Abstract
In D. melanogaster, resistance to starvation and desiccation vary in opposite directions across a geographical gradient in India but there is lack of such clinal variation on other continents. However, it is not clear whether these resistance traits or other correlated traits are the target of natural selection. For resistance to starvation or desiccation in D. melanogaster, we tested the hypothesis whether body color phenotypes and energy metabolites show correlated selection response. Our results are interesting in several respects. First, based on within population analysis, assorted darker and lighter flies from a given population showed that darker flies store higher amount of trehalose and confer greater desiccation resistance as compared with lighter flies. By contrast, lighter flies store higher lipids content and confer increased starvation tolerance. Thus, there is a trade-off for energy metabolites as well as body color phenotypes for starvation and desiccation stress. Further, trait associations within populations reflect similar patterns in geographical populations. Second, we found opposite clines for trehalose and body lipids. Third, coadapated phenotypes have evolved under contrasting climatic conditions i.e. drier and colder northern localities select darker flies with higher trehalose as well as desiccation resistance while hot and humid localities favor lighter flies with higher lipids level and greater starvation tolerance. Thus, the evolution of coadapated phenotypes associated with starvation and desiccation resistance might have resulted due to specific ecological conditions i.e. humidity changes on the Indian subcontinent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantitative traits vary geographically and show rapid response to selection under laboratory or field conditions (Endler 1986; Mousseau et al. 2000). There are parallel latitudinal clines for body size in Drosophila melanogaster on different continents; and thermal changes are considered as the major selective agent (James et al. 1997; Hoffmann and Weeks 2007). Laboratory selection experiments have also resulted in larger flies of D. melanogaster at lower temperatures (Atkinson 1994). Thus, evolutionary responses for body size are similar under laboratory as well as wild conditions. By contrast, there are no consistent patterns for clinal variation in desiccation and starvation resistance along latitude on different continents (Hoffmann and Harshman 1999; Rion and Kawecki 2007). Starvation resistance in D. melanogaster has shown no cline in South American populations (Robinson et al. 2000) as well as in Australian populations (Hoffmann et al. 2001; Hoffmann and Weeks 2007) but there is a positive cline with latitude in eastern North America (Schmidt et al. 2005). For desiccation resistance also, either there are no clines or limited geographical variations in different Drosophila species from the Australian continent (Hoffmann and Harshman 1999; Hoffmann and Weeks 2007). However, there are opposite clines for desiccation and starvation resistance in D. melanogaster as well as several other Drosophila species on the India subcontinent (Parkash and Munjal 1999; Parkash and Munjal 2000). Such contrasting differences in clinal variations for starvation or desiccation resistance may be due to significant differences in the ecological conditions prevailing on the Indian subcontinent as compared with other continents. Further, selection responses differ under laboratory versus field conditions e.g. laboratory selection studies have shown parallel changes in starvation or desiccation resistance (Chippindale et al. 1996; Gibbs et al. 1997; Chippindale et al. 1998; Telonis-Scott et al. 2006). Thus, it is not clear whether between population differences in desiccation and starvation resistance are due to direct or indirect selection response.
For laboratory analysis of quantitative traits in geographical populations, it is generally assumed that natural selection shapes each trait independently. However, for wild populations, ecophysiological and morphological traits might coevolve according to their combined influence on fitness (Angilletta 2009). For example, tropical habitats on the Indian subcontinent select lighter body color phenotype as well as starvation tolerance (Parkash and Munjal 2000) but it is not clear whether these traits are under independent selection or coevolve. Further, there are evidences in favor of coadaption hypothesis (Angilletta 2009) e.g. behavioral thermoregulation and body coloration are coadapted traits in pygmy grasshopper (Tetrix subulata; Forsman 2000). Coadapted traits are also represented by associations between body melanisation and thermo-resistance traits in D. melanogaster (Parkash et al. 2010). If ecophysiological traits and body melanisation coevolve, we may expect within-population associations between body color phenotypes and stress-related traits in Drosophila melanogaster but such traits associations have not been analyzed so far.
On the Indian subcontinent, if natural selection has resulted in opposite clines for desiccation and starvation resistance, we may expect differences in the storage as well as utilization of energy metabolites (carbohydrates and/or lipids) in latitudinal populations of D. melanogaster. Most of the previous studies on clinal variations on desiccation and starvation resistance in D. melanogaster from different continents have not considered changes in the storage of energy metabolites as well as their utilization under stressful conditions (Parkash and Munjal 1999; Hallas et al. 2002; Griffiths et al. 2005; Collinge et al. 2006). Further, a single study on the geographical populations of D. melanogaster from Australia showed lack of correlation between lipid content and starvation resistance but this study did not consider actual utilization of lipids and/or carbohydrates as a function of stress duration (Hoffmann et al. 2001). However, storage and utilization of energy metabolites may differ under desiccation or starvation stress in D. melanogaster.
