Abstract
Although evidence that reptiles exhibit indeterminate growth remains equivocal and based on inadequate data, the assumption that they do is still widely accepted as a general trait of reptiles. We examined patterns of variation in adult growth using long-term mark-recapture data on 13 populations of 9 species representing 3 families of freshwater turtles located in South Carolina, Michigan, and Arizona in the USA and in Ontario, Canada. Across 13 study populations, growth rates of all adults and only those that grew averaged 1.5 and 1.9 mm/yr respectively. Sources of variation in growth rates included species, population, sex, age, and latitude. Most adults of both sexes with recapture intervals greater than 10 years grew, but across all populations an average of 19 % of individuals did not grow (some with recapture intervals up to 30 years). For known-age adults of three species, the highest growth rates occurred during the 10 years following sexual maturity, and the proportions of non-growing individuals increased with age. Growth rates of adults were on average 92 % lower than those of juveniles. Based on linear relationships of clutch size and body size of females at average juvenile and adult growth rates it would take 0.7 (0.2–1.2) years and 8.6 (min–max = 2.3–18.5) years, respectively, to grow enough to increase clutch size by one egg. The majority of within population variation in adult body size in 3 species appeared to be a combination of differences in ages at maturity and juvenile and early adult growth, rather than indeterminate growth. The results from our study populations indicate that increases in body size (and associated reproductive output) that results from indeterminate growth are not substantial enough to represent a major factor in the evolution of life histories in general or the evolution of longevity and aging specifically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among vertebrates, body growth is most rapid in juveniles and then either stops (determinate) or continues at a reduced rate (indeterminate) after individuals reach maturity (Lincoln et al. 1982; Karkach 2006). Although there is widespread acknowledgment of its existence, historical perceptions of the dynamics of indeterminate growth are varied (Table 1; Oliver 1955; Zug et al. 2001). It is surprising that there are so few detailed empirical studies of indeterminate growth given that survivorship and reproductive traits are positively related to body size in a variety of diverse taxa (Congdon and Gibbons 1985; Kingsolver and Diamond 2011). Perhaps one problem is that empirically sufficient data on adult growth (particularly for long-lived organisms) require long-term studies that are logistically difficult to maintain (Tinkle 1979). Although the paucity of long-term studies has resulted in incomplete documentation of potential sources of variation in indeterminate growth, there is no compelling reason why adult growth rates should not vary (within and among individuals, populations, and species) as do other biological traits.
Although Oliver (1955) and Zug et al. (2001) were published almost a half century apart, both authors lament that data are lacking to categorize whether growth is determinate or indeterminate in most species of amphibians and reptiles. Selected quotes from literature spanning 68 years (1944–2011; Table 1) provide a brief history of the varied perceptions of indeterminate growth that are comparable to the parable of the blind men and the elephant. Indeterminate growth has been (and still is) generally perceived as a widespread characteristic of reptiles (quotes 1, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14; Table 1), but data are lacking for categorization of adult growth (quotes 2, 15; Table 1). For some species, adult growth may be minimal or lacking altogether (quotes 1, 8, 11, 17; Table 1), and the last quote in Table 1 (# 17) actually suggests that future research is required to document whether determinate growth is the major pattern in extinct and extant Sauropsida (Woodward et al. 2011).
Life history theories are primarily based on age-specific schedules of fecundity and survival, but adult body size (and therefore indeterminate growth), can also be important in determining life history characteristics (Kirkpatrick 1984). Selection for indeterminate growth can occur when larger body size that results from adult growth is associated with increased survival or increased reproductive output (clutch size, clutch frequency, or egg size; Kingsolver and Pfennig 2004). In life history models for organisms with indeterminate growth, it is usually assumed that allocation to growth is constant with age of adults (Heino and Kaitala 1999; Lika and Kooijman 2003) and that the rate or duration of adult growth (discounted by mortality rates) is sufficient to be a mechanism that allows older individuals to have higher reproductive output (Charnov and Berrigan 1991; Charnov et al. 2001; Kozlowski 1996; Jokela 1997; Heino and Kaitala 1999). If body size of individuals and reproductive output increase as a result of indeterminate growth then proportionally more births can occur later in life and that would be an evolutionary mechanism for extending longevity and delaying the onset or reducing the severity of senescence (Medawar 1952; Williams 1957, 1966; Hamilton 1966; Kirkwood and Rose 1991; Baudisch 2005; Sparkman et al. 2007).
