Abstract
Understanding the evolution and maintenance of within-sex reproductive morphs, or alternative reproductive phenotypes (ARPs), requires in depth understanding of the proximate mechanisms that determine ARP expression. Most species express ARPs in complex ecological environments, yet little is know about how different environmental variables collectively affect ARP expression. Here, I investigated the influence of maternal and developmental nutrition and sire phenotype on ARP expression in bulb mites (Rhizoglyphus robini), where males are either fighters, able to kill other mites, or benign scramblers. In a factorial experiment, females were raised on a rich or a poor diet, and after maturation they were paired to a fighter or a scrambler. Their offspring were put on the rich or poor diet. Females on the rich diet increased investment into eggs when mated to a fighter, but suffered reduced longevity. Females indirectly affected offspring ARP expression as larger eggs developed into larger final instars, which were more likely to develop into a fighter. Final instar size, which also strongly depended on offspring nutrition, was the main cue for morph development: a switch point, or size threshold, existed where development switched from one phenotype to the other. Sire phenotype affected offspring phenotype, but only if offspring were on the poor diet, indicating a gene by environment interaction. Overall, the results revealed that complex environmental effects can underlie ARP expression, with differential maternal investment potentially amplifying genetic effects on offspring morphology. These effects can therefore play an important role in understanding how selection affects ARP expression and, like quantitative genetics models for continuous traits, should be incorporated into models of threshold traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenotypic variation within natural populations is ubiquitous. One of the most extreme and also frequently observed forms of phenotypic variation within single populations is the existence of discrete morphological variants. Examples of such variation are alternative reproductive phenotypes (ARPs), which represent adaptive, phenotypic variation within a single sex (usually males) (Oliveira et al. 2008). Common forms of ARPs are dimorphic variation in structures that act as weapons used in male–male competition such as cerci in earwigs (Eberhard and Gutiérrez 1991) and the third pair of legs in various mite species (Radwan 1993; Tomkins et al. 2004). The consistent variation in ARPs is an evolutionary puzzle (Oliveira et al. 2008): for example, how have ARPs evolved and how is such variation maintained within single populations? Key to understanding the forces that generate these discrete phenotypes, and how the resulting phenotypes will be affected by selection, is to investigate and understand the proximate mechanisms that determine the expression of ARPs (Schlichting and Pigliucci 1995).
The results of the many studies that have investigated the proximate mechanisms responsible for generating variation in ARPs initially revealed two very different determinative regimes. Firstly, ARPs can result from the expression of alternative alleles at one or a few loci, such as male tail morphologies in pigmy swordtail fish (Ryan et al. 1992) and male shape in marine isopods (Shuster and Wade 1991). In these taxa, the phenotypes of individual males are primarily determined by their genotypes, and only minimally by environmental conditions experienced by these individuals during ontogeny. Secondly, production of different ARPs can be largely independent of the inheritance of specific alleles and instead depend predominantly on the environmental conditions experienced during ontogeny (Emlen 2008). Examples include the male horn dimorphism in some scarab beetles (Emlen 1994; Kotiaho et al. 2003) and the male leg dimorphism in some acarid mites (Radwan 1993, 2001). In these taxa, each individual has the potential to develop into each of the alternative phenotypes, but the actual expression of a particular morph depends on the environmental conditions experienced during a critical developmental period (Emlen 2008). Brockmann et al. (2008) recently criticised this rigid classification (e.g. Shuster and Wade 2003), since environmental conditions may affect the expression of genetic polymorphisms, and genetic differences may affect the expression of condition-dependent ARPs (Emlen 2008). This means that focusing on gene by environment interactions is important in understanding the expression of ARPs (e.g. Hazel et al. 1990, 2004; Tomkins and Hazel 2007), rather than merely distinguishing between genetic polymorphism or conditional phenotype. Although this change in approach has improved our understanding of the evolution and maintenance of ARPs (Oliveira et al. 2008), further progress is hampered by the fact that little is known about how different environmental variables collectively affect ARP expression. Most species express ARPs in complex environments affected by many different ecological and demographic factors. However, studies have generally concentrated on assessing the relationship between variation in one environmental variable—such as food quantity, food quality, or population density (Emlen 1994; Radwan 1995; Radwan et al. 2002)—on the expression of ARPs. The results of these studies likely present a limited view on the proximate mechanisms that lead to the production of discrete phenotypic variation. Such studies are also prone to overlook other important sources of variation that may be crucial to our understanding of the ecological context within which species express ARPs.
The aim of this study is to advance our understanding of complex, proximate mechanisms that lead to the production of ARPs. To this end I will investigate: (1) the collective impact of variation in maternal and developmental nutrition and sire phenotype on ARP expression, (2) the relative importance of these environmental and genetic drivers on ARP expression, and (3) the presence of any gene by environment interaction influencing ARP expression. I will investigate each of these three aspects of ARP expression for the bulb mite (Rhizoglyphus robini). Male bulb mites are either ‘fighters’, which have a thickened and sharply terminated third pair of legs with which they can kill other mites (Radwan et al. 2000), or ‘scramblers’, which have unmodified legs and are defenceless (Fig. 1). The male dimorphic bulb mite has traditionally been classified as a species where ARP expression is associated with a genetic polymorphism (Shuster and Wade 2003). Heritability of male morph expression in this species, calculated using the threshold model of quantitative genetics (Falconer 1989), has been found to range from 0.3 (Smallegange and Coulson, in press) to 0.8 (Radwan 2003). However, the fact that male morph expression in this species also depends on environmental quality (Radwan 1995), suggests that gene by environment interactions likely underlie male morph expression.