In a previous study, we analysed clinal variation in desiccation and starvation resistance in mass cultures of latitudinal populations of D. melanogaster from India (Parkash and Munjal 1999) but this study did not consider within population variation, correlated selection responses and changes in the utilization of energy metabolites. In the present study, we have analyzed associations between body color changes, stress-related traits and their energy metabolites in latitudinal populations of D. melanogaster. For this, we considered laboratory populations of D. melanogaster grown at 21°C under common garden conditions. We addressed the following questions (a) Do contrasting patterns of darker and lighter body color phenotypes correlate with opposite clines for desiccation and starvation resistance along a latitudinal gradient in India (b) Whether there are associations between body color phenotypes and desiccation or starvation resistance. (c) Whether resistance to desiccation and starvation in D. melanogaster involve a trade-off for energy metabolites.
We investigated changes in desiccation and starvation resistance in twenty isofemale lines from each of eight geographical populations of D. melanogaster. Stress resistance traits and energy metabolites were analyzed in assorted darker and lighter isofemale lines from a given population (i.e. within population analysis). We tested whether two energy metabolites (trehalose and lipids) covary or show a trade-off for desiccation versus starvation resistance in geographical populations. We compared utilization of energy metabolites (trehalose or lipids) as a function of different stress durations (desiccation or starvation) in assorted body color phenotypes of D. melanogaster. Finally, we examined whether body color phenotypes, energy metabolites and stress resistance represent coadapted traits at within and between population levels in D. melanogaster.
Materials and methods
Collections and cultures
Wild individuals of D. melanogaster (n = 600–650 flies from each site) were collected in October, 2009 by net-sweeping method in a single trip from eight latitudinal sites (8°05′–32°40′ N). Wild-caught females were used to initiate isofemale lines (20 lines per population). All cultures were maintained at low density (60–70 eggs per vial of 40 × 100 mm size) on cornmeal-yeast-agar medium at 21°C. All experiments were performed with G3 and G4 generations on 8 days old virgin female flies. Climatic data for thermal variables of origin of populations were obtained from Indian Institute of Tropical Meteorology (IITM; www.tropmet.res.in). However, data on relative humidity were obtained from ‘Climatological Tables’ published by the Indian Meteorological Department, Govt. of India, New Delhi.
Analysis of body melanisation
We measured percent body melanisation of wild-caught female individuals (400–450) as well as 200 virgin female flies from each isofemale line (n = 20 If lines) per population. Melanisation was estimated with visual scoring with Olympus stereo-zoom microscope SZ-61 (www.olympus.com). Body melanisation was estimated from dorsal as well as lateral views of the female abdomen giving values ranging from 0 (no melanisation) to 10 (complete melanisation) for each of the six abdominal segments (2nd–7th). Since the abdominal segments differ in size (i.e. 0.86, 0.94, 1.0, 0.88, 0.67 and 0.38 for 2nd–7th segments respectively), these relative sizes were multiplied with segment-wise melanisation scores. Data on percent melanisation were calculated as (Σ observed weighted melanisation scores of abdominal segments per fly/Σ relative size of each abdominal segment × 10 per fly) × 100 (Parkash et al. 2008).
Percent distribution of darker and lighter flies
For each population, all the wild-caught females (n = 400–450) were assorted as darker versus lighter flies on the basis of pigmentation score of all the abdominal segments i.e. darker flies (>45% melanisation) and lighter flies (<30% melanisation). Percent distribution was calculated as the number of darker or lighter flies out of total female flies of D. melanogaster collected from a given locality.
Analysis of body size (wing length)
Wing length was measured from the thorax articulation to the tip of third longitudinal vein under Olympus stereo-zoom microscope SZ-61, Japan (www.olympus.com) fitted with a micrometer. We measured wing length in ten flies from each laboratory reared isofemale line (n = 20 If × 10 flies) of eight latitudinal populations. These flies were used for measurement of different stress-related traits.
Within population analysis
For within population analysis, we assorted isofemale lines (n = 70–80 per population) into darker (>45% melanisation) and lighter (<30% melanisation) body color phenotypes from one northern and one southern population of D. melanogaster. Twenty isofemale lines each of dark or light phenotype were used for analyzing within population trait variability. We isolated isofemale lines for larger or smaller body size from northern (Udhampur) population. For each isofemale line, we checked repeatability of trait values (wing size) across six generations and lines with stable trait values were used for analysis of desiccation and starvation resistance.
Measurement of stress resistance
For starvation and desiccation resistance (a) we analyzed ten individuals from each of the 20 isofemale lines per population; (b) Sixty wild-caught individuals of each population; (c) assorted darker or lighter isofemale lines from one northern and one southern population of D. melanogaster. For each assay, we used 200 virgin flies (n = 20 isofemale lines × 10 replicates) of each population.
Starvation resistance
Ten female flies were placed in a dry plastic vial which contained foam sponge impregnated with 2 ml of water + 2 mg sodium benzoate (to prevent any bacterial growth). Such vials with muslin wraps were placed in a humidity chamber (www.metrexinstruments.com; MEC-30) which maintained 80–85% relative humidity at 21 ± 0.5°C. The mortality was scored daily at 8 am and 8 pm (i.e. 12 h interval) for the first 4 days followed by six hourly observations until all flies had died from starvation.
