Abstract
Some phytophagous insects have been known to inoculate certain fungi on plant substrates. In many cases of such insect–fungi relationships it has been considered that fungi contribute to insects by decomposing lignin or polysaccharides, and that the insects feed on the decomposition products or fungi themselves. Females of the leaf-rolling weevil in the genus Euops (Attelabidae) store spores of symbiotic fungi in the mycangia and inoculate them on leaf rolls. To determine the effect of mycangial fungi on larval nutrition in E. lespedezae, the nutritional value was compared between leaves with and without mycangial fungi. Two Penicillium species were isolated from the mycangia. These mycangial fungi showed little effect on the decomposition of lignin and polysaccharides, and showed little effect on enhancement of soluble sugars within leaves. Thus, the mutualism between Euops and its mycangial fungi contrasts with the mainly nutritional mutualisms between wood-infesting insects (termites, bark/ambrosia beetles, and wood wasps) and lignin/polysaccharide-decomposing fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Symbiotic relationships with fungi have evolved in many insect taxa (Batra 1979; Wheeler and Blackwell 1984; Wilding et al. 1989). These insects transfer and inoculate fungi on plant substrates, and feed on fungi or their decomposition products (Batra and Batra 1979; Haack and Slansky 1987; Paine et al. 1997). Most of these insects, i.e., termites, bark/ambrosia beetles, and wood wasps, feed on dead plant tissue and are considered dependent on symbiotic fungi for the decomposition of lignin and cellulose (Morgan 1968; Barras and Hodges 1969; Martin and Martin 1978; Bridges 1983; Kukor and Martin 1983; Madden 1988; Watanabe et al. 1998; Hyodo et al. 2000), which are the main components of plants, but are indigestible by most insect herbivores (Talmadge et al. 1973; Abe and Higashi 1991; Martin 1991; Hochuli 1996; Schoonhoven et al. 1997). Therefore, insects feeding on nutrient-poor plant tissue gain more nutrients by feeding on fungi inoculated on the tissue or their decomposition products than by feeding on the plant tissue directly.
Leaf-cutting ants inoculate fungi on living plant tissue, mainly old leaves (Hölldobler and Wilson 1990), which contain smaller amounts of lignin and cellulose and larger amounts of non-cellulose polysaccharides than dead plant tissue. It is assumed that fungi that are symbiotic with leaf-cutting ants do not decompose lignin (Lee and Wood 1971; Maynard et al. 1979). Rather, these fungi decompose plant polysaccharides that ants cannot digest (Martin and Weber 1969; Bacci et al. 1995; Siqueira et al. 1998; D’Ettorre et al. 2002; Richard et al. 2005), although the ability to decompose cellulose may differ among species (Martin and Weber 1969; Bacci et al. 1995; Siqueira et al. 1998; Abril and Bucher 2002). Workers of these ants have been observed to ingest the juice of cut leaves prior to inoculation with symbiotic fungi (Littledyke and Cherrett 1976). Thus, leaf-cutting ants are considered to feed on soluble sugars and other usable components from plant juice, in addition to products decomposed by fungi and fungi themselves (Quinlan and Cherrett 1979; Silva et al. 2003).
Leaf-rolling weevils of the genus Euops (Coleoptera: Attelabidae), which are mainly distributed in Africa, the Papuan region, and Asia (Riedel 2002), have symbiotic relationships with fungi. Euops females construct small leaf rolls (<5 mm in length and <3 mm in diameter) by cutting ribbon-shaped pieces from the leaf margin (Sakurai 1985; Sawada and Morimoto 1986), and inoculate the leaf pieces with fungal spores stored in the mycangia (Sakurai 1985; Sawada and Morimoto 1986; Riedel 2002). Leaf rolls are constructed from pieces of young leaves, which are considered one of the most nutrient-rich plant parts. Nevertheless, the weevils inoculate the leaf rolls with mycangial fungi. Recently, mycangial fungi of some Euops species were reported to belong to the genus Penicillium (Riedel 2002). Penicillium species are one of the most common soil fungi, having worldwide distribution (Domsch et al. 1993). Colonization on leaf litter and decomposition ability of polysaccharides were reported in some species (Domsch et al. 1993). The role of mycangial fungi in Euops, however, has not yet been determined. It is unknown whether these mycangial fungi can decompose lignin or polysaccharides in the leaf rolls.
