Abstract
Variation in local environments may lead to variation in the selection pressures and differentiation among local populations even at microgeographic scale. We investigated variation in temperature-induced plasticity in larval life-history traits among populations of an isolated pool frog (Rana lessonae) metapopulation in Central Sweden. Successful breeding of this northern fringe metapopulation is highly dependent on early summer temperature, however, the metapopulation shows very little variation in molecular genetic markers suggesting limited potential for local differentiation. We exposed larvae from three closely-located populations to two temperatures (20 and 25°C) in laboratory to investigate their growth and development responses to temperature variation. In general, larvae exposed to warmer temperature experienced higher survival and metamorphosed faster, but at a smaller size than those at low temperature. We found differences among the populations in both trait mean values and in the plastic responses. Among-family variation within populations was found in growth rate and time to metamorphosis, as well as in plasticity suggesting that these traits have a capacity to evolve. Our results indicate ample phenotypic variation within and among these closely-located populations despite the low molecular genetic variation. The differences in pond temperature characteristics detected in the study in the three localities may suggest that differential selection is acting in the populations. The strong differentiation found in the larval traits implies that understanding the factors that influence the potential of the populations to adapt to environmental changes may be essential for successful conservation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenotypic plasticity, the capacity of a genotype to produce distinct phenotypes under different environmental conditions, is a common and powerful method of adaptation in nature (reviews in Scheiner 1993; Pigliucci 2001; DeWitt and Scheiner 2004). Its maintenance relies on the fact that a plastic phenotype will perform better in a range of environments as compared to a habitat specialist with a fixed phenotype. Costs associated with the plastic responses are predicted to prevent the generalisation of plasticity (DeWitt et al. 1998). These costs can be mainly a consequence of maintenance of plastic mechanisms, be related to the development of nonadaptive plastic responses in incorrect environments, or to detrimental effects that plasticity in one trait causes in the expression of other traits in a specific environment (DeWitt et al. 1998; Pigliucci 2001). Differences in environmental heterogeneity may lead to variation among populations, and populations and species experiencing higher environmental variation are expected to show greater amounts of plasticity (Scheiner 1993; Van Buskirk 2002).
Populations have to harbor enough genetic variation to have the potential to respond evolutionarily to environmental variation (Willi et al. 2006), and population isolation from the main distribution area of a species tends to reduce genetic variation especially at the distribution limits (Blows and Hoffmann 1993; Hampe and Petit 2005). The vast majority of studies on plastic responses have been conducted using only one population, and studies examining among-population differentiation have mainly considered populations that are well geographically segregated (reviews in Pigliucci 2001; DeWitt and Scheiner 2004, but see e.g., Van Buskirk and Arioli 2005; Lind and Johansson 2007; Bell and Galloway 2008). Environmental characteristics often vary at small spatial scales, which may promote the evolution of population-specific reaction norms at a finer scale. Hence, studies of plasticity at small spatial scale can contribute to a better understanding of population dynamics and differentiation in heterogeneous environments (Berg et al. 2005; Van Buskirk and Arioli 2005).
Larval amphibians are a good model group for studying the microevolutionary effects of environmental variation (Beebee 2005). Amphibians generally disperse within short distances, and they often show fidelity to the breeding pond (Lemckert 2004). As a result, gene flow between populations can be low, which is one of the main conditions for population differentiation at small geographic scales (Hendry et al. 2001). Breeding ponds of amphibians are also characterised by large variation in environmental conditions (e.g., hydroperiod, temperature, predators) that facilitates the maintenance of plastic life histories, and plastic responses to environmental variation are widespread in amphibian larvae (reviews in Newman 1992; Gotthard and Nylin 1995). However, the majority of these studies have considered only one population or examined populations at large geographic scales, and only few studies have been conducted at smaller spatial scales (e.g., Merilä et al. 2004; Skelly 2004; Van Buskirk and Arioli 2005; Lind and Johansson 2007).
Variation in local temperature conditions is expected to select for increased variation in thermal reaction norms among populations (Kingsolver and Gomulkiewicz 2003; Kingsolver et al. 2007). In larval amphibians, higher water temperature usually leads to earlier metamorphosis, at smaller size, whereas lower water temperatures lead to a later metamorphosis at larger size (Atkinson 1996). This variation in metamorphic traits may have strong effects on later fitness as early metamorphosis and large size at metamorphosis are favoured because of their positive effects on juvenile survival and adult fecundity (Wells 2007). Temperature variation is especially relevant for populations exposed to strong time constraints, such as those living at high latitudes, where time for larval development is limited by the short growth season and pond freezing (Smith-Gill and Berven 1979; Merilä et al. 2004).