Desiccation resistance
To measure desiccation resistance (time to lethal desiccation in dry air), 10 virgin female individuals were isolated in a dry plastic vial, which contained 2 g of silica gel at the bottom and was covered with a disc of foam piece. Such vials with foam plugs were placed in a desiccator chamber (Secador electronic desiccator cabinet; www.tarson.com) which maintains 6–8% relative humidity. The time period to lethal desiccation time in dry air was recorded.
Measurements of energy metabolites
For all the eight geographical populations, we analyzed ten individuals from each of the 20 isofemale lines per population of D. melanogaster. We also analyzed assorted darker or lighter isofemale lines from one northern and one southern population of D. melanogaster. All assays for estimation of total extractable lipids or trehalose or protein content were done on ten replicates.
Lipid assay
The method for determining the total lipid content was adapted from Marron et al. (2003). Individual virgin flies were placed in 2 ml eppendorf tubes (www.tarson.in) and dried at 60°C for 48 h. Dried flies were weighed on Sartorius microbalance (Model-CPA26P; with precision 0.001 mg; www.sartorious.com). Further, 1.5 ml di-ethyl ether was added in each eppendorf tube and kept for 24 h under continuous shaking (200 rpm) at 37°C. Thereafter, the flies were removed from the solvent and again dried at 60°C for 24 h and finally reweighed. Lipid content was calculated per individual by subtracting the lipid free dry mass from initial dry mass per fly. Relative lipid content to dry mass as well as for wet mass were calculated as the percent of lipid mass to initial dry mass and wet mass respectively.
Trehalose assay
Trehalose content was estimated with Megazyme trehalose assay kit (K-Treh 10/10; www.megazyme.com). For trehalose estimation, 10 flies per isofemale line were homogenized with a hand electric mortar and pestle and boiled in a water bath at 95°C for 20 min. After centrifugation at 12,000 rpm for 15 min, aliquots of the supernatant were placed into two tubes (200 μl each); one was taken as blank while other was digested with trehalase at 37°C (Megazyme trehalose assay kit). In this assay, released d-glucose was phosphorylated by hexokinase and ATP to glucose-6-phosphate and ADP which was further coupled with glucose-6-phosphate dehydrogenase and resulted in the reduction of nicotinamide adenine dinucleotide (NADH). The absorbance by NADH was measured at 340 nm. Pre-existing glucose level in the sample was determined in a control reaction lacking trehalase and substracted from total glucose concentration.
Protein assay
Protein content was estimated in groups of 10 virgin female flies per isofemale line (n = 20) from one northern and one southern population of D. melanogaster. Flies were homogenized in 3 ml of distilled water and centrifuged at 10,000 rpm for 5 min. 50 μl of aliquot was taken from supernatant and treated with 2 ml of Sigma BCA reagent and incubated at 25°C for 12 h. Absorbance was recorded at 562 nm and protein concentration was determined by comparing with standard curve.
Statistical analyses
For all the traits (body melanisation, starvation resistance, desiccation resistance, total lipid content and trehalose content), populations mean along with SE were used for illustrations and tabular data. Within as well as between population trait variability were analyzed through ANCOVA (body size as a covariate); and percent of total variance explained by each variable was calculated. Variance (%) due to populations, phenotypes and their interactions were calculated as proportion of Means square × degree of freedom out of the total sum of such values. Correlations between different traits were based on data from isofemale lines as well as for wild-caught flies. ANOVA was used to check differences in ecophysiological traits between darker and lighter body color isofemale lines and the results were shown as F values. Correlation coefficients (r ± SE) between traits were also calculated for within population trait variability. For evaluating differences in the utilization of energy metabolites (lipids or trehalose or proteins) under starvation or desiccation stress in darker and lighter isofemale lines, the data were subjected to ANOVA. In order to find associations between ecophysiological traits and climatic variables, we used multiple regression analysis of trait variability as a simultaneous function of Tave and relative humidity of the sites of origin of populations. Statistical calculations and illustrations were made with the help of Statistica™ 5.0.
Results
Climatic variables
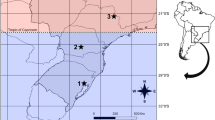
Figure 1 illustrates eight collection sites of Drosophila melanogaster along a large latitudinal transect (8° 29′–32° 40′N) but a narrow longitudinal range (75°33′–77°49′E). Southern and northern localities fall under tropical and subtropical climatic conditions and differ significantly in relative humidity (47.52 vs. 77.35%) and seasonal thermal amplitude (Tcv: coefficient of variation of mean monthly temperatures; Fig. 1b, c and Table 1). In contrast, the average yearly temperature (Tave) along the north–south transect is not much variable (22.21–28.42°C) and poorly correlated with latitude (r = −0.23 ns). Thus, D. melanogaster populations face climatic conditions which vary significantly along latitude on the Indian subcontinent.