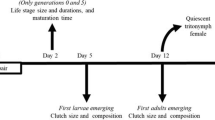
Ventral views of a scrambler and a fighter male (which were raised on yeast); a bulb mite egg; and two quiescent tritonymphs (the final instar stage during which mites develop into adults), one of which was raised on yeast and the other on filter paper. Fighters possess a thickened and sharply terminated third pair of legs (see arrows) which they can use to kill other males. Scramblers have unmodified legs (see arrows) and are defenceless. Because fighters can kill other males and even monopolise access to females, they are considered competitively superior to scramblers (Radwan and Klimas 2001)
In this study, environmental conditions were manipulated across two generations: a maternal generation and the offspring generation. Maternal environmental conditions were manipulated to investigate how variation in environmental conditions experienced by females affects the phenotype of their offspring. This is important because if ARP expression depends on resource availability, maternal effects [which occur when maternal phenotype affects offspring phenotype (Mousseau and Fox 1998)] may influence offspring ARP expression if maternal investment in offspring affects the amount of resources available for offspring development (Hunt and Simmons 2000). Maternal investment in offspring, in turn, critically depends on maternal resource availability. In response to resource limitation, females often produce small eggs (Fox and Czesak 2000), but sometimes fewer, but larger eggs are produced (Brody and Lawlor 1984) if the increased production costs are outweighed by the increased fitness returns of the larger offspring (e.g. Fox et al.1997; Bashey 2006). Maternal investment in offspring may also depend on the phenotypes of the different males that females mate with during their lifetime, if these phenotypes are associated with different direct and/or indirect fitness benefits (differential allocation hypothesis: Burley 1986; Sheldon 2000). Indeed, females adjust investment in offspring to male phenotype across a diverse range of taxa, including birds (Gil et al. 1999; Cunningham and Russell 2000), fishes (Kolm 2001) and insects (Wedell 1996; Kotiaho et al. 2003). The differential allocation hypothesis assumes that this increased investment into offspring is at the expense of the future reproductive events of mothers (Burley 1986, Sheldon 2000). Of the former studies, only Kotiaho et al. (2003) tested this important assumption, but did not find evidence that females suffered a reduction in lifespan or lifetime reproductive output when mated with males of the ‘high-quality’ phenotype. In the main experiment of this study I assessed the effects of maternal nutrition and sire phenotype on maternal investment in egg size, and explored whether offspring nutrition affected the extent at which these effects carried over to influence final instar size and male adult phenotype. Final instar size was measured because, in many species, the sensitive period for developing ARPs occurs during the final larval or nymphal instar stage (Emlen 2008). A second experiment was performed to test the assumption of the differential allocation hypothesis (Burley 1986) that females mated with fighters suffer a reduction in life span and/or lifetime reproductive output compared to females mated with scramblers (following Kotiaho et al. 2003).
Materials and methods
Stock cultures
Stock cultures have been kept in the laboratory at Imperial College since 2008 as a large population (>10,000 individuals, subdivided into nine subpopulations mixed once a month). Stock cultures were originally obtained from the University of Amsterdam, where bulb mites had been collected from flower fields near Anna Paulowna (The Netherlands) and kept in the laboratory since 1992. Subpopulations were kept in plastic containers (l × w × h: 4 × 4 × 2.5 cm) with a plaster of Paris substratum, maintained at 20–27°C, >70% relative humidity, and fed yeast granules (Allison’s active dry yeast) ad libitum. Each week, debris in the containers was removed and each subpopulation reduced by about a quarter by removing food and debris containing several hundred mites at all stages of development.