In this study we focused on microgeographic variation of plastic responses in an isolated anuran metapopulation by examining among- and within-population variation in temperature-induced plasticity in a metapopulation of pool frogs (Rana lessonae) in central Sweden. This northern fringe metapopulation is separated from the main distribution area and shows strongly reduced genetic variation as compared to continental populations (Sjögren 1991b; Zeisset and Beebee 2001). R. lessonae is one of the most warmth-demanding amphibian species in Europe (Sinsch 1984; Sjögren et al. 1988), and temperature-induced plasticity is probably especially important for the severely time-constrained Swedish metapopulation, which is exposed to high variation in temperature both within and between seasons (Sjögren 1991a, b). In this study, we characterized the temperature variation during the larval period in three closely-located ponds and examined population differentiation and the extent of temperature-induced plasticity in metamorphic characteristics in the R. lessonae populations living in these ponds.
Materials and methods
Study species and populations
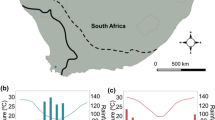
The pool frog is widespread in much of the continental Europe, being present also in two isolated areas of Scandinavia (southern Norway and east-central Sweden; Zeisset and Beebee 2001; Snell et al. 2005). In Sweden, the species is restricted to ca. 140 breeding ponds along a ca. 70 km stretch of Baltic coast in Uppland County, more than 300 km from the closest continental populations in Estonia (Sjögren 1991b; Länsstyrelsen i Uppsala län 2007; Fig. 1a). These ponds are of relatively recent origin as postglacial land uplifting along this coastline is strong (ca 6 mm/year), and most of the metapopulation’s distribution area was under the sea 1,000 years ago. Start of reproduction requires high water temperatures (16°C) and reproduction in this area usually begins in late May (Sjögren et al. 1988). As R. lessonae larvae cannot over winter, they are exposed to strong time constraints to complete metamorphosis before water temperature falls below levels that prevent further development. As a consequence, the Swedish metapopulation is severely time-constrained in cold years and a delayed start of the breeding season may lead to an almost complete reproductive failure in the metapopulation (Sjögren et al. 1988; Sjögren 1991b).
The study animals originated from three localities situated within a range of about 5 km: Björkfjärden (60°29′N, 18°0′E, henceforth BJ), Klungsten (60°32′N, 18°1′E, henceforth KL) and Klungsten Hamn (60°32′N, 18°1′E, henceforth KLH; Fig. 1b). The study was not meant as exhaustive sampling of the localities occupied in the area (which would also be prevented by the protection status of the species in Sweden), but instead we selected a subset of populations representative of the thermal conditions experienced by R. lessonae in the study area (G. Orizaola and A. Laurila, unpublished data). These localities range from small to medium-sized permanent ponds situated close to the Baltic Sea (<1 km, <10 m asl). The ponds are completely or partially surrounded by a thin reedbed (Phragmites communis) and mixed coniferous-deciduous forest, and are therefore differentially exposed to sea winds and shading. Water temperature in these ponds was recorded in May–August 2007. In each pond we placed a HOBO U22 Pro v2 temperature logger in two areas of maximum breeding activity. The loggers were placed 10 cm below the surface, and temperature was recorded every 15 min. The larvae exhibit strong basking behavior on pond surface and are likely able to select the highest temperatures available in the ponds (Sjögren et al. 1988; Orizaola and Laurila personal observations).
Experimental procedures
Eggs from freshly laid clumps (<24 h-old) were collected in the three localities (15 clumps in BJ, 12 in KLH and 10 in KL) in the spring of 2005. Date of egg collection differs slightly among the localities as a consequence of cold weather in early summer, which caused differences on the onset of the breeding period among populations. However, all the clumps were collected at the peak of the breeding season for each locality, and differences among populations due to seasonal differences among populations are unlikely. Ca. 25 eggs were collected per clump. Pool frog females can lay multiple clumps close to each other (Tietje and Reyer 2004), and therefore we always sampled eggs from clumps separated by at least 5 m to minimize the risk of collecting multiple clumps laid by the same parents. Eggs were brought to the laboratory in Uppsala and maintained in 1 l plastic vials at room temperature (20°C). After hatching, larvae were kept in the same plastic vials in groups of 20 individuals before starting the experiment.
