Abstract
Species in similar habitats are often similar in morphology or behaviour, attributed to adaptation to similar environmental selection pressures, sometimes mediated by competitive interactions. For passerine songs, similarity of phenotype in identical habitats and character displacement have been documented, the former due to adaptation to the acoustics of the habitat, and the latter due to competition for acoustic space among species. If these phenomena are widespread, they should lead to community convergence of bird songs. Here, we test if passerine communities in similar habitats converge in song attributes or in acoustic differentiation among species. We compared the songs of European and North American Mediterranean climate passerine communities in open and closed habitats. Song frequency varied across different habitats but not continents. This was independent of both phylogeny and body size, indicating community convergence due to acoustic adaptation, rather than species sorting or similarity as a by-product of another type of ecological convergence. We found little evidence for regular spacing in song features among species, as would be expected if acoustic competition shapes within-community structure. However, for one of five song components, the open habitat communities showed a similar distribution of phenotypes on each continent. The proportion of interspecific variation in song explained by these effects was small. The fact that songs are complex signals that vary in many dimensions may explain why competition for acoustic space seems to be of small importance in structuring songs in these passerine communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Similar habitats often promote convergence, defined as evolution towards similar phenotypes from distinct ancestral forms. Spectacular examples of morphological convergence among species abound (for example the New World and Old World vultures: Wink 1994; Seibold and Helbig 1995). Convergence may also happen at the level of the ecological community and, although ancestral states of communities are hard to infer, it is possible to test for convergence by comparing communities of two or more habitat types in different regions of the world (e.g. Schluter 1986; Pavoine et al. 2004). On the assumption that each region’s communities established independently, any parallel differences across habitats within regions is strong evidence for convergence. An extreme result of community convergence is the production of communities that are significantly similar in the distribution of phenotypes. For example, finch communities in similar habitats of different continents have distributions of body size and beak shape that are more similar than expected by chance (Schluter 1986). Such similarity of distributions implicates competition among species (Schluter 1986, 1990).
Community convergence and community structure have been studied especially regarding ecomorphological traits (Emerson and Gillespie 2008), but both phenomena may apply to communication signals (Gröning and Hochkirch 2008). Physical characteristics, such as light filtering or acoustic properties, vary among habitats and influence how effectively different signals are broadcast and perceived (Bradbury and Vehrencamp 1998). While the role of the environment generally favours the same kind of signal for all species in the community, competition between species may result in dispersion of signals. This may happen either indirectly because morphological changes driven by ecological competition impact the signal (e.g. body size affects the frequency of acoustic signals; Wallschläger 1980; Ryan and Brenowitz 1985), or because of direct signal interference or similarity (reviewed in Gröning and Hochkirch 2008). In the latter case, species may compete for “signal space” to avoid signal masking or species misidentification, which is especially relevant for long-range mating and territorial signals (Miller 1982; Nelson and Marler 1990; Chek et al. 2003). For example, in some South-American frog communities the vocalizations of the different species may be more widely and regularly spaced in acoustic space than random, suggesting community structure due to interspecific acoustic competition (Chek et al. 2003). Here, we test for community convergence and similarity of spacing among species in an important class of communication signals, birdsongs.
Passerine songs are acoustic signals used mostly in long-range communication for mate attraction and territoriality (Catchpole and Slater 2008) and can potentially be shaped by interspecific interactions. Some birds avoid singing when other species that use the same frequency range are singing (Planqué and Slabbekoorn 2008), suggesting acoustic masking by heterospecific song. Species singing similar songs may also suffer fitness costs due to ambiguity in species recognition. For example, blue tits (Cyanistes caeruleus) suffer from territorial aggressiveness by the larger bodied great tits (Parus major), and in sympatry have character displacement of song (Doutrelant and Lambrechts 2001), which reduces interspecific aggression (Doutrelant et al. 2000). Collared flycatchers (Ficedula albicollis) in sympatry with pied flycatchers (F. hypoleuca) also show character displacement of song, which in these species is primarily an epigamic signal, putatively to avoid hybridisation (Wallin 1986), but many male pied flycatchers learn mixed song types in sympatry (i.e. song containing both pied and collared syllables), which leads to hybridisation (Qvarnström et al. 2006). Sympatric antbird species have also diverged in song more than allopatric species (Seddon 2005). More general acoustic competition from the community may also influence the optimal song phenotype of a species. For example, Bewick’s wrens (Thryomanes bewickii) and American tree sparrows (Spizella arborea) have increased song variability where fewer sympatric passerine species exist (Kroodsma 1985; Naugler and Ratcliffe 1994), although this may be a consequence of other environmental factors associated with the decrease in species richness (e.g. higher local densities, Price 2008, chapter 12). While interspecific competition for acoustic space may affect birdsong, some studies did not find effects of sympatric species on song (Hunter and Krebs 1979; Espmark 1999), no character displacement when closely related species coexist in sympatry (Lohr 2008), and no consistent within-species reduction of variation in the song traits that are potentially more informative for species recognition (Nelson 1989). Also, interspecific territoriality sometimes leads to convergent, rather than divergent, character displacement of song (Cody 1969; Qvarnström et al. 2006; Price 2008, chapter 14). Thus, the importance of interspecific acoustic competition in structuring song phenotypes within communities remains unknown.