However, not all of the variation in body size and associated reproductive traits is necessarily the result of indeterminate growth (Carr and Goodman 1970; Halliday and Verrell 1988; Congdon and Gibbons 1990) but can also be due to differences in juvenile growth rates and the length of time individuals delay maturity (Carr and Goodman 1970; Congdon and van Loben Sels 1993). Therefore, quantification of the occurrence and sources of variation in indeterminate growth is required to understand the importance of adult growth in shaping the adaptive landscapes that determine the evolution of life histories in general and the evolution of longevity and aging specifically (Williams 1957, 1966).
As data from long-term studies of a few species of turtles have accumulated, the idea that indeterminate growth applies equally to adults of all species has continued to erode. Among the few species examined for example, indeterminate growth has been reported for the Ornate box turtle (Terrapene ornata, Legler 1960), Wood turtle (Glyptemys insculpta, Lovich et al. (1990, 1998), Yellow-bellied slider (Trachemys scripta; Dunham and Gibbons, 1990), and Midland Painted turtles (Congdon et al. 2003), but lifetime growth was questioned in Mud turtles (Kinosternon subrubrum; Gibbons, 1983). In populations of Blanding’s turtles (Emydoidea blandingii, Congdon and van Loben Sels, 1991, 1993; Pappas et al. 2000) and Soft shell turtles (Trionyx muticus; Plummer 1977) some adults grew and others did not.
We combined empirically robust data sets on adult growth from four studies spanning 20–30 years to first assess the frequency of occurrence and sources of variation in indeterminate growth within and among 9 species (13 populations) of freshwater turtles. Second, we explored whether increases in reproductive output that are associated with indeterminate growth appear to be a substantial factor in the evolution and maintenance of longevity.
To achieve the research goals, we sought to answer six basic questions about the occurrence and sources of variation in patterns of adult growth in our study populations of turtles. (1) Is indeterminate growth a universal characteristic of turtles? (2) Do all adults in a population continue to grow? (3) Are the proportions of adult males and females that grow or do not grow different within and among populations of a species? (4) What are the growth rates of adult turtles? (5) Are differences in adult growth rates concordant with expectations based on sexual size dimorphism (i.e., does the larger sex have higher or lower adult growth rates)? (6) Are growth rates or proportions of adults that grow or not grow related to ages of individuals?
Where adequate data on hatchling size, minimum size and age at maturity, and the relationship between body size and clutch size were all available, we also answer four additional questions about potential mechanisms influencing the adaptive value of adult growth. (7) How much are adult growth rates reduced compared to growth rates of juveniles? (8) How many years would adults have to grow to account for the range of body sizes of adults in each population? (9) What proportion of the total range of body size within a population is represented in the youngest individuals? (10) At average juvenile or adult growth rates, how many years would it take to grow enough to add one egg to a clutch?
Materials and methods
From north to south, growth data are from the long-term studies of: (1) R. Brooks (1988–2006) on the Algonquin Provincial Park, Ontario, Canada; (2) O. Sexton, H. Wilbur, D. Tinkle, and J. Congdon (1953–2007) on the E. S. George Reserve, Michigan; (3) W. Gibbons (1964–2000) at Sherriffs’ Marsh, Michigan, and (1967–2009) at the Savannah River Site, and (1983–2009) at Kiawah Island, South Carolina, and (4) R. van Loben Sels and J. Congdon (1990–2005) in southeastern Arizona (Table 2).