The experiment was started when larvae had reached developmental stage 25 (Gosner 1960; complete absorption of gills and active feeding). Since eggs were collected within a few hours after laying and both eggs and larvae were maintained under common standardised conditions, the families experienced identical environmental conditions before the start of the experiment. Eight randomly chosen larvae from each family were placed individually in 1 l opaque plastic vials in each experimental temperature (day 0 of the experiment, eight vials per family and temperature in total). The experiment was conducted in a 20°C constant temperature room, which served as low temperature treatment (mean water temperature in the vials ±SE throughout the experiment was 20.31 ± 0.02°C). Within the room, half of the vials were placed in four glass-aquaria (120 × 120 × 25 cm), which served as high temperature treatment. The aquaria were filled with water to the depth of 6 cm and heated with aquarium heaters (100 W, four per aquarium) to 25°C (mean water temperature in the vials ± SE throughout the experiment 25.38 ± 0.03°C). These two temperatures are within temperature variation in natural pools (Sjögren et al. 1988; this study). To account for a temperature gradient within the room, larvae in the low temperature treatment were placed in eight vertically ordered blocks of three shelves each, so that one individual of each family was present in each of the eight blocks. In the high temperature treatment, the position of the vials was rotated within the four aquaria (blocks), each containing two replicates per family.
We used reconstituted soft water (deionised and aerated water plus 48 mg l−1 NaHCO3, 30 mg l−1 CaSO4 · 2H2O, 61.5 mg l−1 MgSO4 · 7H2O, and 2 mg l−1 KCl; APHA 1985) to standardise water quality during all the experiment (eggs and larvae). Photoperiod during larval development was constant 18 h light:6 h dark, corresponding to the situation in central Sweden in May. Larvae were fed ad libitum with chopped boiled spinach. Water in the vials was completely changed every third day. Individual survival was recorded daily throughout the experiment.
We checked daily for metamorphs, determined by the emergence of forelimbs (stage 42; Gosner 1960). Time to metamorphosis was defined as the number of days elapsed between day 0 and reaching Gosner stage 42. The metamorphs were weighed to the nearest 0.1 mg in a digital balance, after gently rolling them in paper towel to remove extra water. Growth rate was calculated as the weight at metamorphosis divided by the time to metamorphosis. A dorsal image of every larva was taken with a digital camera and snout-vent length (hereafter body length) was then measured from the digital images with Image-Pro Plus 1.1 software for Windows (Media Cybernetics 1993).
Statistical analyses
We used a nested block design, where the terms population and temperature treatment were considered as fixed effects and the effects of family (nested within the population) and experimental block (nested within the temperature treatment) as random effects. Interactions between fixed and random effects were regarded as random effects. Population was considered as a fixed factor as they were selected to cover a range of variation in temperature conditions. Tadpole survival was analysed as a binomial response variable with a generalised linear mixed model using the Glimmix procedure in SAS (Littell et al. 2006). The effects of the different factors on metamorphic traits (time to metamorphosis, weight at metamorphosis, body length and growth rate) were analysed with mixed model ANOVAs using restricted maximum likelihood (REML) estimation procedures and type III sum of squares using SPSS 13.0 software package (SPSS 2004). Variables were log-transformed prior to the analyses to meet the assumption of normality. Temperature variation among ponds was examined with a general linear model ANOVA and type III sum of squares, using population and period (seven periods of half a month starting 15 May) as fixed factors.
To investigate the genetic relationships between fitness traits, we estimated broad-sense genetic correlations (r G , estimated as Pearson product-moment correlations) between time to metamorphosis and mass at metamorphosis using family means for every combination of population and temperature treatments. Similar analyses were performed to examine the correlations between survival and larval period and survival and growth rate. As variation in larval survival was only detected in low temperature and in KL and KLH populations, the analyses were only conducted for these parts of the data set. The survival values were arcsin-squareroot transformed before the analyses.