a Map of India showing geographical locations of collection sites of D. melanogaster. b Change in mean monthly temperature of one northern (Udhampur), central (Wardha) and southern (Trivandrum) locality. c Changes in relative humidity (RH) as a function of latitude of origin of populations. Data on geographical and climatic variables are given in Table 1
Clinal variation
Data on between-population differences for ecophysiological traits in eight latitudinal populations of D. melanogaster are shown in Table 1. Twenty isofemale lines each of the eight geographical populations were grown at 21°C (under common garden conditions) so as to find genetic differences and to minimize the effects due to environmental differences between collection sites of D. melanogaster. All the traits showed clinal variation with about twofold increase or decrease in trait values along the latitudinal transect (Table 1). Three traits (body melanisation, desiccation resistance and trehalose level) were positively correlated with latitude. However, both starvation resistance and lipid content decreased along latitude. Northern population (Udhampur) showed higher levels of body melanisation (54.25 ± 2.19 percent), desiccation resistance (28.47 ± 1.67 h) and trehalose content (105.24 ± 3.24 μg fly−1; Table 1). In contrast, such traits values are about two times lower (melanisation = 25.18 ± 0.12; desiccation = 15.47 ± 0.73 h; trehalose = 51.27 ± 1.69 μg fly−1; Table 1) in southern population (Trivendrum) of D. melanogaster. However, negative clines were observed for starvation resistance and lipid content i.e. higher trait values for southern population (starvation = 189.21 ± 3.34 h; lipids = 107.04 ± 2.01 μg fly−1) as compared with northern population (starvation = 102.25 ± 4.19 h; lipids = 59.26 ± 2.98 μg fly−1; Table 1). For all the traits, latitudinal slope values (b) are highly significant (but vary in sign) and show changes in trait values per degree latitude (Table 1).
In order to compare trait variability at within and between population levels, the data were subjected to ANCOVA (with body size as a covariate) and the results are shown in Table 2. For all traits, there is significant within population variance (~30%) while between population variance is quite high (~60%; Table 2). Further, percent variance is quite similar for all the traits at both the within and between population levels.
Trait associations with climatic variables
In order to find possible relationships between phenotypes (darker and lighter flies) and climatic variables of origin of wild populations of D. melanogaster, the results of linear regression analyses are shown in Fig. 2a and b. Percent distribution of lighter flies significantly correlated with relative humidity and both showed a significant negative cline along latitude (Fig. 2a). Thus, a decrease of humidity along latitude covaries with lower percent distribution of lighter flies. In contrast, darker flies are abundant in the north where there are higher seasonal variations in temperature and lower relative humidity (Fig. 2b). Such trait associations suggest climatic selection of body color phenotypes along latitude.
Parallel changes in a relative humidity and percent distribution of lighter flies; b Tcv and darker flies as a function of latitude of origin of D. melanogaster. Regression equations (y = a + bx) are also shown. c–d correlations of melanisation with starvation resistance (c) and with desiccation resistance (d) based on population mean values of D. melanogaster. Tcv = coefficient of variation of mean monthly temperatures
Trait correlations (between population level)
Data on stress resistance traits (desiccation or starvation) as a function of changes in body melanisation of wild-caught flies (n = 60 flies each) from eight latitudinal populations of D. melanogaster are shown in Fig. 2c and d. Based on between population differences, there is significant negative correlation (r = −0.89 ± 0.09; P < 0.001) between body melanisation and starvation resistance (Fig. 2c). In contrast, there is a significant positive correlation (r = 0.94 ± 0.08; P < 0.001; Fig. 2d) between melanisation and desiccation resistance. Thus, data on wild-caught flies of D. melanogaster showed contrasting relationships of body melanisation with desiccation or starvation resistance.
Trait correlations (within population level)
Further, correlations between body melanisation (of assorted darker and lighter isofemale lines from north Indian population) and desiccation or starvation resistance; and also with energy metabolites (trehalose or lipid content) are shown in Fig. 3. Both starvation and lipid content have shown negative correlation with body melanisation (r ≥ −0.85; P < 0.001; Fig. 3a, b). However, desiccation resistance and trehalose level are positively correlated with body melanisation (r ≥ 0.87; P < 0.001; Fig. 3c, d). Further, there is a positive correlation between starvation resistance and lipid content (r ≥ 0.85; P < 0.001; Fig. 4a); and also between desiccation resistance and trehalose content (r ≥ 0.83; P < 0.001; Fig. 4c). By contrast, assorted flies with smaller or larger body size showed a negative correlation with starvation resistance (r ≥ −0.79; P < 0.001; Fig. 4b) but no correlation with desiccation resistance (r ≤ 0.14 ns; Fig. 3d).