We documented adult growth in three populations of Snapping turtles (Chelydra serpentina, Linnaeus, 1758) and Painted turtles, (Chrysemys picta, Gray, 1844) and one population each of Chicken turtles (Deirochelys reticularia, Agassiz, 1857), Blanding’s turtles (Emydoidea blandingii, Holbrook, 1838), Wood turtles (Glyptemys insculpta, LeConte, 1829)), Sonoran mud turtles (Kinosternon sonoriense, Gilmore, 1922), Eastern mud turtles (Kinosternon subrubrum, Lacépède, 1788), Diamondback terrapins (Malaclemys terrapin, Schoepff, 1793), and Yellow-bellied sliders (Trachemys scripta, Thunberg in Schoepff, 1792).
Because turtles are long-lived, they are particularly good models for determining patterns of variation in adult growth over inter-capture intervals that are far longer than needed to rule out measurement errors. Turtles were captured, individually marked, measured (either plastron length or carapace length), released at their capture location, and recaptured at least once. Ages were determined for individuals of three species (Chrysemys picta, E. blandingii, and Chelydra serpentina) on the E. S. George Reserve by marking them as hatchlings or by counting clear growth rings on the shells of young juveniles. Known age individuals of both sexes were used to examine the relationships between age and adult growth rates. In addition, known age primiparous females of all three species were indentified using extensive recapture records and first captures with eggs to compare the ranges of body sizes of primiparous females and young adult males to all adults in the population.
Data and analyses
Because adults grow slowly, we set minimum inter-capture intervals at 2 years for the short-lived chicken turtle and 4 years for all other species to allow changes in adult body sizes sufficient to minimize the effects of measurement errors. Small negative changes in body size were assumed to be measurement errors and were set to zero, whereas large negative changes were assumed to be errors in the identification of individuals or data recording and were deleted from data sets.
Two analyses were used to examine aspects of adult growth. First, growth rates of individual adult males and females in each population were determined using regression analyses of body size of all recaptures with intervals equal to or greater than the minimums defined above (i.e., we measured the rate of increase in body length, not the increase in body volume or mass). Individual slope values were averaged for each population for both sexes and for individuals with slopes that were (those that had grown) and were not (those that had not grown) significantly different from zero. We used a one-tailed P value to assess the significance of the slope, as our expectation was one-sided (i.e., individuals could not shrink). Second, G-tests were used to compare the proportions of individuals of each sex assigned to growth and no growth categories.
Three approaches were used to determine the potential adaptive value of indeterminate growth. First, we compared the range of variation of young adults (those close to ages at maturity) to that of all adults in each of three populations on the ESGR. Second, we calculated how many years an average adult female would have to grow to account for the entire range of body sizes in a population (maximum body size − minimum size at maturity). And third, we compared the time necessary for females to add one egg to a clutch at juvenile versus adult growth rates. Juvenile growth rates were calculated as the difference between the average hatchling body size and minimum size at maturity/minimum age at maturity for females of each species. We also used the linear relationships between clutch size and body size for each population to calculate how long it would take to attain a change in body size necessary to add one egg at mean juvenile or adult growth rates.
Results
Question 1.—We found that indeterminate growth is a common characteristic of turtles, as some adults of both sexes in all populations grew between captures (Table 2).
Question 2.—Among all populations 19 % of all adults did not grow, even those with recapture intervals greater than 20 years. Although the percent of non-growing females was similar to the other populations in the short-lived D. reticularia, no non-growing male were detected.
Question 3.—Proportionally more males (25.5 %, min–max = 8–60 %) than females (13.0 %, min–max = 0–33 %) did not grow (Gadj = 50.9, P < 0.05). The proportions of males not growing were significantly greater than females in four populations (Chrysemys. picta at Sherriffs’ Marsh, Chelydra. serpentina at Algonquin Park, K. subrubrum at SREL, and M. terrapin at Kiawah Island; G-adj., Ps < 0.05). Among four species with different proportions of non-growing males and females, males were smaller (Chrysemys. picta and M. terrapin), larger (Chelydra. serpentina), and similar in size (K subrubrum). In no case was the proportion of non-growing females significantly greater than that of males.