Results
Average temperature differed among the ponds (F 2,306 = 7.24, P = 0.0008), KLH having lower temperatures than BJ and KL (Tukey test, P < 0.01; Fig. 2a). BJ had higher temperature than the other two ponds at the onset of the breeding season (early June; P > 0.01, Fig 2a). Temperature varied during the breeding season, being lower at the beginning and the end of the season (F 6,306 = 54.44, P < 0.0001; Tukey test, P < 0.0001; Fig. 2a). Daily temperature range also differed among ponds (F 2,306 = 3.66, P = 0.027), KLH experiencing higher temperature variation (P < 0.0001; Fig. 2b).
Survival was higher at high temperature treatment (F 1,34.84 = 37.40, P < 0.001; Fig. 3a) and in BJ than in KL and KLH (F 2,34.81 = 5.65, P = 0.008). In addition, survival at low temperature was much lower in KL and KLH than in BJ, bringing about a significant population × temperature interaction (F 1,34.74 = 5.96, P = 0.006; Fig. 3a). Survival probabilities differed among families (Z = 2.18, P = 0.014), however, the interaction between family and temperature treatment was not significant (Z = 1.00, P = 0.159).
Full-sib reaction norms for metamorphic characteristics for larvae of three Rana lessonae populations reared under two temperature environments. a Survival to metamorphosis, b time to metamorphosis, c weigh at metamorphosis, d body length at metamorphosis, and e growth rate during larval period. BJ Björkfjärden, KL Klungsten; KLH Klungsten Hamn. Vertical bars in all the graphs are ±1 SE
There was considerable population differentiation in trait means as well as in temperature-induced plasticity in most of the studied traits. Temperature treatment had a strong effect on the duration of the larval period, high temperature leading to shorter time to metamorphosis (Table 1; Fig. 3b). BJ had the shortest larval period metamorphosing ca. 13 days earlier than the other two populations (Tukey test, P < 0.001 for both temperatures). Weight and body length at metamorphosis also showed high plasticity in response to temperature. Larvae metamorphosed at a smaller size and a lower weight in the high temperature treatment (Table 1; Fig. 3c, d).
Weight at metamorphosis also differed among the populations, but as indicated by the significant population × temperature interaction (Table 1), differences among populations were not uniform across temperature treatments. Weight at metamorphosis was highest in KLH and lowest in BJ at low temperature (P < 0.01), whereas the populations did not differ in metamorphic weight at high temperature (P > 0.2; Fig. 3c). Overall, body length did not differ among the populations, but a significant population x temperature interaction indicated heterogeneity in the responses among the populations (Table 1; Fig. 3d). Larvae had longest bodies in KLH at low temperature (P < 0.01), whereas the populations did not differ in body length at high temperature (P > 0.5; Fig. 3d).
Growth rate was significantly influenced by population origin, but not by temperature treatment main effect. A significant population × temperature interaction indicated variation in plastic responses among the populations (Table 1). BJ showed a 20% increase in growth rate with increasing temperature (Fig. 3e). On the contrary, KLH experienced a 14% reduction in growth from low to high temperature treatment (Fig. 3e). Consequently, KLH individuals had high growth rate at low temperature but low growth at high temperature (roughly 30% lower than in BJ individuals; Fig. 3e).
Significant family effects were detected for time to metamorphosis and growth rate, and significant family × temperature interactions for larval period and growth rate (Table 1). However, ANOVAs conducted within populations showed significant family effects only within BJ population (in all trait means and growth rate plasticity; analyses not shown).
Broad-sense genetic correlations between time and mass at metamorphosis differed among the populations and temperatures. Significant correlations were found at high temperature for BJ (r G = −0.62, P = 0.014) and KL (r G = 0.75, P = 0.013), but for KLH a significant correlation (r G = 0.70, P = 0.011) was only found at low temperature. The duration of the larval period was significantly correlated with larval survival at low temperature in both KL and KLH populations, families with longer developmental periods experiencing higher mortality at low temperature (r = −0.71, P = 0.021 for KL; r = −0.70, P = 0.012 for KLH). Growth rate was negatively associated with mortality at low temperature in KL (r = 0.68, P = 0.029), families with faster growth rate having lower mortality.