We use passerine communities of open and closed habitats in two Mediterranean climate regions—southern Europe and California—to ask if songs have converged between communities in identical habitats. Testing for convergence of phenotypes is straightforward: in a two-way ANOVA (habitat × continent) songs should differ among habitat types, but not among continents (Schluter 1986). We test this controlling for phylogeny, so that similarity between identical-habitat communities reflects evolution rather than related species colonizing similar habitats (Ryan and Brenowitz 1985). To test for similarity in community structure we compare the distribution of phenotypes between communities that inhabit matched habitats (Schluter 1990). We also ask if song phenotypes are uniformly distributed within each community, as could result if song evolves due to acoustic competition among sympatric species.
Methods
Passerine communities
We studied the passerine communities of open and closed Mediterranean climate habitats in California and Provence described by Cody (1975) and Blondel (1979, 1981), respectively, and compiled in Blondel et al. (1984). These communities are representative of mainland Californian and European Mediterranean habitats. We chose to study these communities because they were previously used in several studies of ecomorphology and community convergence (e.g. Cody and Mooney 1978; Blondel et al. 1984; Pavoine et al. 2004), and because song recordings are available for all species.
We used the species lists for the open (sagebrush, matorral) and closed (woodland) habitats (codes 2 and 5 in Blondel et al. 1984). Lists for two habitat types of intermediate vegetation density are also available, but we did not use them because most species in these intermediate habitats also occur in either the open or closed habitats. We excluded the Corvidae because corvids in these areas do not have long-range songs, and because they are much larger than the other passerines so that their calls sound distinctively different and should not be important for acoustic competition with the smaller passerines. The dataset comprises 7 and 23 species for open and closed habitats in California, two of which are common to both habitat types, and 8 and 17 species for these habitats in Europe, one of which is common to both habitat types (Fig. 1). There are no species in common between the European and Californian communities.
Phylogeny of the passerine species studied, obtained collating information from different molecular phylogenies (see text for sources). Symbols indicate open (●○) or closed (■□) habitat, and shading indicates Europe (□○) or California (■●). Lengths from each node to tips are drawn as number of daughter species minus one
Song measurements
We created spectrograms (FFT length of 512 and overlap of 87.5% on sound files with a sampling frequency of 22,050 Hz, corresponding to a resolution of 43 Hz and 2.9 ms) for all songs (not other vocalizations) in the recordings of Perrins (1998) and the Cornell Laboratory of Ornithology (1992) with the software Avisoft-SASLab Pro v.4.40 (Avisoft Bioacoustics, Berlin). Songs were identified as groups of syllables separated from other songs by more than 0.5 s and, within-songs, syllables were identified as isolated sound elements or groups of elements closely spaced comparatively to other syllables. On average we measured four songs and 47 syllables per species (Appendix Table 4).