Question 4.—Average growth rate of all adults was 1.5 mm/yr and of only those that grew was 1.9 mm/yr (N = 13 populations). For adult Painted and Snapping turtles, growth rates were higher at lower latitudes. However, although the sites are essentially at the same latitude in Michigan, Painted turtles grew faster at Sherriffs’ Marsh (a large marsh associated with a lake; see also Frazer et al. 1991a) than at the ESGR (a complex of small swamps, ponds, and marshes). Growth rates of males, females, or both sexes combined were <1.0 mm/yr in five species (Table 4); three were from the northern limit of the range of turtles at Algonquin Park in Canada (Chrysemys. picta, Chelydra. serpentina, and G. insculpta), one was from Michigan (E. blandingii), and one was from the coast of South Carolina (M. terrapin). Wood turtles and Blanding’s turtles have similar life history trait values of delayed sexual maturity, low annual fecundity, and extended longevity (Brooks et al. 1992; Congdon and van Loben Sels 1993; Congdon et al. 2001), traits that may have co-evolved with low adult growth rates regardless of location.
Question 5.—Growth rates of all males and females averaged 1.3 and 1.7 mm/yr, respectively, and for just those individuals that grew averaged 1.7 and 2.2 mm/yr, respectively (Table 2). Male snapping turtles are substantially larger than females in all three study populations, and males grew an average of 0.40 mm/yr faster than did females. In populations of the four species that have substantially larger adult females (C. picta, D. reticularia, T. scripta, M. terrapin), females grew 0.8 mm/yr faster than males, but at Algonquin Park (Canada) and the ESGR (Michigan) Painted turtle males grew 0.3 mm/yr faster than females.
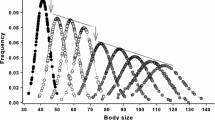
Question 6.—The highest growth rates of known age males and females of all three species (Chrysemys. picta, E. blandingii and Chelydra. serpentina) on the ESGR occurred during the 10 years following sexual maturity; however, a few individuals of both sexes did not grow during that period (Fig. 1a–f). In a sample of 466 male and 418 female Painted turtles of known age, the proportion of older adults that did not was greater than for young individuals (Gadj, P < 0.05).
Question 7.—Growth rates of adult females and males averaged 7.7 % (min–max = 3.0–12.2 %) and 8.5 % (min–max = 1.0–11.3 %), respectively, of juvenile growth rates (N = 11 populations; Table 4).
Question 8.—For adult growth rates to account for all of the body size variation in the 13 populations examined, growth would have to continue over an average of 68 years (min–max = 24–117; Table 4).
Question 9.—Among all known-age adult females of three species on the ESGR, the range of body sizes of the youngest females (those within the range of ages that individuals reach sexual maturity) encompassed 95–98 % of the entire range of body sizes in the population. The range of body sizes of young males of all three species (within 5 years of the best estimates of minimum age at sexual maturity) bracketed 83–100 % of body sizes of adult males for the entire population (Table 3).
Question 10.—Based on relationships between clutch size and body size of females, it would take individuals with the average growth rates of juveniles 0.7 (min–max = 0.2–1.2) years versus adults 8.6 (min–max = 2.3–18.5) years to increase clutch size by one egg (Table 4); the benefit would be discounted by the respective mortality rates of juveniles and adults.