Experimental procedures
The main experiment consisted of three treatments: two maternal diets (poor and rich), two sire morphs (fighter and scrambler), and two offspring diets (poor and rich). The poor diet was ad lib access to filter paper and the rich diet was ad lib access to yeast. Yeast is rich in protein, whereas filter paper contains only cellulose on which the mites feed. These two diets represent extremes in terms of food quality and for this reason are commonly used in life table studies to assess effects of food quality on growth, development, and ARP expression of mites of the family Acaridae (Gerson et al. 1983, 1991; Radwan 2001; Smallegange and Coulson in press). Although these two diets differ qualitatively, for logistic reasons it was not possible to offer individual mites different amounts of food of the same kind (e.g. yeast) in a controlled manner. The experiment started by taking eggs from the stock cultures and putting them in individual tubes on the poor or the rich diet. The morph of the sire of these eggs was unknown. At maturation, 97 females were photographed using a Lumenera Infinity 3.1 camera connected to a Zenith SRZ-4500 (7-45x) stereomicroscope and their body length (without mouthparts) measured to the nearest 0.1 μm using Infinity Analyze Imaging Software. After mating females with a fighter or scrambler (taken from the stock cultures), 1,097 eggs were collected (10–15 eggs per female and 99–150 eggs per treatment combination), their width (a) and length (b) measured to calculate egg volume as \( \frac{1}{6}\pi a^{2} b \) (eggs resemble a prolate spheroid: Fig. 1), and put in individual tubes on the poor or rich diet. After the offspring had developed into larvae, tubes were checked daily and when they had developed into a quiescent tritonymph (the final instar stage during which mites moult into adults: Fig. 1), the length of the quiescent tritonymph was measured (as before). In the soil mite Sancassania berlesei (where males exhibit the same male dimorphism) both tritonymph length and weight are predictors of male morph expression, although of the two predictors, tritonymph weight is the more accurate one (Radwan et al. 2002). Here, however, due to logistic reasons, weight of individual mites could not be measured and I therefore resorted to measuring the length of individuals. However, given the fact that tritonymph length and weight are strongly and positively correlated (Radwan et al. 2002), this should still adequately capture the relationship between the size of male mites and male morph expression. Upon reaching maturity (usually the next day), age (to the nearest day), sex and morph of the mites were scored and body size was measured (as before). Throughout the experiment, all mites were kept in individual tubes, which were numbered to be able to identify mites individually.
The second experiment was conducted to explore the assumption of the differential allocation hypothesis (Burley 1986) that females with fighters suffer a reduction in life span and/or lifetime reproductive output compared to females mated with scramblers. On average, longevity of female bulb mites on a yeast diet is 43 days, but on a filter paper diet, longevity is 61 days as development lasts significantly longer (although adult lifetime is shorter than that of females on yeast) (Smallegange and Coulson, in press). This experiment consisted of one treatment: sire morph (fighter or scrambler). The experiment was begun by collecting 25 quiescent tritonymphs from the stock cultures and putting each tritonymph in an individual tube. The next day, when the mites emerged as adults, 10 females were isolated and their body size measured (as before). Males were collected from the stock cultures to form five mating pairs with a fighter, and five mating pairs with a scrambler. This procedure was repeated twice more over the next 2 days, resulting in a total of 30 mating pairs. Each mating pair was put in an individual tube and fed yeast ad libitum for the duration of the experiment. Each working day, until she died, the number of eggs laid was counted. Two males died during the experiment and were replaced by new males from the stock cultures.
In both experiments, the tubes the mites were kept in (all tubes had unique numbers) were all 10 mm diameter plastic tubes with a plaster of Paris and powdered charcoal base, which was kept moist to avoid desiccation of the mites. Tops of tubes were sealed by a circle of filter paper (allowing gaseous diffusion), which was held in place by the tubes’ standard plastic caps with ventilation holes cut into them. Mites were kept in an unlit incubator at 27°C and >70% relative humidity.
Statistical analyses
First, using a general linear mixed-effects model (GLMM) with Gaussian errors and with maternal identity included as a random factor, I investigated maternal investment in egg size by assessing how egg volume depended on maternal nutrition, maternal size and sire morph (Table 1). Probability plots showed that the residuals of this model were normally distributed. Next, I assessed if effects of maternal investment in egg size carried over to affect final instar size and male phenotype, and if offspring environmental conditions affected the extent at which effects of maternal environmental conditions (nutrition, sire phenotype) carried over to affect final instar size and male phenotype. To this end, effects of egg volume, maternal and offspring diet, sire morph and maternal size were tested on quiescent tritonymph size (final instar size) using a GLMM with Gaussian errors and with maternal identity as a random factor (Table 1). Probability plots showed that the residuals of this model were normally distributed. Then, treatment effects were tested on male morph expression, firstly from the perspective of the mother by expressing male morph expression as the proportion of offspring that developed into a fighter (Table 1), and secondly from the perspective of the offspring by expressing male morph expression as a binary response variable, denoting whether or not a male mite developed into a fighter (Table 1). The difference between these two analyses is that the ‘maternal model’ included the covariates egg volume averaged per dam and maternal size, and the ‘offspring model’ included egg volume, maternal size and also quiescent tritonymph size as covariates (Table 1). Treatment effects on male morph expression expressed as a proportion (maternal perspective) were analysed using a generalized linear model (GLM) with quasibinomial errors (to correct for overdispersion). The binary response variable male morph expression (offspring perspective) was analysed using a GLMM with binomial errors, and with maternal identity as a random factor. Finally, I focused on the reaction norm between age and size at maturity. Reaction norms describe how phenotypic traits of individuals change with changes in the environment. Here I assessed if the reaction norm between age and size at maturity was diet- or morph-specific by testing the effects of age at maturity and its interaction with maternal diet, offspring diet, sire morph and morph of offspring on male size at maturity, using a GLMM with Gaussian errors and with maternal identity as a random factor (Table 1). Probability plots showed that the residuals of this model were normally distributed.
In the second experiment, I assessed if females incurred a cost in terms of longevity if they were mated with fighters by testing the effects of total lifetime egg production, age at peak daily egg production and increase in adult size and their two-way interactions with sire morph on female longevity. Age at peak daily egg production was included to assess if earlier maximum egg production might result in a shorter lifespan. Increase in adult size was measured as the difference in size at 7 days old and at maturation. Female size at maturity and the fixed blocking factor day were also included in the model. Treatment effects on female longevity were analysed using a GLM with Gaussian errors. To analyse female longevity, the GLM was fitted through the origin because newly born females lay zero eggs (including an intercept in the model gave qualitatively the same results). Probability plots showed that the residuals of this model were normally distributed.