The objective of this study was to determine whether mycangial fungi of Euops species enhance the nutritive value of leaf rolls by decomposing lignin or polysaccharides. First, we isolated fungi from mycangia and leaf rolls of E. lespedezae Sharp and monitored the succession of fungal flora on the leaf-rolls. We then calibrated the ability of mycangial fungi, epiphytes, and endophytes to decompose lignin, polysaccharides, and soluble sugars. If the mycangial fungi are good at decomposing lignin or polysaccharides, the decomposition of these indigestible substances would be accelerated in leaf rolls inoculated with the mycangial fungi. Consequently, soluble sugar content would be high in leaf rolls inoculated with mycangial fungi. In addition, the ability of the mycangial fungi to enhance the nitrogen content of leaf rolls was also measured.
Our results suggest that there is a different important role of mycangial fungi than enhancing nutrients by decomposing lignin or polysaccharides, viz. suppressing antagonistic bacteria or fungi.
Material and methods
Insect species
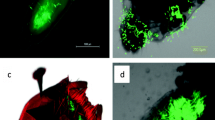
Euops lespedezae is distributed in Japan and Korea, and females of this species construct leaf rolls from the leaves of Fabaceae and Polygonaceae (Suzuki and Uehara 1997). The most common host plant in Kyoto, Japan, is Lespedeza cyrtobotrya Miq. (Fabaceae), which has trifoliate leaves. The weevil cuts ribbon-shaped pieces of leaf from the leaflet (hereafter, leaf) margin, inoculates them with the mycangial fungi, and then forms leaf rolls (Fig. 1a–c). One egg is oviposited per leaf roll, and the hatched larva feeds on the molded leaf roll until emergence. This weevil species is univoltine in Kyoto.
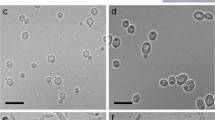
Leaf rolls, adults, and mycangial fungi of E. lespedezae. (a) Leaf-roll construction by a female weevil. (b) Leaf roll. (c) Ventral side of female thorax and abdomen with an arrow showing the entrance to the mycangia. Colony of (d) Penicillium sp. 1 and (e) P. sp. 2 cultured on 2% malt extract agar medium for 9 months. Bars in photos represent (b) 1 mm, (c) 200 μm, (d, e) 1 cm
Isolation of mycangial fungi
Ten female E. lespedezae were collected from the host shrub L. cyrtobotrya at Mt. Daimonji (35.2°N, 135.48°E), Kyoto, Japan, on 13 and 25 May 2005. Spores stored in the mycangia of females were removed using a sterile needle under a microscope and inoculated on 2% malt-extract agar (MA) medium at room temperature, and then on LCA medium (0.1% glucose, 0.1% MgSO4 · 7H2O, 0.02% KCl, 0.2% NaNO3, 0.02% yeast extract, and 1.3% agar (w/v); Miura and Kudo 1970) at 25°C in the dark. Sporulating colonies were used to identify the fungi.
A molecular method was also used to identify the mycangial fungi. DNA was extracted from mycelia of these isolates following the methods of Gardes and Bruns (1993). The 28s rDNA (D1 and D2 regions) was amplified using D1 (Peterson 2000) and NL4 primers (O’Donnell 1993). DNA base sequences were determined according to the method of Iwamoto et al. (2002). The accession numbers for the DNA sequences are AB253796 and AB253797. Sequenced isolates were compared to known species using a BLAST search.
Isolation of leaf-roll-associated fungi
Leaf rolls recently constructed by weevils were collected from Mt. Daimonji (ten leaf rolls) on 3 June 2005 and from weevils in the laboratory that had been collected at Mt. Daimonji (14 leaf rolls). Fungi from these 24 leaf rolls were isolated just after construction (ten each from the field and laboratory) and 1 month after construction (four from the laboratory). The leaf rolls were unrolled and eggs or larvae inside were removed. Five 2 × 2 mm pieces were cut from each leaf roll using a razor and placed on 2% MA medium. Isolated fungi were cultured at room temperature for >2 weeks in natural light, and then identified by microscopic observation. To determine the fungal composition, the dominance of each fungus within a leaf roll was determined as the number of pieces from which the fungus was isolated. One-way analysis of variance (ANOVA) with a Tukey–Kramer test at P = 0.05 was used to compare the dominance within a leaf roll among fungi using JMP ver. 5 software (SAS Institute, Cary, North Carolina, USA).
Contribution of mycangial fungi to the nutritional value of leaf rolls
Fungi isolated from the mycangia of E. lespedezae females were cultured on 2% MA medium for 1 month. Before the inoculation experiment, 78 pieces of 1 × 1 mm in size per fungal species were cut from the culture and placed separately on 1.5% plain agar plates using forceps.