Discussion
Occurrence and variation of adult growth in turtles
Our results support the general consensus that indeterminate growth is a common feature of turtles (i.e., the majority of individuals grew in all 13 populations examined; Table 1). However, because an average of almost 20 % of adults of both sexes ceased growing for periods of a decade or more the simplistic expectation that all adults continue to grow was not supported. In four populations proportionally more males than females stopped growing; however, in no case did proportionally more females than males stop growing. Growth rates of all adults of all species averaged 1.5 mm/year and the minimal differences in adult growth rates of males and females does not support the hypothesis that indeterminate growth is a mechanism that contributes substantially to sexual size dimorphism within the populations examined. The increased proportion of older individuals that did not grow coupled with a negative relationship between growth rates and ages of individuals indicates that indeterminate growth is more prevalent in young versus older adults.
The adaptive value of adult growth rates
The 92 % reduction in adult growth rates compared to those of juveniles was presumably due to increased direct allocation to reproduction by females and to reproductive activities by males. We recognize that some reduction in adult growth rates associated with age is the result of allometry associated with body size (i.e., for a given level of investment, smaller individuals grow faster in length than do larger bodied ones). However, the proportion of individuals that did not grow at all (even at intervals from 10 to 30 years) increased with age, providing strong evidence that they effectively ceased allocation to growth.
If the entire range of adult body sizes in the populations examined was the result of adult growth, rather than variation in size at maturity among individuals in a population, growth would have to continue for an average of 68 (24–117) years. For some species, such durations are longer than the maximum lifetimes expected based on adult survivorships. In a particularly insightful study of adult growth in green turtles (Chelonia mydas), Carr and Goodman (1970) found that at adult growth rates, it would take more than 80 years to account for the range of body sizes of females. Our data on Painted turtles, Blanding’s turtles, and Snapping turtles support the conclusions of Carr and Goodman (1970) and indicate that the majority of variation in adult body size was due to variation in sizes at which maturity was reached (Table 3).
Among species, juvenile females would have to postpone maturity for an average of approximately one year to attain an increase in body size sufficient to add one egg to a clutch; whereas adults would have to grow an average of 9 years to attain the same result (Table 3). At the growth rates of adults of shorter-lived female D. reticularia, it would require about 3 years and for longer-lived female E. blandingii and K. subrubrum over 15 years to increase body size enough to add one egg to a clutch. In all cases the benefits attained by adding one egg would be discounted by mortality rates over the periods of growth (e.g., a higher mortality rate over a short period for Chicken turtles; Buhlmann et al. 2009, and a low mortality rate over a long period for Painted turtles, Blanding’s turtles, and Mud turtles; Samson 2003; Congdon and van Loben Sels 1993; Frazer et al. 1991b). For adults, reproduction continues over the period of growth required to add one egg, whereas for juveniles, death during the shorter period of delaying maturity results in no lifetime reproductive success (i.e., a serious cost of error).
Issues related to the concept of indeterminate growth (Table 1)
We suggest that interest in indeterminate growth has been dampened because the historical literature on either side of the issue generally presented the concept with little or no consideration of the potential importance of variation in the trait. Even with the general acceptance that indeterminate growth is a trait of reptiles and amphibians, two book chapters on growth in reptiles (Andrews 1982) and turtles (Dunham and Gibbons 1990), and recent herpetology books (Zug et al. 2001; Pough et al. 2003; Vitt and Caldwell 2009) do not have a section heading for indeterminate growth, and only mention the trait in relation to other topics.
The acceptance of the general concept of indeterminate growth (i.e., growth continues throughout adult life) may be applicable in some shorter-lived lizards (Olsson and Shine 1996). However, the lack of long-term growth data on long-lived ectotherms has allowed unsupported perceptions of the importance of indeterminate growth to persist (Oliver 1955; Zug et al. 2001). For example, one perception, that a bigger adult turtle is older than a smaller one, is widely accepted in spite of a few empirical studies that have questioned the assumption (Carr and Goodman 1970; Plummer 1977; Gibbons 1983; Congdon and Gibbons 1990; Congdon and van Loben Sels, 1991; Pappas et al. 2000).