In each analysis a model simplification procedure was used whereby the full model was fitted, after which the least significant term was removed (starting with the highest order interaction) if the deletion caused an insignificant increase in deviance (significance was assessed by performing a likelihood ratio test). This procedure was repeated until the model only contained significant terms (P < 0.05). All non-significant terms removed during model simplification, as well as significant terms that were part of a significant higher interaction, are given in the Online Appendix. The random factor maternal identity was never removed during model simplification. Models were fitted by maximum likelihood in the ‘lme4’ package in R. For the GLMMs with Gaussian errors this means that the parameter estimates in each model are maximum likelihood estimates. Significance of these estimates was assessed using 95% confidence limits based on the underlying Gaussian distribution of the model residuals. To this end, a Markov Chain Monte Carlo (MCMC) sample was generated from the posterior distribution of each parameter estimate. This used the function mcmcsamp in the R package lme4 (Bates and Sarkar 2007). Then, the Bayesian highest posterior density (HPD) 95% confidence intervals of the MCMC sample for each parameter estimate (\( \hat{e} \)) was computed using the function HPDinterval in the R package coda (Plummer et al. 2006). If the HPD interval of a parameter estimate overlaps with zero, then the associated factor has no significant effect on the response variable. Although this method does not generate P values, it is currently the most reliable way to assess the uncertainty in the parameter estimates for this type of GLMM (Baayen et al. 2008). Having run all analyses, the relative importance of significant treatment effects on male morph expression were established by comparing effect sizes. Effect sizes between two means \( \bar{x}_{1} \) and \( \bar{x}_{2} \) were measured as Cohen’s d (Cohen 1988): \( d = \left( {\bar{x}_{1} - \bar{x}_{2} } \right)/s \), where the pooled standard deviation is: \( s = \sqrt {{{(n_{1} - 1)s_{1}^{2} + (n_{2} - 1)s_{2}^{2} } \mathord{\left/ {\vphantom {{(n_{1} - 1)s_{1}^{2} + (n_{2} - 1)s_{2}^{2} } {(n_{1} + n_{2} )}}} \right. \kern-\nulldelimiterspace} {(n_{1} + n_{2} )}}} \).
Results
Egg volume and tritonymph size
Egg volume was significantly affected by sire morph, but differently for dams on different diets (DM * MS: \( \hat{e} \) = −1.12 · 105; n = 1,071; HPD interval: −2.24 · 105 to −0.13 · 105) (\( \hat{e} \) stands for parameter estimate, n is the sample size, which is also given in Fig. 3): eggs from fighter sires were larger than eggs from scrambler sires, but only if dams were on the rich diet (Fig. 2a). The significant effect of maternal diet (DM: \( \hat{e} \)=25.0 · 104; n = 1,071, HPD interval: 17.1 · 104 to 32.0 · 104) emphasized that females produced larger eggs on the rich than on the poor diet (Fig. 2a). There was no effect of maternal size on egg volume (Online Appendix: Table A1).
Results of the main experiment, testing effects of maternal and offspring nutrition and sire phenotype on offspring phenotype. a When dams were on the rich diet, egg volume was higher when they were mated to a fighter than to a scrambler (squares); egg volume was lowest when dams were on the poor diet. b, c Probability of males developing into a fighter in relation to offspring diet and sire morph (fighter: triangles, scrambler: squares) (b), and as a function of quiescent tritonymph length (a slight jitter was added) (c). Raw data are shown ±SEM. Numbers in figure a and b are sample sizes
Egg volume positively affected the size of quiescent tritonymphs (significant effect of E: \( \hat{e} \) = 2.88 · 10−5; n = 266; HPD interval: 1.18 · 10−5 to 4.46 · 10−5). In addition, quiescent tritonymphs were larger if mites were on the rich diet (572.2 ± 2.5 μm SEM, n = 180) than if mites were on the poor diet (379.6 ± 4.1 μm SEM, n = 86) (significant effect of DO: \( \hat{e} \) = 1.82 · 102; HPD interval: 1.81 · 102 to 1.99 · 102). There was no direct effect of maternal size, maternal nutrition or sire morph on quiescent tritonymph size (Online Appendix: Table A1).
Male morph expression
Analysing male morph expression from the perspective of the mother, i.e. the proportion of her offspring that developed into a fighter, revealed a significant interaction between offspring diet and sire morph (DO * MS: n = 89, t = -2.46, P = 0.016): if offspring were on the poor diet, the proportion of fighters was higher if their sire was a fighter than if their sire was a scrambler (Fig. 2b). For offspring on the rich diet there was no sire effect on male morph expression (Fig. 2b). The strong effect of offspring diet (DO: t = 5.02, P < 0.001) showed that the fraction of males that developed into fighters was higher for mites on the rich than on the poor diet (Fig. 2b). There was no direct effect of maternal size, maternal nutrition or egg volume on male morph expression (Online Appendix: Table A2).