Negative correlation between body melanisation and starvation tolerance (a); and with lipid content per fly (b); and positive correlation between melanisation with desiccation tolerance (c); and with trehalose content per fly (d) in assorted darker (>45% melanisation) and lighter body color phenotypes (<30% melanisation) isofemale lines from northern population (Udhampur) of D. melanogaster
Correlations between traits based on 10 isofemale lines each of darker and lighter phenotypes of a north Indian (Udhampur) population of D. melanogaster. Positive correlations are shown: a between starvation resistance and lipids content; c between desiccation resistance and trehalose content. There is a negative relationship between body size (wing length) and starvation resistance (b); and no correlation of body size with desiccation resistance (d) in seven isofemale lines
Trait analysis of darker and lighter flies
Data on differences in trait values between assorted darker and lighter flies from two geographical (one northern and one southern) populations of D. melanogaster are shown in Table 3. Results of ANOVA (F values) have shown significant differences for each trait between darker and lighter body color isofemale lines (Table 3). Darker flies have shown higher values of body melanisation, desiccation hours and trehalose content. However, lighter flies are characterized by high level of lipid content as well as starvation tolerance (Table 3). The trait values for both darker and lighter flies of northern versus southern populations differ significantly but the magnitude of differences between darker and lighter flies is about twofold (Table 3). Thus, there are significant differences in trait values (energy metabolites and stress traits) between body color phenotypes.
Storage of energy metabolites
Data on quantitative differences for energy metabolites in darker versus lighter flies from two geographical populations (one northern and one southern) of D. melanogaster are shown in Table 4. For each population, the level of metabolites is shown as percent content per dry mass as well as per wet mass. For both populations, darker and lighter flies did not vary in protein content (Table 4). However, lipid content was (~2-fold) higher in lighter flies as compared with darker flies while trehalose level was significantly higher in darker flies (Table 4). For lipid or trehalose, results of ANOVA have shown significant differences between darker and lighter body color isofemale lines (Table 4).
Utilization of energy metabolites
Figure 5 illustrates changes in energy metabolite level (lipids or trehalose) when darker or lighter isofemale lines of northern population (Udhampur) were exposed to different duration of desiccation or starvation stress. For starvation stress experiment, there were significant differences (~2-fold) in lipid content between lighter versus darker flies. In lighter flies, higher lipid content (99.14 ± 0.54 μg fly−1) sustained longer starvation hours (up to 180 h) while darker flies showed about fifty percent lesser starvation tolerance (starvation 88.61 ± 0.98 h; lipids = 48.31 ± 0.77 μg fly−1; Table 3; Fig. 5a). However, there were no changes in trehalose level when darker or lighter flies were exposed to starvation stress (Table 5). In contrast, when darker and lighter flies were exposed to different durations of desiccation stress, darker flies with higher trehalose level (119.57 ± 1.38 μg fly−1) showed longer survival up to 30 h as compared with 15 h in case of lighter flies (trehalose = 54.17 ± 0.92 μg fly−1). However, there was no utilization of lipids by darker or lighter flies under desiccation stress (Table 5). Thus, we found a clear trade-off in the utilization of either trehalose during desiccation stress or lipids under starvation stress. Further, experiments on protein utilization with lighter and darker flies exposed to desiccation or starvation stress showed no utilization of proteins. Lack of protein utilization is evident from non-significant F values for both the color phenotypes when exposed to desiccation or starvation stress (Table 5). For lipids utilization, F values are highly significant for starvation stress but not under desiccation stress. In contrast, trehalose utilization is highly significant under desiccation stress but not with starvation stress (Table 5).
Changes (mean ± SE) in the lipid content as a function of starvation stress durations (0–180 h with 30 h intervals (a); and changes in trehalose content under desiccation stress (0–30 h with 5 h intervals (b) in darker and lighter body color isofemale lines of D.melanogaster. Bars represent metabolite content before and after utilization under a given stress duration
Relationships with climatic variables
In order to test whether trait variability reflects adaptive changes due to climatic conditions, we considered multiple regression analysis of trait variability as a simultaneous function of Tave. and relative humidity of sites of origin of D. melanogaster populations (Table 6). For each trait, correlation (r) and slope (b) values are highly significant with relative humidity but nonsignificant for Tave. The R2 values (genetic determination of trait variability) are highly significant for all the traits. Thus, Clinal variations for energy metabolites and stress-related traits are better explained by geographical changes in relative humidity on the Indian subcontinent.
Discussion
Indian populations of D. melanogaster harbour significant levels of within population variation for desiccation as well as starvation tolerance. Between population differences in desiccation and starvation resistance as well as energy metabolites (trehalose and lipids content) are ordered into opposite clines along latitude in India. Trait divergence at within population level may have led to adaptive differences between geographical populations of D. melanogaster. This is evident from coadapted changes for body color phenotypes (dark or light) and energy metabolites (trehalose or lipids content) for desiccation or starvation resistance at the level of within as well as between populations. We found a trade-off in the storage as well as utilization of energy metabolites i.e. higher trehalose content in darker flies confers longer survival under desiccation stress. In contrast, lighter flies store more lipids and sustain longer starvation tolerance. Within population analysis has shown that body size changes are associated with starvation resistance but not with desiccation resistance. Finally, adaptive differences for starvation and desiccation resistance in D. melanogaster are linked with regular but significant changes in relative humidity along latitude in India.