Discussion
We found significant differentiation in trait means at a microgeographic scale in an amphibian metapopulation characterized by low molecular genetic variation (Sjögren 1991b; Zeisset and Beebee 2001). Moreover, we detected generalised effects of temperature on metamorphic traits and strong differentiation in phenotypic plasticity. Significant among-family effects within populations suggest a genetic basis for both population differentiation and temperature-induced plasticity, and significant family × temperature interactions in some traits also indicates genetic variation in plastic responses within populations, however, our experimental design cannot exclude differences in non-genetic maternal effects. We also found that, at low temperature, families showing longer developmental periods and slower growth experienced higher mortality in some of the populations.
One of the most interesting results in the present study is the high inter-population variation found in larval life history and plastic responses, especially when considering the small geographic scale examined and the isolatedness of the Swedish R. lessonae metapopulation. The high level of inter-population variation in quantitative traits contrasts with the almost total absence of molecular genetic diversity within this metapopulation (Sjögren 1991b; Zeisset and Beebee 2001; Snell et al. 2005; A. Laurila et al. unpublished data) suggesting that natural selection may be the causative factor behind the variation, as reported in a number of studies on population differentiation (reviewed by Leinonen et al. 2008). Possible selective factors include variation in water temperature and predation risk among the ponds, but more studies are required to establish the causal relationships. Alternative explanations for the large population differentiation in larval life history traits are population-specific maternal effects or varying degree of inbreeding depression across populations (Roff 1997; Merilä and Crnokrak 2001).
In general, we found that R. lessonae larvae reared at high temperature metamorphosed earlier and at smaller size than larvae from the low temperature environment. Temperature also generally increased larval growth rate. These results are in accordance with general rules for ectotherms predicting slow development and large body size in low temperature environments (Atkinson 1996).
Interestingly, we found that the genetic correlations differed between populations, with size at and time to metamorphosis being negatively correlated in BJ (at high temperature) and positively correlated in KL (high temperature) and KLH (low temperature). These results indicate that the relationship between body size and development time was fundamentally different among the populations: in BJ fast-developing individuals reached the largest body size, whereas in KL and KLH individuals a long development time was needed to reach large body size. High positive correlations between development time and size at metamorphosis were also reported in a Swiss R. lessonae population (Semlitsch 1993).
Few previous studies have examined population differences in plasticity at a small geographic scale. However, these studies have revealed microgeographic variation in plastic responses to temperature, desiccation and predation risk (Freidenburg and Skelly 2004; Skelly 2004; Van Buskirk and Arioli 2005; Lind and Johansson 2007). In a series of studies of the wood frog (Lithobates sylvaticus) both embryonic development and thermal preference of the larvae were related to source pond temperatures suggesting that local climate can select for adaptive microgeographic variation in these traits (Freidenburg and Skelly 2004; Skelly 2004). We found that KLH population showed signs of adaptation to low temperature in terms of high growth rate and increased body weight and body length at metamorphosis. KLH population also showed a strong correlation between larval period and mass at metamorphosis at low temperature, resulting in large body size with prolonged larval period. Indeed, only KLH had higher growth rate at low temperature. Patterns of plasticity in the KLH population may suggest adaptation to the prevailing temperature regime: this pond is situated close to the sea and has lower mean temperature and higher daily temperature variation than the other localities surrounded by forest. However, the lower developmental rate and survival (at low temperature) in KLH oppose this interpretation (see below). Interestingly, families with higher growth at low temperature experienced lower mortality, indicating that only a subset of KLH families showed signs of adaptation to the cooler environment. Clearly, the results are thus far inconclusive and studies including more localities are required to fully clarify the adaptive value of temperature-induced plasticity on this northern R. lessonae metapopulation.
Higher survival detected at high temperature agrees with the view of R. lessonae being one of the most warmth-demanding amphibians in Europe (Sinsch 1984). The negative relationship between the duration of the larval period and survival for KL and KLH, families with longer developmental period exhibiting lower survival, also reflects the stressfulness of the cool temperatures, at least for some populations. Differences in survival among the populations and families suggest a different likelihood of population decline if several cool summers occur in succession, with KL and KLH being at higher risk. Populations living on the margins of the distribution range of the species, like the Swedish R. lessonae metapopulation, are likely to be more severely affected by climatic variation because of their lower population size and lower adaptive potential in edge populations (Blows and Hoffmann 1993; Hampe and Petit 2005; Willi et al. 2006). Our results suggest that conservation strategies should therefore consider the potential of the different subpopulations to face changes in the climatic conditions.