The results from the perspective of the offspring were similar, except for the significant effect of maternal diet on male morph expression (DM: \( \hat{e} \) = −1.013, n = 319, z = −2.116, P = 0.034): offspring had a higher probability of developing into a fighter if dams were on the rich diet (0.55 ± 0.04 SEM, n = 167) than if dams were on the poor diet (0.48 ± 0.04 SEM, n = 152). Apart from that, there was the same significant interaction between offspring diet and sire (DO * MS: \( \hat{e} \) = 3.111, n = 319, z = 2.634, P = 0.008). There was also a strong positive effect of quiescent tritonymph size (ST: \( \hat{e} \) = 0.024, z = 4.137, P < 0.001, n = 266): the probability of developing into a fighter increased with increasing tritonymph size (Fig. 2c). There was no significant effect of offspring diet (DO: \( \hat{e} \) = −1.668, z = −1.406, P = 0.160, n = 319), but offspring diet and tritonymph size were significantly correlated (ρ = 0.83), from which I infer that the significant effect of tritonymph size captured the effect of offspring diet. Comparing effect sizes revealed that offspring diet was the most influential determinant of male morph expression (see also Fig. 2b): the effect size of maternal diet was 0.14, the effect size of sire morph for males on the poor offspring diet was 0.59, and the effect size of offspring diet was 2.05. Figure 3 summarises the effects of maternal and offspring environmental conditions on male morph expression.
Flowchart summarising how maternal environmental conditions (diet and morph of mate), offspring diet, and paternity influence male morph expression of offspring. Maternal females produced larger eggs on the rich than on the poor diet, but females on the rich diet that were mated with a fighter produced the largest eggs. This differential investment in egg size carried over to influence final instar size: larger eggs resulted in larger tritonymphs. Offspring nutrition additionally influenced tritonymph size as tritonymphs on the rich diet were larger than tritonymphs on the poor diet. Larger tritonymphs, in turn, were more likely to develop into a fighter. As a result of these developmental pathways, the proportion of fighters was higher if offspring were on the rich diet than if offspring were on the poor diet. There was also a direct relationship between maternal nutrition and the proportion of offspring that emerged as fighters. Paternal (genetic) effects were apparent when offspring were on the poor diet, in which case the probability of developing into a fighter was higher if the sire was a fighter than if he was a scrambler. This suggests the presence of a gene by environment interaction
The point at which the probability of being a scrambler equalled the probability of being a fighter was when the length of the quiescent tritonymph equalled 490.9 ± 58.6 (SE) μm (Fig. 2c). This switch point did not differ between mites from a fighter sire or scrambler sire (fighter sire: 494.2 ± 76.5 (SE) μm; scrambler sire: 500.9 ± 41.1 (SE) μm), nor did it differ between offspring diets (poor: 522.6 ± 67.8 (SE) μm; rich: 515.8 ± 56.6 (SE) μm) or between maternal diets (poor: 479.3 ± 58.5 (SE) μm; rich: 529.8 ± 52.1 (SE) μm) (non-significance was inferred from overlapping standard errors).
Reaction norm of age and size at maturity
There was an interactive effect between age at maturity and morph of male offspring on size at maturity (age * MO: \( \hat{e} \) = −1.15; n = 318; HPD interval: −2.37 to −0.34). The slope of the linear relationship between age and size at maturity was more shallow for fighters than for scramblers (Fig. 4a), implying that to mature at the same size, fighters required a longer development time than scramblers. The reaction norm also differed between offspring diets (age * DO: \( \hat{e} \) = −6.03; HPD interval: −11.24 to −0.82): the slope of the relationship between age and size at maturity was more shallow for males on the poor diet than for males on the rich diet (Fig. 4b), showing that as food quality is reduced, more and more individuals are forced to delay maturity and to mature at the minimum threshold size. There was no effect of maternal nutrition or sire morph on the reaction norm (Online Appendix: Table A1).
Reaction norms between age and size at maturity for fighters (triangles, dashed line) and scramblers (squares, solid line) (a), and for males on the two offspring diets, filter paper (triangles, dashed line) and yeast (squares, solid line) (b), as observed in the main experiment. Note that the axes are on a log scale (to increase clarity) in panel a but not in panel b. c Female longevity in relation to total lifetime egg production and morph of mate (fighter: triangles, dashed line; scrambler: squares, solid line) as observed in the second experiment: females mated to a fighter showed a reduction in longevity
Cost of reproduction
The interaction between the total number of eggs laid during a female’s lifetime and the morph of a female’s mate significantly affected female longevity (\( \hat{e} \) = 0.042, t = 3.66, P = 0.001, n = 28). Total lifetime egg production was positively correlated to female lifespan, but this relationship was steeper for females mated to scramblers than for females mated to fighters (Fig. 4c), implying that increased egg production reduced longevity more if females were mated to a fighter.