Contrasting patterns of humidity variation
In the present study, we have considered between populations differences in starvation as well as desiccation in D. melanogaster in relation to regional differences in ecological conditions on the Indian subcontinent. Southern and northern localities fall under tropical and subtropical climatic conditions because tropic of cancer passes almost midway through the subcontinent. Average temperature does not vary significantly along latitude in India (r = 0.22 ± 0.29 ns). Thus, temperature variation may not be solely responsible for geographical variation in starvation and desiccation resistance in D. melanogaster from India. In contrast, Tave(s) vary significantly along latitude on other continents but are not correlated with desiccation or starvation resistance (Robinson et al. 2000; Hoffman et al. 2001). Interestingly, there are significant changes in relative humidity along latitude in India (r = 0.91 ± 0.08). However, humidity changes along latitude are far less on the Australian as well as on the South American continents which also lack clinal variations in desiccation and starvation resistance (Robinson et al. 2000; Hallas et al. 2002). Thus, there are specific climatic conditions (a steeper humidity gradient) on the Indian subcontinent which contrast with those occurring on South American and Australian continents. On the Indian subcontinent, significant changes in humidity can act as selection agent for starvation and desiccation resistance in D. melanogaster.
Selection of coadapted phenotypes
In the present study, we found evolution of coadapted phenotypes for starvation and desiccation resistance. The distribution of lighter flies and their starvation resistance level have shown negative clines along latitude in India. Interestingly, lighter flies have shown higher level of stored lipid content. Thus, there are significant associations between lighter body color phenotype, higher lipid content and starvation resistance. For desiccation resistance, our results agree with an earlier study on association between darker body color phenotypes and desiccation resistance in D. melanogaster (Parkash et al. 2008). However, no previous study has reported a positive cline for trehalose in latitudinal populations of D. melanogaster. Further, there are associations between trehalose level and body melanisation of darker flies. Selection responses due to dry versus wet climatic conditions match opposite clines for desiccation and starvation resistance in D. melanogaster on the Indian subcontinent. In the subtropics, drier conditions select darker flies with higher trehalose level for sustaining desiccation resistance. By contrast, wet conditions, favor coadapated changes i.e. lighter body color phenotypes with higher lipid level to support starvation resistance.
Storage of energy metabolites
In order to cope with physiological stresses (starvation or desiccation), adaptive responses in insects involve storage of energy rich metabolites (Edney 1977; Lim and Lee 1981; Satake et al. 2000). Storage of energy metabolites (carbohydrates and lipids) have been analysed in laboratory selection studies (Graves et al. 1992; Hoffmann and Parsons 1993; Chippindale et al. 1996). However, energetic basis of desiccation and starvation resistance in geographical populations has received lesser attention so far (Hoffmann et al. 2001; Rion and Kawecki 2007). For desiccation resistance, most of the earlier studies analyzed glycogen level in laboratory selected strains (Clark et al. 1990; Djawdan et al. 1998; Chippindale et al. 1998; Bradley et al. 1999; Gefen et al. 2006). However, we analysed trehalose content because it is the most abundant sugar in insect hemolymph and helps to cope with desiccation stress (Ring and Danks 1998). One study on laboratory selection in D. melanogaster has also provided an indirect evidence for increased trehalose level in desiccation-resistant flies (Folk et al. 2001). In the present study, we found a positive latitudinal cline for trehalose content and a negative cline for lipids in D. melanogaster. To the best of our knowledge, this is the first report which has shown a trade-off of energy metabolites for resistance to starvation and desiccation in D. melanogaster.
Utilization of energy metabolites
Most of the previous studies on laboratory selection as well as comparative studies did not consider the utilization of energy metabolites under stressful conditions (Hoffmann and Parsons 1993; Chippindale et al. 1998; Gibbs and Matzkin 2001). A single study has analyzed utilization of energy metabolites under desiccation or starvation stress (Marron et al. 2003). The rates of lipid and protein metabolism did not vary for starvation or desiccation stress but carbohydrates metabolism was sevenfold higher under desiccation stress in five Drosophila species (Marron et al. 2003). However, in the present study, we found utilization of trehalose under desiccation stress; and of lipids under starvation stress in D. melanogaster (Fig. 5).
Impact of body size on stress resistance
Few studies have examined the relationship between body size and stress resistance in D. melanogaster (Hoffmann and Parsons 1993). Comparative studies of desiccation resistance and body mass changes across 29 desert and mesic Drosophila species have shown positive correlation (Gibbs and Matzkin 2001). In contrast, no correlated changes in body size were found in laboratory selected desiccation resistance strains in D. melanogaster (Hoffmann and Parsons 1993). However, in the present study, there is lack of body size-desiccation resistance relationship (Fig. 4d). Further, within population differences in body size are negatively correlated with starvation resistance (Fig. 4b).
Laboratory selected starvation resistance strains of D. melanogaster have shown increase in dry as well as wet weight (Chippindale et al. 1996). In contrast, for starvation resistance, laboratory selection on lipid storage in D. melanogaster has shown a negative relationship between body size and lipid content i.e. body size of D. melanogaster decreased when lipid mass increased (Clark et al. 1990). Thus, for starvation resistance, body size changes differ in laboratory versus natural selection in D. melanogaster.