Conclusions
We found strong population differentiation at a microgeographic scale in life history characteristics and temperature-induced plasticity in an isolated amphibian metapopulation. Despite the near absence of molecular genetic variation within this metapopulation, our study detected strong variation in trait means and plastic responses both among and within populations, possibly suggesting that natural selection is shaping life history traits of the local populations. However, a more comprehensive assessment of the role that maternal effects could play on population differentiation in this metapopulation will be required to fully address this point. These results highlight the importance of combining both ecological and molecular genetic analyses to obtain useful information for management of small and isolated populations. For example, detailed knowledge about population differentiation and adaptation to local environmental conditions can be crucial for the successful development of translocations and reintroduction programs (Storfer 1996). In the current scenario of climate change, understanding the potential of different populations to adapt to changes in environmental conditions will be critical for their conservation.
References
APHA (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Heath Association, Washington
Atkinson D (1996) Ectotherm life-history responses to developmental temperature. In: Johnston IA, Benett AF (eds) Animals and temperature: phenotypic and evolutionary adaptation. Cambridge Univ Press, Cambridge, pp 183–204
Beebee TJC (2005) Conservation genetics of amphibians. Heredity 95:423–427. doi:10.1038/sj.hdy.6800736
Bell DL, Galloway LF (2008) Population differentiation for plasticity to light in an annual herb: adaptation and cost. Am J Bot 95:59–65. doi:10.3732/ajb.95.1.59
Berg H, Becker U, Matthies D (2005) Phenotypic plasticity in Carlina vulgaris: effects of geographical origin, population size, and population isolation. Oecologia 143:220–231. doi:10.1007/s00442-004-1801-2
Blows MW, Hoffmann AA (1993) The genetics of central and marginal populations of Drosophila serrata I. genetic variation for stress resistance and species borders. Evol Int J Org Evol 47:1255–1270. doi:10.2307/2409990
DeWitt TJ, Scheiner SM (2004) Phenotypic plasticity: functional and conceptual approaches. Oxford Univ Press, Oxford
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81. doi:10.1016/S0169-5347(97)01274-3
Freidenburg LK, Skelly DK (2004) Microgeographical variation in thermal preference by an amphibian. Ecol Lett 7:369–373. doi:10.1111/j.1461-0248.2004.00587.x
Gosner KN (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gotthard K, Nylin S (1995) Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life-history. Oikos 74:3–17. doi:10.2307/3545669
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. doi:10.1111/j.1461-0248.2005.00739.x
Hendry AP, Kinnison MT, Day T, Taylor EB (2001) Population mixing and the adaptive divergence of quantitative traits in discrete populations: a theoretical framework for empirical test. Evol Int J Org Evol 55:459–466. doi:10.1554/0014-3820(2001)055[0459:PMATAD]2.0.CO;2
Kingsolver JG, Gomulkiewicz R (2003) Environmental variation and selection on performance curves. Integr Comp Biol 43:470–477. doi:10.1093/icb/43.3.470
Kingsolver JG, Massie KR, Shlichta JG, Smith MH, Ragland GJ, Gomulkiewicz R (2007) Relating environmental variation to selection on reaction norms: an experimental test. Am Nat 169:163–174. doi:10.1086/510631
Länsstyrelsen i Uppsala län (2007) 2005 års inventering av gölgroda längs Nordupplands kustband samt utvärdering av gölgrodans åtgärdsprogram. Länsstyrelsens meddelandeserie 2007: 1. Länsstyrelsen i Uppsala län, Uppsala, Sweden
Leinonen T, O’Hara RB, Cano JM, Merilä J (2008) Comparative studies of quantitative trait and neutral marker divergence: A meta-analysis. J Evol Biol 21:1–17
Lemckert FL (2004) Variations in anuran movement and habitat use: implications for conservation. Appl Herpetol 1:165–181. doi:10.1163/157075403323012179
Lind MI, Johansson F (2007) The degree of adaptive phenotypic plasticity is correlated with the spatial environmental heterogeneity experienced by island populations of Rana temporaria. J Evol Biol 20:1288–1297. doi:10.1111/j.1420-9101.2007.01353.x
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS® for mixed models, vol 2. SAS Institute Inc, Cary