Since adult growth is a trait that results in an individual being larger as it becomes older, why aren’t larger adult turtles necessarily older individuals? Variation in adult body size apparently may often be due to differences in growth rates and ages at maturity of juveniles (within and among hatchling cohorts) that results in substantial variation in the body sizes of young adults. Therefore, although the assumption that larger individuals are older is often correct in rapidly growing young juveniles, the relationship weakens with age of juveniles and continues to decay as the ages of adults increase.
Understanding the evolution of life histories of long-lived organisms with indeterminate growth requires information on the sources and amounts of variability in adult and juvenile growth rates. For example, many reproductive traits of females are related to body size, so understanding how variation in body sizes of females in a population arises will change our perception of how body size determines the adaptive landscape of life history traits. If most body size variation among females results from indeterminate growth, then natural selection (through associated increases in reproductive output) can oppose senescence and increase longevity by increasing the proportion of births late versus early in life (Williams 1957, 1966). Because growth rates diminish as adults become older, life history models that assume an equal allocation rule at all ages of adults over estimate allocation to growth in adults, particularly if 19 % of adult turtles (this study) cease growth altogether. However, if the variation in body size is due to a combination of differences in juvenile growth rates, early adult growth rates, and ages at which individuals mature, selection for longevity and delaying the onset and severity of senescence will be associated with age specific traits rather than with adult growth (Congdon et al. 2001, 2003).
In an excellent review of individual growth, Karkach (2006) pointed out that current definitions of growth patterns are based on age (determinate if the organism reaches its maximum size at some age that is usually around sexual maturity and indeterminate if growth continues throughout life) may not be adequate for the questions important to life-history studies. Two definitions that fit evolutionary concepts were proposed by Karkach (2006) that could replace the traditional definitions of determinate and indeterminate growth. The first is based on survival; if asymptotic size of individuals is reached while many individuals are still alive, growth is determinate, and if few are still alive growth is indeterminate. The second is based on reproductive value; if asymptotic size of individuals is reached before most reproduction has occurred growth is determinate and after most reproduction has occurred growth is indeterminate. Depending on the questions asked, either definition more accurately describes the importance of indeterminate growth than the classic definition. Both definitions improve the way evolutionary questions can be framed about how tradeoffs in age-specific resource allocation patterns are shaped by survival, reproductive tactics, and aging, because the evolutionary currency of death (or survivorship) is births (Williams 1957).
References
Andrews RM (1982) Patterns of growth in reptiles. In: Gans C, Pough FH (eds) Biology of the reptilia, vol 13. Cornell University, Ithaca
Baudisch A (2005) Hamilton’s indicators of the force of selection. Proc Nat Acad Sci USA 102:8263–8268
Bellairs A (1970) The Life of Reptiles. Volume II. The Universe Natural History Series. Universe Books, New York
Bjorndal KA (1980) Demography of the breeding population of the green turtle Chelonia mydas, at Tortuguero, Costa Rica. Copeia 3:525–530
Brooks RJ, Shilton CM, Brown GP et al (1992) Body size, age distribution, and reproduction in a northern population of Wood Turtles (Clemmys insculpta). Can J Zool 70:462–469