Climatic selection of starvation tolerance
The ecological aspects of starvation resistance in wild populations of D. melanogaster have received lesser attention so far. On the Indian subcontinent, there is no gradient in food availability (decaying fruits) which could indicate strong selection for starvation tolerance in D. melanogaster. In fact, D. melanogaster flies are unlikely to face food shortage throughout the year due to extensive banana plantations in the southern localities. Further, some studies on Drosophila population ecology have suggested that larval food rarely becomes a limiting factor under wild conditions and competition is unlikely to be a strong limiting factor under field conditions (Shorrocks et al. 1984; Atkinson and Shorrocks 1984). Alternatively, wild populations of D. melanogaster may be limited by abiotic stresses such as humidity and temperature. Low humidity habitats select desiccation resistance but it is not clear whether high humidity and warm conditions affect starvation resistance.
Laboratory selection experiments have shown correlated changes in both desiccation and starvation resistance (Chippindale et al. 1996; 1998). These studies have suggested reduction in metabolic rate under laboratory conditions and such changes may induce a positive correlation between desiccation and starvation resistance. By contrast, in nature, ecological conditions which promote starvation resistance may differ from those responsible for desiccation resistance. There are two possible ways which may cause starvation stress to D. melanogaster. First, larger population size of tropical insect species result in species interaction, competition and higher dispersal rates which can cause starvation stress (Barrera 1996). Another study has shown higher starvation resistance associated with dispersal behaviour in spider mites (Li and Margolies 1994). On the India subcontinent, wind velocity in the peninsular southern localities are quite high (~10 to 12 km/h) and this may cause higher dispersal rate of D. melanogaster leading to longer duration of starvation stress. Second, temperature and water availability affect metabolic rate in some insects taxa (Davis et al. 2000). At higher ambient temperature in the tropics, smaller flies (1,200 μg/fly) may face starvation stress due to higher rate of metabolism per unit body weight. Thus, warm and humid climatic conditions in the southern peninsular Indian localities may promote starvation tolerance in D. melanogaster.
Conclusions
A steeper humidity gradient along latitude in India has selected coadapted phenotypes (body color phenotype and energy metabolites) for greater starvation resistance in the tropics as compared with higher desiccation resistance in the subtropics. The evidence for coadapted phenotypes is based on within population analysis i.e. darker flies store higher trehalose level which confers greater desiccation resistance. In contrast, higher lipid content in lighter flies sustains higher starvation tolerance. Based on between population differences in trait values, we found opposite clines for body color phenotypes (darker vs. lighter flies); energy metabolites (trehalose vs. lipid content) and desiccation versus starvation resistance. We have shown that flies actually metabolize trehalose under desiccation stress; and lipids under starvation stress. Thus, there is a trade-off for storage and utilization of energy metabolites for desiccation versus starvation resistance. We found negative correlation between body size and starvation resistance. However, there is lack of relationship between body size and desiccation resistance. We may suggest that desiccation and starvation resistance have evolved due to selection of coadapted phenotypes.
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Atkinson D (1994) Temperature and organism size—a biological law for ectotherms? Adv Ecol Res 25:1–58
Atkinson WD, Shorrocks BC (1984) Aggregation of larval Diptera over discrete and ephemeral breeding sites: the implications for coexistence. Am Nat 124:336–352
Barrera R (1996) Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol 21:112–127
Bradley TJ, Williams AE, Rose MR (1999) Physiological responses to selection for desiccation resistance in Drosophila melanogaster. Am Zool 39:337–345
Chippindale AK, Chu TJF, Rose MR (1996) Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50:753–766
Chippindale AK, Gibbs AG, Sheik M, Yee KJ, Djawdan M, Bradley TJ, Rose MR (1998) Resource allocation and the evolution of desiccation resistance in laboratory-selected Drosophila melanogaster. Evolution 52:1342–1352
Clark AG, Szumski FM, Bell KA, Keith LE, Houtz S, Merriwether DA (1990) Direct and correlated responses to artificial selection on lipid and glycogen contents in Drosophila melanogaster. Genet Res 56:49–56
Collinge JE, Hoffmann AA, McKechnie SW (2006) Altitudinal patterns for latitudinally varying traits and polymorphic markers in Drosophila melanogaster from eastern Australia. J Evol Biol 19:473–482
Davis ALV, Chown SL, McGeoch MA, Scholtz CH (2000) A comparative analysis of metabolic rate in six Scarabaeus species (Coleoptera: Scarabaeidae) from southern Africa: further caveats when inferring adaptations. J Insect Physiol 46:553–562