Media Cybernetics (1993) Image Pro Plus 1.1 for Windows. Media Cybernetics, Bethesda, USA
Merilä J, Crnokrak P (2001) Comparison of genetic differentiation at marker loci and quantitative traits. J Evol Biol 14:892–903. doi:10.1046/j.1420-9101.2001.00348.x
Merilä J, Laurila A, Lindgren B (2004) Variation in the degree and costs of adaptive phenotypic plasticity among Rana temporaria populations. J Evol Biol 17:1132–1140. doi:10.1111/j.1420-9101.2004.00744.x
Newman RA (1992) Adaptive plasticity in anuran metamorphosis. Bioscience 42:671–678. doi:10.2307/1312173
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. The Johns Hopkins Univ Press, Baltimore
Roff DA (1997) Evolutionary quantitative genetics. Chapman & Hall, New York
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Annu Rev Ecol Syst 24:35–68. doi:10.1146/annurev.es.24.110193.000343
Semlitsch RD (1993) Adaptive genetic variation in growth and development of tadpoles of the hybridogenetic Rana esculenta complex. Evol Int J Org Evol 47:1805–1818. doi:10.2307/2410223
Sinsch U (1984) Thermal influences on the habitual preference and the diurnal activity in three European Rana species. Oecologia 64:125–131. doi:10.1007/BF00377554
Sjögren P (1991a) Extinction and isolation gradients in metapopulations: the case if the pool frog (Rana lessonae). Biol J Linn Soc Lond 42:135–147. doi:10.1111/j.1095-8312.1991.tb00556.x
Sjögren P (1991b) Genetic variation in relation to demography of peripheral pool frog populations (Rana lessonae). Evol Ecol 5:248–271. doi:10.1007/BF02214231
Sjögren P, Elmberg J, Berglind S-Å (1988) Thermal preferences in the pool frog Rana lessonae: impact on the reproductive behaviour of a northern fringe population. Holarct Ecol 11:178–184
Skelly DK (2004) Microgeographic countergradient variation in the wood frog, Rana sylvatica. Evol Int J Org Evol 58:160–165
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–585. doi:10.1086/283413
Snell C, Tetteh J, Evans IH (2005) Phylogeography of the pool frog (Rana lessonae Camerano) in Europe: evidence for native status in Great Britain and for an unusual postglacial colonization route. Biol J Linn Soc Lond 85:41–51. doi:10.1111/j.1095-8312.2005.00471.x
SPSS (2004) SPSS 13.0 release for Windows. SPSS Inc, Chicago
Storfer A (1996) Quantitative genetics: a promising approach for the assessment of genetic variation in endangered species. Trends Ecol Evol 11:343–348. doi:10.1016/0169-5347(96)20051-5
Tietje GA, Reyer H-U (2004) Larval development and recruitment of juveniles in a natural population of Rana lessonae and Rana esculenta. Copeia 2004:638–646. doi:10.1643/CE-03-273R1
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160:87–102. doi:10.1086/340599
Van Buskirk J, Arioli M (2005) Habitat specialization and adaptive divergence of anuran populations. J Evol Biol 18:596–608. doi:10.1111/j.1420-9101.2004.00869.x
Wells KD (2007) The ecology and behaviour of amphibians. The Univ of Chicago Press, Chicago
Willi Y, Van Buskirk J, Hoffmann AA (2006) Limits to the adaptive potential of small populations. Annu Rev Ecol Evol Syst 37:433–458. doi:10.1146/annurev.ecolsys.37.091305.110145
Zeisset I, Beebee TJC (2001) Determination of biogeographical range: an application of molecular phylogeography to the European pool frog Rana lessonae. Proc R Soc Lond B Biol Sci 268:933–938. doi:10.1098/rspb.2001.1600
Acknowledgments
Emma Dahl and Anaelisa Valdés gave us invaluable help during the field and laboratory work. We thank Miguel Tejedo and Céline Teplitsky for constructive comments on an earlier version of the manuscript. Our research was supported by a post-doctoral fellowship of the Spanish Ministry of Education and Science and fellowships from Fundación Caja Madrid and Fundación Ramón Areces (to G.O.), and by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (to A.L.). The experiments were conducted in agreement with the national laws and the eggs were collected with permission from the county authorities (Länsstyrelsen i Uppsala Län).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orizaola, G., Laurila, A. Microgeographic variation in temperature-induced plasticity in an isolated amphibian metapopulation. Evol Ecol 23, 979–991 (2009). https://doi.org/10.1007/s10682-008-9285-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-008-9285-x