Discussion
The results of this study revealed complex environmental effects on ARP expression in the bulb mite, with maternal environmental conditions affecting male morph expression directly, but also indirectly through differential resource investment into eggs. Specifically, females that were on the rich food diet manipulated mean egg size and laid the largest eggs when mated to a competitively superior fighter—in line with Burley’s hypothesis (Burley 1986; Sheldon 2000). Females mated to fighters lived less long than females mated to scramblers. Although I did not test for a causal relationship between egg investment and longevity, this result suggests that the females, and not the males, controlled the differential resource allocation into eggs, fulfilling an important, underlying assumption of the differential allocation hypothesis (Burley 1986). Females that were on the rich diet also directly affected male morph expression, but whether this effect was due to the differential resource investment into eggs or caused by another mechanism is unclear.
The ramifications of the differential investment into eggs are that any observed sire effect on male morph expression could actually be due to females allocating resources differentially in response to the morph of their mate (Kotiaho et al. 2003). Indeed, larger eggs developed into larger tritonymphs, which were in turn more likely to develop into fighters. As a result, heritability estimates of male morph expression (e.g. Radwan 1995) could be amplified by maternal effects so that caution should be exercised against using heritability analyses where maternal effects are not factored out. Incorporating maternal effects into heritability analyses is standard practice in ecological studies on mammals (Falconer and Mackay 1996; Kruuk 2004), but not yet in studies on threshold characters (Falconer and Mackay 1996). Theory states that maternal effects can complicate evolutionary dynamics by slowing down or increasing the rate of evolutionary change of traits (Kirkpatrick and Lande 1989). Here, this means that differential maternal investment into eggs could fuel a response to sexual selection on secondary sexual traits (cf. Kotiaho et al. 2003). It is therefore important to consider maternal effects prior to attributing differences in offspring morphology to paternal effects in evolutionary studies. Also, differential maternal investment into eggs can carry over to influence the population dynamics of future generations (Benton et al. 2005). To what extent differential investment into eggs might similarly influence the demographic structure of populations with ARPs remains to be investigated. This is crucial, however, in understanding the role of maternal effects in phenotypic trait and population dynamics (Coulson et al. 2010).
Maternal investment in offspring size is also known to influence the phenotypic plasticity of offspring traits, such as the trade-off between egg size and number (Bashey 2006) or the developmental threshold of different intraspecific morphs (Michimae et al. 2009). Here, however, the developmental threshold for male morph expression, i.e. the size at which male tritonymphs are equally likely to develop into a fighter or a scrambler, was unaffected by maternal (or paternal) effects and offspring nutrition. I did find evidence of phenotypic plasticity in the reaction norm between age and size at maturity. This reaction norm was of a typical L-shape (Day and Rowe 2002): mites delayed maturation under poor food conditions to mature at a minimum size, showing low variation in size at maturity, but considerable variation in age at maturity. I found no maternal (nor paternal) effects on this reaction norm. Instead, this reaction norm only differed between scramblers and fighters, with fighters requiring a longer time to mature at the same size than scramblers. Because fighters and scramblers responded in different ways to good and bad developmental conditions, these differing responses determined the degree of male dimorphism: under favourable growth conditions, there was little male dimorphism in body size, whereas male dimorphism increased as growth conditions deteriorated (Fig. 4a). Natural selection should favour rapid development to sexual maturity, because the sooner an organism matures, the more likely it is to reproduce before dying (Williams 1966). This means that in poor environments, scramblers may be at a selective advantage as they can reach sexual maturity faster than fighters, but this advantage is less pronounced as environmental conditions improve. These morph-specific responses to changes in environmental conditions may have important implications for understanding the variation in the proportion of fighters and scramblers in bulb mite populations.
Direct paternal (genetic) effects were evident only when offspring were on the poor food diet, suggesting a gene by environment interaction. Radwan (2009) poses that male morph expression in the bulb mite is determined by a gene of large effect, but that its effect can be obscured by additional genetic and environmental impacts. However, in contrast to Radwan’s studies (Radwan 1995, 2003), the heritabilities of male morph expression in our lab populations are relatively low (0.3–0.4: Smallegange and Coulson, in press). The proportion of fighters in our lab populations varies between 70 and 80% of all males (cf. Fig. 3b), and is similar to that observed in field populations (69–72%: Radwan 1995; Radwan and Klimas 2001). Since the proportion of fighters has not increased to near fixation, the low heritabilities for our lab populations are unlikely due to lack of genetic variation (such as observed by Radwan et al. 2004), but instead indicate that the male dimorphism is a polygenic or quantitative trait.