Djawdan M, Chippindale AK, Rose MR, Bradley TJ (1998) Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiol Zool 71:584–594
Edney EB (1977) Water balance in land arthropods. Springer, Berlin
Endler JA (1986) Natural selection in the wild. Princeton University Press, New Jersey. USA
Folk DG, Han C, Bradley TJ (2001) Water acquisition and partitioning in Drosophila melanogaster: effects of selection for desiccation resistance. J Exp Biol 204:3323–3331
Forsman A (2000) Some like it hot: intra-population variation in behavioural thermo-regulation in colour-polymorphic pygmy grasshoppers. Evol Ecol 14:25–38
Gefen E, Marlon AJ, Gibbs AG (2006) Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation. J Exp Biol 209:3293–3300
Gibbs AG, Matzkin LM (2001) Evolution of water balance in the genus Drosophila. J Exp Biol 204:2331–2338
Gibbs AG, Chippindale AK, Rose MR (1997) Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J Exp Biol 200:1821–1832
Graves JL, Toolson EC, Jeong C, Vu LN, Rose MR (1992) Desiccation, flight, glycogen, and postponed senescence in Drosophila melanogaster. Physiol Zool 65:268–286
Griffiths JA, Schiffer M, Hoffmann AA (2005) Clinal variation and laboratory adaptation in the rainforest species Drosophila birchii for stress resistance, wing size, wing shape and development time. J Evol Biol 18:213–222
Hallas R, Schiffer M, Hoffmann AA (2002) Clinal variation in Drosophila serrata for stress resistance and body size. Genet Res Camb 79:141–148
Hoffmann AA, Harshman LG (1999) Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity 83:637–643
Hoffmann AA, Parsons PA (1993) Selection for adult desiccation resistance in Drosophila melanogaster-fitness components, larval resistance and stress correlations. Biol J Linnean Soc 48:43–54
Hoffmann AA, Weeks AR (2007) Climatic selection on genes and traits after a 100 year -old invasion: a critical look at the temperature-tropical clines in Drosophila melanogaster from eastern Australia. Genetica 129:133–147
Hoffmann AA, Hallas R, Sinclair C, Mitrovski P (2001) Levels of variation in stress resistance in Drosophila among strains, local populations, and geographic regions: patterns for desiccation, starvation, cold resistance, and associated traits. Evolution 55:1621–1630
James AC, Azevedo RBR, Partridge L (1997) Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 146:881–890
Li JB, Margolies DC (1994) Responses to direct and indirect selection on aerial dispersal behavior in Tetranychus urticae. Heredity 72:10–22
Lim SJ, Lee SS (1981) The effect of starvation on haemolymph metabolites, fat body and ovarian development in Oxya japonica (Acrididae: Orthoptera). J Insect Physiol 27:93–96
Marron MT, Markow TA, Kain KJ, Gibbs AG (2003) Effects of starvation and desiccation on energy metabolism in desert and mesic Drosophila. J Insect Physiol 49:261–270
Mousseau TA, Sinervo B, Endler JA (2000) Adaptive genetic variation in the wild. Oxford University Press, New york
Parkash R, Munjal AK (1999) Climatic selection of starvation and desiccation resistance in populations of some tropical Drosophilids. J Zool Syst Evol Res 37:195–202
Parkash R, Munjal AK (2000) Evidence of independent climatic selection for desiccation and starvation tolerance in Indian tropical populations of D. melanogaster. Evol Ecol Res 2:685–699
Parkash R, Rajpurohit S, Ramniwas S (2008) Changes in body melanisation and desiccation resistance in highland vs. lowland populations of Drosophila melanogaster. J Insect Physiol 54:1050–1056
Parkash R, Sharma V, Kalra B (2010) Correlated changes in thermotolerance traits and body color phenotypes in montane populations of Drosophila melanogaster: analysis of within-and between-population variations. J Zool 280:49–59
Ring RA, Danks HV (1998) The role of trehalose in cold-hardiness and desiccation. Cryo Letters 19:275–282
Rion S, Kawecki TJ (2007) Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol 20:1655–1664
Robinson SJW, Zwaan B, Partridge L (2000) Starvation resistance and adult body composition in a latitudinal cline of Drosophila melanogaster. Evolution 54:1819–1824
Satake S, Kawabe Y, Mizoguchi A (2000) Carbohydrate metabolism during starvation in the silkworm Bombyx mori. Arch Ins Biochem Physiol 44:90–98
Schmidt PS, Matzkin L, Ippolito M, Eanes WF (2005) Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution 59:1721–1732
Shorrocks BC, Rosewell J, Edwards K, Atkinson W (1984) Interspecific competition is not a major organising force in many insect communities. Nature 310:310–312
Telonis-Scott M, Guthridge KM, Hoffmann AA (2006) A new set of laboratory selected Drosophila melanogaster lines for the analysis of desiccation resistance: response to selection, physiology and correlated responses. J Exp Biol 209:1837–1847
Acknowledgments
Financial assistance from University Grants Commission, New Delhi is gratefully acknowledged. D. D. Aggarwal is thankful to Council of Scientific and Industrial Research, New Delhi for senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parkash, R., Aggarwal, D.D. & Kalra, B. Coadapted changes in energy metabolites and body color phenotypes for resistance to starvation and desiccation in latitudinal populations of D. melanogaster . Evol Ecol 26, 149–169 (2012). https://doi.org/10.1007/s10682-011-9482-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9482-x