We measured 11 song traits that together quantify variation in song phonology among species. Syntax was quantified as the proportion of syllables that are repeated (i.e. trilled) and song length measured as the average duration of songs. The remaining measurements were made for every syllable and then averaged for each species. Peak frequency was measured as the frequency with maximum amplitude in the power spectrum of the syllable. Frequency bandwidth was computed as the maximum minus minimum frequencies, where maximum and minimum frequencies are the frequencies at which sound amplitude drops below minus 24 dB relatively to maximum amplitude in the power spectra (e.g. Podos 1997). The other measurements were made on spectrograms following the methods in Cardoso and Mota (2007). Briefly, length of syllables and length of intervals are the durations of syllables and of intervals separating syllables within songs, elements per syllable is the number of temporally separated sounds within each syllable, number of frequency inflections is the number of times a rising frequency modulation is followed by a descending one or vice versa, and two voiced sounds, harmonics, and rattles are the proportion of each syllable’s length that contains, respectively, two voices (two simultaneous sounds produced by the two sides of the syrinx and modulated in frequency independently of each other), harmonics (octaves of the fundamental frequency), or rattles (sometimes referred to as ‘buzzes’: broadband and harsh sounding fast modulations or repetitions within syllables, e.g. last syllable in Fig. 4D of Cardoso and Mota 2007). In two species (Anthus campestris and Lanius meridionalis) all songs were monosyllabic, and therefore no intervals between syllables could be measured. In these two species the length of intervals was set to the threshold to identify different songs (0.5 s) because intervals between syllables (in this case equivalent to intervals between songs) are comparatively very long. Average values of all measurements for each species and sample sizes are given in the Appendix Table 4.
Most song measurements were approximately normally distributed, but song length, syllable duration, interval duration and number of elements were not (Kolmogorov–Smirnov tests against a normal distribution: all Z > 1.47, all N = 52, all P < 0.03). These four measurements were log transformed (after transformation, all Z < 1.26, all P > 0.08), and the transformed values used instead of the original ones. We reduced the 11 measurements into a set of orthogonal axes by principal component analysis (PCA) on the correlation matrix of the 11 measurements across all species.
Relations with body size
We obtained body masses from Dunning (2008) (male values were used when body mass is given separately for males and females, Appendix Table 4). The distribution of body masses was right-skewed (Kolmogorov–Smirnov Z = 2.09, N = 52, P < 0.01), and therefore we log transformed it (after transformation, Z = 0.95, P = 0.33). We tested for a relation between body mass and each of the song principal components (PCs) using general least squares (GLS) regressions controlling for phylogeny (Pagel 1999). GLS regressions were run with the software BayesTraits (M. Pagel and A. Meade, available from www.evolution.rdg.ac.uk), each time estimating λ to adjust the phylogenetic correction to the degree of phylogenetic signal in the data (Pagel 1999; Freckleton et al. 2002).
To run the GLS regressions we assembled a phylogeny (Fig. 1) based primarily on Barker et al. (2004) and expanded using several other molecular phylogenies (Alström et al. 2006; Barhoum and Burns 2002; Bleiweiss 2007; Blondel et al. 1996; Carson and Spicer 2003; Groth 1998; Johnson and Lanyon 1999; Lovette and Bermingham 2002; Sibley and Ahlquist 1990; Voelker and Spellman 2004; Yuri and Mindell 2002). Because this phylogeny collates information from multiple sources, the original branch lengths cannot be used. As an approximation, as a first step we set branch lengths at each node proportionally to the number of daughter species of that node (e.g. Grafen 1989; Garland et al. 1992), as drawn in the Fig. 1, and then estimated δ (a parameter that scales relative lengths from root to tips to fit the phenotypic data, Pagel 1999) for each regression.
Differences among habitat types and continents
We tested for differences in song among habitat types and among continents using multiple GLS regressions controlling for phylogeny. In these multiple GLS regressions the dependent variable was a song PC or body mass, and the independent variables were habitat type, continent, and their interaction term. The three species that occur both in open and closed habitats were not used in this analysis, and the phylogeny in the Fig. 1 was modified accordingly. We also repeated the song regressions adding body mass as an independent variable, to check if song convergence is mediated by body size.
Distribution of phenotypes
We compared the distribution of phenotypes between communities in the same habitat type using 2-sample Kolmogorov–Smirnov tests and testing for significance at the lower tail (Schluter 1990). Unlike its most common usage, which looks at the upper tail to test for differences between samples, we look at the lower tail to test for “significant similarity”. A Kolmogorov–Smirnov Z lower than the 5th percentile of its distribution indicates that the two samples have distributions more similar than random (at α = 0.05).