Currently the best model available to understand the evolution and maintenance of condition-dependent, polygenic or quantitative ARPs is the environmentally cued threshold model (ET model) (Hazel et al. 1990, 2004; Tomkins and Hazel 2007). This model is based on the notion that there is genetic variation between individuals in the switch point at which development switches from one phenotypic alternative to another, in response to environmental cues. So what cues affect male morph expression in bulb mites? Population density does not directly influence male morph expression in bulb mites, unlike for example in S. berlesei and R. echinopsus where the concentration of colony pheromones directly influences male morph expression (Radwan 2001; Radwan et al. 2002). It is furthermore unlikely that direct social interaction and assessment is involved in morph determination. All juvenile mites in this study were individually isolated, but still a positive relationship between quiescent tritonymph size and probability of developing into a fighter was observed. Females could have passed information on current environmental quality onto their male offspring, since there was a direct maternal effect on male morph expression, as well as an indirect effect, as females on the rich diet laid larger eggs, which were more likely to develop into fighters. However, the most likely candidate cue is body size, because the effect size of offspring nutrition on male morph expression was the largest, and offspring nutrition and quiescent tritonymph size were highly correlated. Like bulb mite fighters, S. berlesei fighters emerge from relatively large quiescent tritonymphs (Radwan et al. 2002). S. berlesei fighters achieve this relatively greater weight gain during their tritonymph stage and not as protonymphs (the instar stage preceding the tritonymph stage) (Radwan et al. 2002). Here, bulb mite tritonymph size was predominantly determined by offspring nutrition and not by parental effects. If, like S. berlesei and other invertebrates (Emlen 2008), weight gain during the final instar stage determines male morph expression in bulb mites, it could be that under poor food conditions most male tritonymphs are unable to gain sufficient weight to reach the critical tritonymph size, or switch point (cf. Fig. 3c), to develop into a fighter. Since poor food conditions could also arise through exploitation competition at high population densities, population density could therefore also indirectly affect male morph expression.
Investigating and understanding the proximate mechanisms that underlie ARP expression is key to understanding how the resulting phenotypes will be affected by selection (Schlichting and Pigliucci 1995). The results of this study showed that complex environmental effects can underlie ARP expression. Although not yet put into practice, it is possible to include such effects in the ET model (Tomkins and Hazel 2007). According to the ET model, evolutionary change in ARP expression arises through changes in the distribution of switch points. Predictions on the direction of selection could therefore be tested by perturbing the ratio of ARPs in a population and assess whether fitness changes as predicted by the ET model, and whether the switch point evolves in the predicted direction (Brockmann and Taborsky 2008). To this end, one should first quantify the exact fitness functions of the different phenotypes (e.g. Hunt and Simmons 2001) as well the frequency distribution of body sizes (Tomkins and Hazel 2007). The ET model furthermore states that the average fitnesses of different condition-dependent ARPs are not necessarily equal (although the fitnesses of the alternatives at the switch point are equal: Hazel et al. 2004) because each individual follows a conditional, developmental pathway that maximises its fitness, which makes it unlikely that different phenotypes have equal success (Repka and Gross 1995). Hence in studies on ARPs that are influenced by gene by environment interactions, the focus should change from searching for fitness equality of ARPs (Radwan and Klimas 2001; Shuster and Wade 2003; Smallegange and Coulson, in press) to assessing the fitness functions of those ARPs. Intriguingly though, recent findings revealed that the ARPs of male bulb mites have equal, long-run stochastic growth rates across a wide range of environments characterised by different temporal sequences of good and bad habitats (Smallegange and Coulson, in press). The results of this study also revealed that the different male morphs face different trade-offs between age and size at maturity. Future research should also include the role of such trade-offs, if any, in the maintenance of ARPs as well as the scarcely investigated role of frequency-dependence (Tomkins and Hazel 2007) that can maintain the coexistence of ARPs if their expression has a condition-dependent and -independent component (Hazel et al. 2004).
References
Baayen RH, Davidson DJ, Bates DM (2008) Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 59:390–412
Bashey F (2006) Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Ecology 60:348–361
Bates D, Sarkar D (2007) lme4: linear mixed-effects models using S4 classes. R package version 0.9975-13
Benton TG, Plaistow SJ, Beckerman AP et al (2005) Changes in maternal investment in eggs can affect population dynamics. Proc R Soc Lond B 272:1351–1356
Brockmann HJ, Taborsky M (2008) Alternative reproductive tactics and the evolution of alternative allocation phenotypes. In: Oliveira RF, Taborsky M, Brockmann HJ (eds) Alternative reproductive tactics. Cambridge University Press, Cambridge, pp 25–51
Brockmann HJ, Oliveira RF, Taborsky M (2008) In: Oliveira RF, Taborsky M, Brockmann HJ (eds) Alternative reproductive tactics. Cambridge University Press, Cambridge, pp 471–489
Brody MS, Lawlor LR (1984) Adaptive variation in offspring size in the terrestrial isopod, Armadillidium vulgare. Oecologia 61:55–59
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Cohen J (1988) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