Buhlmann KA, Congdon JD, Gibbons JW et al (2009) Ecology of chicken turtles (Deirochelys reticularia) in a seasonal wetland ecosystem: exploiting resource and refuge environments. Herpetologica 65:39–53
Carr A, Goodman D (1970) Ecological implications of size and growth in Chelonia. Copeia 1970:783–786
Charnov EL, Berrigan D (1991) Evolution of life history parameters in animals with indeterminate growth, particularly fish. Evol Ecol 5:63–68
Charnov EL, Turner TF, Winemiller KO (2001) Reproductive constraints and the evolution of life histories with indeterminate growth. Proc Natl Acad Sci USA 98(16):9460–9464
Congdon JD, Gibbons JW (1985) Egg components and reproductive characteristics of turtles: relationships to body size. Herpetologica 41:194–205
Congdon JD, Gibbons JW (1990) The evolution of turtle life histories. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Congdon JD, van Loben Sels RC (1991) Growth and body size in Blanding’s turtles (Emydoidea blandingii): relationships to reproduction. Can J Zool 69:239–245
Congdon JD, van Loben Sels RC (1993) Reproductive characteristics and body size: relationships with attainment of sexual maturity and age in Blanding’s turtles (Emydoidea blandingii). J Evol Biol 6:547–557
Congdon JD, Nagle RD, Kinney OM et al (2001) Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Exp Geront 36(2001):813–827
Congdon JD, Nagle RD, Kinney OM et al (2003) Testing hypotheses of aging in long-lived painted turtles (Chrysemys picta). Exp Gerontol 38:765–772
Dunham AE, Gibbons JW (1990) Growth of the slider turtle. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Ernst CH, Barbour RW (1972) Turtles of the United States. University Press of Kentucky, Kentucky
Flower SS (1944) Persistent growth in the tortoise, Testudo graeca. Proc Zool Soc London 114:451–455
Frazer NB, Gibbons JW, Greene JL (1991a) Growth, survivorship and longevity of Painted Turtles Chrysemys picta in a southwestern Michigan marsh. Am Midl Nat 125:245–258
Frazer NB, Gibbons JW, Greene JL (1991b) Life history and demography of the common mud turtle Kinosternon subrubrum in South Carolina, USA. Ecology 72:2218–2231
Gibbons JW (1983) Reproductive characteristics and ecology of the mud turtle, Kinosternon subrubrum (Lacépède). Herpetologica 39:254–271
Gibbons JW (1990) Turtle studies at SREL: a research perspective. In: Gibbons JW (ed) Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, DC
Goode J (1967) Freshwater Tortoises of Australia and New Guinea (in the Family Chelidae). Lansdowne Press, Melbourne
Halliday TR, Verrell PA (1988) Body size and age in amphibians and reptiles. J Herpetol 22:253–265
Hamilton WD (1966) The moulding of senescence by natural selection. J. Theoret Biol 12:12–45
Heatwole H (1976) Reptile Ecology. University of Queensland Press, Brisbane
Heino M, Kaitala V (1999) Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. J Evol Biol 12:423–429
Jokela J (1997) Optimal energy allocation tactics and indeterminate growth: life-history evolution of long-lived bivalves. In: Streit B, Stadler T, Lively CM (eds) Evolutionary Ecology of Freshwater Animals: Concepts and Case Studies. Birkhauser Verlag Basel, Switzerland
Karkach AS (2006) Trajectories and models of individual growth. Demog Res 15:347–400. doi:10.4054/DemRes.2006.15.12
Kingsolver JG, Diamond SE (2011) Phenotypic selection in natural populations: what limits directional selection? Am Nat 177:346–357
Kingsolver JG, Pfennig DW (2004) Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58:1608–1612
Kirkpatrick M (1984) Demographic models based on size, not age, for organisms with indeterminate growth. Ecology 65:1874–1884
Kirkwood T, Rose M (1991) Evolution of senescence: late survival sacrificed for reproduction. Philosophical Trans Biol Sci 332:15–24
Kozlowski J (1996) Optimal allocation of resources explains interspecific life-history patterns in animals with indeterminate growth. Proc Biol Sci 263:559–566
Legler JM (1960) Natural history of the ornate box turtle, Terrapene ornata ornata Agassiz. Univ Kansas Publ Mus Nat Hist 11:527–669