We also tested if the distribution of phenotypes in each community is significantly similar to a uniform distribution using 1-sample Kolmogorov–Smirnov tests. As above, we evaluated significance at the lower tail, which tests if distribution is significantly similar to uniformity. This would indicate that the spacing of phenotypes within a community is significantly more even than random draws from a uniform distribution, as could happen if song evolves due to acoustic competition among sympatric species. Apart from the GLS regressions, all statistical tests were done in SPSS v13.0.
Results
Song PCA
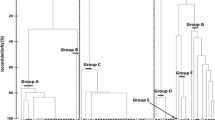
A PCA on the correlation matrix of the 11 song measurements returned five PCs with eigenvalues larger than one, which together explained 76% of the variation in measurements among species (Table 1). PC1 reflects mostly songs with long and complex syllables. PC2 quantifies songs with harmonics, large bandwidths, and short syllables. PC3 quantifies songs with few syllable repetitions and frequency inflections, but many rattles. PC4 is related mostly to high song frequency, and also to two voiced sounds (two simultaneous and independently modulated sounds). PC5 is characterized by long songs and long intervals between syllables. We use these five PCs in the following analyses. Figure 2 shows the distribution of these five PCs in each of the four communities.
Relations with body size
Body mass was negatively related to PC4 (GLS regression: standardized β = −0.44, N = 52, P = 0.004); i.e. larger species sing lower frequency songs. Body mass also tended to vary with PC1 (standardized β = 0.33, P = 0.02) and PC2 (standardized β = −0.28, P = 0.03), but these trends are not significant after correcting for multiple comparisons (5 PCs, Bonferroni adjusted α = 0.01). Body mass was not related with PC3 and PC5 (both |β| < 0.15, P > 0.30).
Differences among habitat types and continents
Table 2 shows the results of multiple GLS regressions of each song PC or body mass on habitat type and continent. For song PC4 the full regression model was significant (F 3,45 = 11.79, P = 0.001), and explained 21% of the variation. This was due to an effect of habitat type on song PC4 (standardized partial β = −0.45, P = 0.002). Song PC 4 did not differ among continents and the interaction term was not significant either (Table 2). The regression of song PC1 was also significant (R 2 = 0.18, F 3,45 = 10.04, P = 0.003) due to the interaction term only (P = 0.01), not to any of the main effects (both |β| < 0.13, both P > 0.40). For the remaining song PCs and also for body mass there were no differences between habitat types or continents (all |β| < 0.17, all P > 0.26, Table 2).
These results were not due to differences in body mass among communities. When including body mass as a covariate the above results are stronger (song PC4: F 4,44 = 28.08, P < 0.001, β of habitat = −0.57, P < 0.001; song PC1: F 4,44 = 19.52, P < 0.001, interaction of continent and habitat, P = 0.006). Including body mass in the regression there was also a tendency for the model of song PC2 to be significant (F 4,44 = 6.58, P = 0.014) due to the effect of mass (β of mass = −0.28, P = 0.051, all other |β| < 0.11 and P > 0.21), but this was not significant after correcting for multiple comparisons (5 PCs, Bonferroni adjusted α = 0.01).
Distributions of phenotypes
The distribution of song PC5 was significantly similar between the two open habitat communities (2-samples Kolmogorov–Smirnov test, Z = 0.41, lower-tail P = 0.005, Bonferroni adjusted α for six tests in this pair of communities = 0.008), and PC4 had a non-significant tendency to be identically distributed in these two communities (Z = 0.55, lower-tail P = 0.08, Table 3, Fig. 2). In all other cases, the distributions of song PCs and also body sizes were not significantly similar between communities in identical habitat type (all Z > 0.58, all lower-tail P > 0.11, Table 2).
The distribution of song PC3 and PC5 were similar to a perfectly spaced uniform distribution in the California open habitat community (1-sample Kolmogorov–Smirnov, Z = 0.49, lower-tail P = 0.03, and Z = 0.44, lower-tail P = 0.01, respectively), but these results do not withstand correction for multiple tests. For the other communities, PCs and body mass distributions were not significantly similar to a uniform distribution (all Z > 0.54, all P > 0.07).
Discussion
We asked if birdsongs converged between passerine communities in similar Mediterranean climate habitats, and if within communities song phenotypes are structured so as to reduce song similarity between sympatric species.
