-mating barriers to hybridisation in field crickets. Mol Ecol
Vamosi SM, Schluter D (1999) Sexual selection against hybrids between sympatric stickleback species: evidence from a field experiment. Evolution 53:874–879
van der Sluijs I, van Dooren TJM, Hofker KD, van Alphen JJM, Stelkens RB, Seehausen O (2008) Female mating preference functions predict sexual selection against hybrids between sibling species of cichlid fish. Philos Trans R Soc Lond B Biol Sci 363:2871–2877
Veech JA, Benedix JH, Howard DJ (1996) Lack of calling song displacement between two closely related ground crickets. Evolution 50:1982–1989
Veen T, Borge T, Griffith SC, Sætre G-P, Bureš S, Gustafsson L, Sheldon BC (2001) Hybridization and adaptive mate choice in flycatchers. Nature 411:45–50
Veen T, Faulks J, Rodríguez-Muñoz R, Tregenza T (2011) Premating reproductive barriers between hybridising cricket species differing in their degree of polyandry. PLoS ONE 6:1–7
Veen T, Faulks J, Tyler F, Lloyd J, Tregenza T (2012) Data from: diverse reproductive barriers in hybridising crickets suggests extensive variation in the evolution and maintenance of isolation. Data deposited in the Dryad Repository. http://dx.doi.org/10.5061/dryad.sh53j
von Hörmann-Heck VS (1955) Untersuchungen über den Erbgang einiger verhaltensweisen bei Grillenbastarden (Gryllus campestris und Gryllus bimaculatus De Geer). Zeitschrift für Tierpsychologie 14:10–183
Wagner WE (1998) Measuring female mating preferences. Anim Behav 55:1029–1042
Weissing FJ, Edelaar P, van Doorn GS (2011) Adaptive speciation theory: a conceptual review. Behav Ecol Sociobiol 65:461–480
Wiley C, Qvarnström A, Andersson G, Borge T, Sætre G-P (2009) Postzygotic isolation over multiple generations of hybrid descendents in a natural hybrid zone: how well do single-generation estimates reflect reproductive isolation? Evolution 63:1731–1739
Wirtz P (1999) Mother species—father species: unidirectional hybridization in animals with female choice. Anim Behav 58:1–12
Acknowledgments
We would like to thank Gina Conte, Seth Rudman and Rolando Rodríguez-Muñoz for valuable advice and Rolando for cricket collecting. Four anonymous Peerage of Science reviewers gave very constructive advice for which we are very thankful. T. V. was supported by a Rubicon grant from the Netherlands Organization for Scientific Research and the NSERC-CREATE Training Program in Biodiversity Research from Canada. F.T. was supported by the European Social Fund. T. T. was supported by grants from the Natural Environment Research Council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Veen, T., Faulks, J., Tyler, F. et al. Diverse reproductive barriers in hybridising crickets suggests extensive variation in the evolution and maintenance of isolation. Evol Ecol 27, 993–1015 (2013). https://doi.org/10.1007/s10682-012-9610-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9610-2