Coulson T, Tuljapurkar S, Childs DZ (2010) Using evolutionary demography to link life history theory, quantitative genetics and population ecology. J Anim Ecol 79:1226–1240
Cunningham EJ, Russell AF (2000) Egg investment is influenced by male attractiveness in the mallard. Nature 404:74–77
Day T, Rowe L (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat 159:338–350
Eberhard WG, Gutiérrez EE (1991) Male dimorphisms in beetles and earwigs and the question of developmental constraints. Evolution 45:18–28
Emlen DJ (1994) Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera, Scarabaeidae). Proc R Soc Lond B 256:131–136
Emlen DJ (2008) The roles of genes and the environment in the expression and evolution of alternative tactics. In: Oliveira RF, Taborsky M, Brockmann HJ (eds) Alternative reproductive tactics. Cambridge University Press, Cambridge, pp 83–108
Falconer DS (1989) Introduction to quantitative genetics, 3rd edn. Wiley, New York
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Pearson Education Ltd, Harlow
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369
Fox CW, Thakar MS, Mousseau TA (1997) Egg size plasticity in a seed beetle: an adaptive maternal effect. Am Nat 149:149–163
Gerson U, Capua S, Thorens D (1983) Life history and life tables of Rhizoglyphus robini Claparède (Acari: Astigmata: Acaridae). Acarologia 24:439–448
Gerson U, Cohen E, Capua S (1991) Bulb mite, Rhizglyphus robini (Astigmata: Acaridae) as an experimental animal. Exp Appl Acarol 12:103–110
Gil D, Graves J, Hazon N et al (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Hazel WN, Smock R, Johnson MD (1990) A polygenic model for the evolution and maintenance of conditional strategies. Proc R Soc Lond B 242:181–187
Hazel WN, Smock R, Lively CM (2004) The ecological genetics of conditional strategies. Am Nat 163:888–900
Hunt J, Simmons LW (2000) Maternal and paternal effects on offspring phenotype in the dung beetle Onthophagus taurus. Evolution 54:936–941
Hunt J, Simmons LW (2001) Status-dependent selection in the dimorphic beetle Onthophagus taurus. Proc R Soc Lond B 268:2409–2414
Kirkpatrick LM, Lande R (1989) The evolution of maternal characters. Evolution 43:485–503
Kolm N (2001) Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc R Soc Lond B 268:2229–2234
Kotiaho JS, Simmons LW, Hunt J et al (2003) Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am Nat 161:852–859
Kruuk LEB (2004) Estimating genetic parameters in wild populations using the ‘animal model’. Philos Trans R Soc Lond B 359:873–890
Michimae H, Nishimura K, Tamori Y, Wakahara M (2009) Maternal effects on phenotypic plasticity in larvae of the salamander Hynobius retardatus. Oecologia 160:601–608
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, Oxford
Oliveira RF, Taborsky M, Brockmann HJ (eds) (2008) Alternative reproductive tactics. Cambridge University Press, Cambridge
Plummer M, Best N, Cowles K et al. (2006) Coda: output analysis and diagnostics for MCMC. R package version 0.10-7
Radwan J (1993) The adaptive significance of male polymorphism in the acarid mite Caloglyphus berlesei. Behav Ecol Sociobiol 33:201–208
Radwan J (1995) Male morph determination in 2 species of acarid mites. Heredity 74:669–673
Radwan J (2001) Male morph determination in Rhizoglyphus echinopus. Exp Appl Acarol 25:143–149
Radwan J (2003) Heritability of male morph in the bulb mite, Rhizoglyphus robini (Astigmata, Acaridae). Exp Appl Acarol 29:109–114
Radwan J (2009) Alternative mating tactics in acarid mites. Adv Stud Behav 39:185–208
Radwan J, Klimas M (2001) Male dimorphism in the bulb mite, Rhizoglyphus robini: fighters survive better. Ethol Ecol Evol 12:69–79
Radwan J, Czyż M, Konior M et al (2000) Aggressiveness in two male morphs of the bulb mite Rhizoglyphus robini. Ethology 106:53–62
Radwan J, Unrug J, Tomkins JL (2002) Status-dependence and morphological trade-offs in the expression of a sexually selected character in the mite, Sancassania berlesei. J Evol Biol 15:744–752
Radwan J, Unrug J, Śnigórska K et al (2004) Effectiveness of sexual selection in preventing fitness deterioration in bulb mite populations under relaxed natural selection. J Evol Biol 17:94–99
Repka J, Gross MR (1995) The evolutionarily stable strategy under individual condition and tactic frequency. J Theor Biol 176:27–31
Ryan MJ, Pease CM, Morris MR (1992) A genetic polymorphism in the swordtail Xiphophorus nigrensis. Testing the prediction of equal fitnesses. Am Nat 139:21–31
Schlichting CD, Pigliucci M (1995) Gene regulation, quantitative genetics and the evolution of reaction norms. Evol Ecol 9:154–168
Sheldon B (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Shuster SM, Wade MJ (1991) Equal mating success among male reproductive strategies in a marine isopod. Nature 350:608–610
Shuster S, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Smallegange IM, Coulson T (in press) The stochastic demography of two coexisting male morphs. Ecology
Tomkins JL, Hazel W (2007) The status of the conditional evolutionarily stable strategy. Trends Ecol Evol 22:522–528
Tomkins JL, LeBas NR, Unrug J et al (2004) Testing the status-dependent ESS model: population variation in fighter expression in the mite Sancassania berlesei. J Evol Biol 17:1377–1388
Wedell N (1996) Mate quality affects reproductive effort in a paternally investing species. Am Nat 148:1075–1088
Williams GC (1966) Adaptation and natural selection. Princeton University Press, Princeton
Acknowledgments
I thank Tim Coulson and Mark Roberts for discussion and comments, and The Netherlands Organisation for Scientific Research for funding (Rubicon Fellowship). All experimental work conducted in this study conforms to the legal requirements of the UK.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smallegange, I.M. Complex environmental effects on the expression of alternative reproductive phenotypes in the bulb mite. Evol Ecol 25, 857–873 (2011). https://doi.org/10.1007/s10682-010-9446-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-010-9446-6