One axis of song variation, song PC4, related to song frequency, differed significantly between open and closed habitat communities (lower frequency songs in closed habitats), but not among the two continents. Song PC4 was also correlated with the species’ body size (larger species singing lower frequency songs, e.g. Wallschläger 1980; Ryan and Brenowitz 1985), but the difference between habitats was independent of body size, which did not differ between habitat types. This result was also independent of phylogeny, implying that songs converged between communities in similar habitats in the two continents, rather than those habitats being colonized by related species. Compared to open habitats, closed habitats propagate low frequency sounds more efficiently than high frequency sounds, mostly because of vegetation scattering high frequencies (Morton 1975; Bradbury and Vehrencamp 1998), and noise profiles of forested environments usually contain more insect-made noise at high frequencies (Ryan and Brenowitz 1985). Both these factors are known to influence the optimal frequency for birdsong. For example, grey-breasted wood-wrens (Henicorhina leucophrys) in environments with high frequency cicada choruses sing lower-frequency songs than in otherwise acoustically identical habitats (Dingle et al. 2008). Accordingly, studies in different parts of the world have consistently found that species inhabiting closed habitats sing at lower frequencies (Chappuis 1971; Morton 1975; Ryan and Brenowitz 1985; reviewed in Boncaraglio and Saino 2007). Therefore, the similarities we found between communities (both closed habitat communities having lower frequencies, and the opposite for open-habitat communities) can be explained by acoustic adaptation of the individual species to their habitat.
We found only sparse evidence of structuring at the community level. We found that the distribution of phenotypes across one axis of song variation, characterized by the length of songs and of intervals between syllables, was significantly similar in the two open habitat communities. But in all other cases the distribution of songs among species was not more similar for communities in identical habitats than expected by chance. Acoustic competition between species could result in regularly spaced phenotypes within communities (e.g. Chek et al. 2003), even if that does not translate into strong similarity across communities. But we only found suggestive evidence of uniformly distributed song phenotypes for two of the axes of song variation in the smallest of the four passerine communities.
These results suggest that community-level effects are not of major importance in structuring song phenotypes within passerine communities. There are examples in the literature of acoustic competition among species either causing character shift (Wallin 1986; Doutrelant and Lambrechts 2001; Seddon 2005; see also Kirschel et al. 2009, for an example with a non-passerine bird) or limiting song variation (Kroodsma 1985; Naugler and Ratcliffe 1994) that, if common, could shape community structure. There are also some studies that failed to find such interspecific effects (Hunter and Krebs 1979; Espmark 1999; Lohr 2008). A whole-community comparison had not been done to evaluate the importance of heterospecific acoustic competition in structuring song phenotypes within communities, and ours suggests it may play a minor role. We suggest that song evolution due to community composition, or species sorting based on song, may happen but generally be of a localised nature that does not shape the community overall. For example, acoustic competition could affect some closely related species (as in Ficedula flycatchers, Wallin 1986) and, since passerine songs are often complex signals that vary in many dimensions, most other species be sufficiently dissimilar that problems of species recognition are not important. Although similar songs can evolve in different lineages (e.g. Price et al. 2007), song evolution by sexual selection generally makes species diverge from each other rather than converge (e.g. Irwin 2000), and therefore the chances that unrelated species evolve songs to be very alike should be small. In addition, it was argued that, due to how vertebrates perceive and discriminate stimuli, song similarities are more likely to interfere with detection (when heterospecifics sing simultaneously using similar frequencies) than to cause species misidentification (Brumm and Slabbekoorn 2005), and temporal avoidance of heterospecifics may alleviate this problem (Planqué and Slabbekoorn 2008; Luther 2008).
We conclude that some song traits converge between communities in identical habitats, attributable to acoustic adaptation of the species individually. However, we did not find evidence for important effects of interspecific acoustic competition in these communities. Nevertheless, further research may be warranted in more diverse and densely populated passerine communities, such as tropical rainforests where many species sing simultaneously (Planqué and Slabbekoorn 2008; Luther 2009), and where interspecific acoustic competition or interference with detection may be more important selection pressures.