Lika K, Kooijman SALM (2003) Life history implications of allocation to growth versus reproduction in dynamic energy budgets. Bull Math Biol 65:809–834
Lincoln RJ, Boxshall GA, Clark PF (1982) A dictionary of ecology, evolution and systematics. Cambridge University Press, Cambridge
Lovich JE, Ernst CH, McBreen JF (1990) Growth, maturity, and sexual dimorphism in the wood turtle, Clemmys insculpta. Can J Zool 68:672–677
Lovich JE, Ernst CH, Herman DW (1998) Geographic variation in growth and sexual size dimorphism of bog turtles (Clemmys muhlenbergii). Am Midl Nat 139:69–78
Medawar P (1952) An Unsolved Problem of Biology. HK Lewis, London
Oliver JA (1955) The natural history of North American amphibians and reptiles. D. Van Nostrand Company, Inc, Princeton, NJ
Olsson M, Shine R (1996) Does reproductive success increase with age or with size in species with indeterminate growth? A case study using sand lizards (Lacerta agilis). Oecologia 105:175–178
Pappas MJ, Brecke BJ, Congdon JD (2000) The Blanding’s turtle of Weaver Dunes, Minnesota. Chelon Conserv Biol 3:557–568
Plummer MV (1977) Reproduction and growth in the turtle Trionyx muticus. Copeia 1977:440–447
Pough HF, Andrews RM, Cadle JE, Crump ML, Savitzky AH, Wells KD (2003) Herpetology, 3rd edn. Prentice Hall, Upper Saddle River, NJ
Samson J (2003) The life history strategy of a northern population of midland painted turtles, Chrysemys picta marginata. M.Sc. thesis, Department of Zoology, University of Guelph, ON, Canada
Sebens KP (1987) The ecology of indeterminate growth in animals. Ann Rev Ecol Syst 18:371–407
Sparkman AS, Arnold J, Bronikowski AM (2007) An empirical test of evolutionary theories for reproductive senescence and reproductive effort in the garter snake Thamnophis elegans. Proc R Soc Lond B 274:943–950
Stearns SC (1992) The Evolution of Life Histories. Oxford University Press, Oxford
Tinkle DW (1979) Long-term field studies. Bioscience 29:717
Vitt LJ, Caldwell JP (2009) Herpetology: an introductory biology of amphibians and reptiles. Academic Press, Burlington, MA
Williams GC (1957) Pleiotropy natural selection evolution of senescence. Evol 11:398–411
Williams GC (1966) Adaptation and Natural Selection. Princeton University Press, Princeton
Woodward HN, Horner JR, Farlow JO (2011) Osteohistological evidence for determinate growth in the American alligator. J Herpetol 45:339–342
Zug GR, Vitt LJ, Caldwell JL (2001) Herpetology: an Introductory Biology of Amphibians and Reptiles, 2nd edn. Academic Press, San Diego
Acknowledgments
Roy Nagle and Owen Kinney, Richard van Loben Sels, and Todd Quinter provided many years of field assistance and continuity to the ESGR study. Judy Greene, Peggy Burkman, and Ruth Estes provided invaluable assistance with managing the extensive data sets from SREL and the ESGR. We thank Tony Tucker, Judy Greene, and Mike Dorcas for providing data on Malaclemys terrapin. R. G. Farmer provided assistance with analyses of growth rates. National Science Foundation grants supported some of the long-term research conducted by J.W.G. (DEB-79-04758) and J.D.C. (DEB-74-070631, DEB-79-06301, BSR-84-00861 and BSR-90-19771). The last half of the E. S. George Reserve study was primarily funded by J. Congdon and N. Dickson. Manuscript preparation was aided by the Environmental Remediation Sciences Division of the Office of Biological and Environmental Research, U.S. Department of Energy through the Financial Assistant Award no. DE-FC09-96SR18546 to the University of Georgia Research Foundation. Previous drafts of the manuscript were improved by comments from N. Dickson, M. Pappas, and L Vitt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Congdon, J.D., Gibbons, J.W., Brooks, R.J. et al. Indeterminate growth in long-lived freshwater turtles as a component of individual fitness. Evol Ecol 27, 445–459 (2013). https://doi.org/10.1007/s10682-012-9595-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-012-9595-x