References
Alström P, Ericson PGP, Olsson U, Sundberg P (2006) Phylogeny and classification of the avian superfamily Sylvioidea. Mol Phylogenet Evol 38:381–397
Barhoum DN, Burns KJ (2002) Phylogenetic relationships of the wrentit based on mitochondrial cytochrome b sequences. Condor 104:740–749
Barker FK, Cibois A, Schikler P, Feinstein J, Cracraft J (2004) Phylogeny and diversification of the largest avian radiation. Proc Nat Acad of Sci USA 101:11040–11045
Bleiweiss R (2007) On the ecological basis of interspecific homoplasy in carotenoid-bearing signals. Evolution 61:2861–2878
Blondel J (1979) Biogéographie et écologie. Masson, Paris
Blondel J (1981) Structure and dynamics of bird communities in Mediterranean habitats. In: di Castri F, Goodall DW, Specht RL (eds) Mediterranean-type shrublands. Elsevier, Amsterdam, pp 361–385
Blondel J, Vuilleumier F, Marcus LF, Terouanne E (1984) Is there ecomorphological convergence among mediterranean bird communities of Chile, California, and France? In: Hecht MK, Wallave B, MacIntyre RJ (eds) Evolutionary biology. Plenum Press, New York, pp 141–213
Blondel J, Catzeflis F, Perret P (1996) Molecular phylogeny and the historical biogeography of the warblers of the genus Sylvia (Aves). J Evol Biol 9:871–891
Boncaraglio G, Saino N (2007) Habitat structure and the evolution of birdsong: a meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct Ecol 21:134–142
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer Associates, Sunderland
Brumm H, Slabbekoorn, H (2005) Acoustic communication in noise. Adv Stud Behav 35:151–209
Cardoso GC, Mota PG (2007) Song diversification and complexity in canaries and seedeaters (Serinus spp.). Biol J Linn Soc 92:183–194
Carson RJ, Spicer GS (2003) A phylogenetic analysis of the emberizid sparrows based on three mitochondrial genes. Mol Phylogenet Evol 29:43–57
Catchpole CK, Slater PBJ (2008) Bird song. Biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Chappuis C (1971) Un exemple de l’influence du milieu sur les émissions vocales des oiseaux: l’évolution des chants en fôret équatoriale. Terre et Vie 25:183–202
Chek AA, Bogart JP, Lougheed SC (2003) Mating signal partitioning in multi-species assemblages: a null model test using frogs. Ecol Lett 6:235–247
Cody ML (1969) Convergent characteristics in sympatric species: a possible relation to interspecific competition and aggression. Condor 71:222–239
Cody ML (1975) Towards a theory of continental species diversities: bird distributions over mediterranean habitat gradients. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press, Cambridge, MA, pp 214–257
Cody ML, Mooney HA (1978) Convergence versus nonconvergence in mediterranean-climate ecosystems. Ann Rev Ecol System 9:265–321
Cornell Laboratory of Ornithology (1992) Peterson Field Guides, Western Bird Songs. Houghton Mifflin, Boston, MA, and Cornell Laboratory of Ornithology, Ithaca, NY
Dingle C, Halfwerk W, Slabbekoorn H (2008) Habitat-dependent song divergence at subspecies level in the grey-breasted wood-wren. J Evol Biol 21:1079–1089
Doutrelant C, Lambrechts MM (2001) Macrogeographic variation in song–a test of competition and habitat effects in blue tits. Ethology 107:533–544
Doutrelant C, Leitão A, Otter K, Lambrechts MM (2000) Effect of blue tit song syntax on great tit territorial responsiveness—an experimental test of the character shift hypothesis. Behav Ecol Sociobiol 48:119–124
Dunning JB (2008) CRC handbook of avian body masses. Taylor & Francis, Boca Raton
Emerson BC, Gillespie RG (2008) Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol Evol 23:619–630
Espmark Y (1999) Song of the snow bunting (Plectrophenax nivalis) in areas with and without sympatric passerines. Can J Zool 77:1385–1392
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726
Garland T Jr, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Grafen A (1989) The phylogenetic regression. Phil Trans Royal Soc Lond B 326:119–157
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Quart Rev Biol 83:257–282
Groth JG (1998) Molecular phylogenetics of finches and sparrows: consequences of character state removal in cytochrome b sequences. Mol Phylogenet Evol 10:377–390
Hunter ML, Krebs JR (1979) Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. J Anim Ecol 48:759–785
Irwin DE (2000) Song variation in an avian ring species. Evolution Int J org Evolution 54:998–1010
Johnson KP, Lanyon SM (1999) Molecular systematics of the grackles and allies, and the effect of additional sequence (cyt b and nd2). Auk 116:759–768
Kirschel ANG, Blumstein DT, Smith TB (2009) Character displacement of song and morphology in African tinkerbirds. Proc Nat Acad Sci USA 106:8256–8261
Kroodsma DE (1985) Geographic variation in songs of the Bewick’s wren: a search for correlations with avifaunal complexity. Behav Ecol Sociobiol 16:143–150
Lohr B (2008) Pitch-related cues in the songs of sympatric mountain and black-capped chickadees. Behav Proc 77:156–165
Lovette IJ, Bermingham E (2002) What is a wood-warbler? Molecular characterization of a monophyletic Parulidae. Auk 119:695–714
Luther DA (2008) Signaller: receiver coordination and the timing of communication in Amazonian birds. Biol Lett 4:651–654
Luther DA (2009) The influence of the acoustic community on songs of birds in a neotropical rain forest. Behav Ecol 20:864–871
Miller EH (1982) Charater and variance shift in acoustic signals in birds. In: Kroodsma DE, Miller EH (eds) Acoustic Communication in Birds. Academic Press, New York & London, pp 253–295
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Naugler CT, Ratcliffe L (1994) Character release in bird song: a test of the acoustic competition hypothesis using American tree sparrows Spizella arborea. J Avian Biol 25:142–148
Nelson DA (1989) The importance of invariant and distinctive features in species recognition of bird song. Condor 91:120–130
Nelson DA, Marler PE (1990) The perception of birdsong and an ecological concept of signal space. In: Berkley MA, Stebbins WC (eds) Comparative Perception Vol. II: Complex Signals. John Wiley & Sons, New York, pp 443–478
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Pavoine S, Dufour A-B, Chessel D (2004) From dissimilarities among species to dissimilarities among communities: a double principal coordinate analysis. J Theor Biol 228:523–537
Perrins CM (ed) (1998) The Complete Birds of the Western Paleartic CD-ROM Version 1.0. Oxford University Press, Oxford
Planqué R, Slabbekoorn H (2008) Spectral overlap in songs and temporal avoidance in a Peruvian bird assemblage. Ethology 114:262–271
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551
Price TD (2008) Speciation in birds. Robert & Company Publishers, Greenwood
Price JJ, Friedman NR, Omland EO (2007) Song and plumage evolution in the new world orioles (Icterus) show similar lability and convergence in patterns. Evolution 61:850–863
Qvarnström A, Haavie J, Saether SA, Eriksson D, Part T (2006) Song similarity predicts hybridization in flycatchers. J Evol Biol 19:1202–1209
Ryan MJ, Brenowitz EA (1985) The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat 126:87–100
Schluter D (1986) Tests for similarity and convergence of finch communities. Ecology 67:1073–1085
Schluter D (1990) Species-for-species matching. Am Nat 136:560–568
Seddon N (2005) Ecological adaptation and species recognition drives vocal evolution in neotropical suboscine birds. Evolution 59:200–215
Seibold I, Helbig AJ (1995) Evolutionary history of New and Old World vultures inferred from nucleotide sequences of the mitochondrial cytochrome b gene. Phil Trans Royal Soc Lond B 350:163–178
Sibley CG, Ahlquist JE (1990) Phylogeny and classification of birds: a study in molecular evolution. Yale University Press, New Haven
Voelker G, Spellman GM (2004) Nuclear and mitochondrial DNA evidence of polyphyly in the avian superfamily Muscicapoidea. Mol Phylogenet Evol 30:386–394
Wallin L (1986) Divergent character displacement in the song of two allospecies: the pied flycatcher Ficedula hypoleuca, and the collared flycather Ficedula albicollis. Ibis 128:251–259
Wallschläger D (1980) Correlation of song frequency and body weight in passerine birds. Experientia 36:412
Wink M (1994) Phylogeny of old and new world vultures (Aves: Accipitridae and Cathartidae) inferred from nucleotide sequences of the mitochondrial cytochrome b gene. Z Naturforschung C 50:868–882
Yuri T, Mindell DP (2002) Molecular phylogenetic analysis of Fringillidae, “New World nine-primaried oscines” (Aves: Passeriformes). Mol Phylogenet Evol 23:229–243
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Cardoso, G.C., Price, T.D. Community convergence in bird song. Evol Ecol 24, 447–461 (2010). https://doi.org/10.1007/s10682-009-9317-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-009-9317-1