As inoculation substrates, leaves were collected from one L. cyrtobotrya shrub at Mt. Daimonji on 23 June 2005. Ribbon-shaped pieces were cut from the leaves, and each piece was weighed within 24 h after collection. Leaf pieces were treated in three ways: sterilized by ethylene oxide gas at 40°C for 6 h to kill both endophytes and epiphytes; surfaces-sterilized using 70% ethanol for 1 min, 15% hydrogen peroxide for 1 min, and 70% ethanol for 1 min to kill only epiphytes; or left untreated. Each leaf piece was deposited over each fungal colony on plain agar. As a control, each leaf piece was similarly deposited on an uninoculated 1.5% plain agar plate. Leaf pieces were stored at 20°C for 3 months.
Leaf pieces were then oven-dried at 40°C for 24 h, weighed, and ground to a powder. Powdered leaves were then used to determine the lignin, total carbohydrate, soluble sugar, and nitrogen content.
To determine the amount of water, lignin, polysaccharide, soluble sugar, and nitrogen content without inoculation, 30 leaf pieces per treatment (gas sterilized, surface-sterilized, and untreated) were weighed fresh and after air-drying for 3 months, and used for chemical analysis similar to the inoculated leaf pieces. The initial dry masses of inoculated leaf pieces were estimated using the uninoculated leaf pieces.
The decomposition abilities of fungi were determined as the residue of each chemical component divided by the initial dry weight of the leaf piece.
Chemical analysis
Lignin content was determined using hot sulfuric acid digestion according to the standard method. After the extraction of soluble compounds using alcohol-benzene at room temperature, the residue was treated with 72% sulfuric acid for 2 h. The mixture was diluted with distilled water to 2.5% sulfuric acid solution, autoclaved at 120°C for 60 min, and then filtered and washed with water. After drying at 105°C, the filtered residue was weighed. The filtrate was used to determine total carbohydrates. The ability of each fungus to decompose lignin was compared using a two-way ANOVA for untreated and surface-sterilized leaf pieces, and one-way ANOVA for gas-sterilized leaf pieces.
Total carbohydrate content was determined using the phenol-sulfuric acid method. The 5% phenol and 98% sulfuric acid were added to the filtrate obtained from the lignin analysis. The optical density was measured with a spectrometer at 490 nm, using glucose as a standard.
The soluble sugar content was determined using the phenol-sulfuric acid method. Powdered leaf pieces were extracted with 50% methanol at 75°C for 60 min and filtered, and 5% phenol and 98% sulfuric acid were added to the filtrate. The optical density of the filtrate was measured with a spectrometer at 490 nm, using glucose as a standard. The polysaccharide content was estimated as the difference between the total carbohydrate and soluble sugar contents.
Nitrogen content was measured using an automatic gas chromatograph (NC analyzer, Sumitomo Chemical Co., Osaka, Japan). Powdered leaf pieces were completely burned in a furnace carried by helium gas at 830°C for 1 min, transforming all nitrogen into N2. N2 was quantitatively measured in the chromatograph tube using hippuric acid as a standard.
For the comparison of the amount of polysaccharide, soluble sugar, and nitrogen content among each treatment, no statistic was done because all leaf pieces of the same treatment were unified and powdered together for the technical necessity.
Results
Isolation of fungi from mycangia
Of the ten observed females, eight had spores stored in the mycangia, but two did not. The mycangial fungus spores inoculated on MA media formed orange and white colonies (Fig. 1d, e). Both isolates sporulated 2 weeks after inoculation on LCA medium and were identified as Penicillium spp. by the morphological characters of the conidia, conidiogenous cells and conidiophores. Blast searches of the two isolates indicated that the two isolates represented probably different species of the genus Penicillium. Thus, we identified orange and white colonies as P. sp. 1 and P. sp. 2, respectively. P. sp. 1 and P. sp. 2 were isolated from three and five females, respectively (Table 1). No fungus was isolated from the mycangia of two females. No female had both Penicillium species.
Isolation of fungi from leaf rolls
A total of 22 fungal species, including P. sp. 1 and P. sp. 2, were isolated from the 24 leaf rolls. For leaf rolls constructed in the laboratory, either P. sp. 1 or P. sp. 2 was isolated from all leaf rolls (Table 2). P. sp. 1 or P. sp. 2 was isolated from 60% of leaf rolls constructed in the field. Cladosporium sp. 1 was isolated from all leaf rolls at the larval stage, although it was only isolated from 30% of leaf rolls at the egg stage.
The dominance within a leaf roll of eight fungal species isolated from two or more leaf rolls was analyzed, with P. sp. 1 and P. sp. 2 grouped together. The dominance within a leaf roll was significantly different among fungi (one-way ANOVA; field, egg stage, df = 7, F = 5.13, P < 0.0001; laboratory, egg stage, df = 7, F = 68.01, P < 0.0001; laboratory, larval stage, df = 7, F = 5.85, P < 0.001). The dominance within a leaf roll of P. sp. 1 or P. sp. 2 in leaf rolls at the egg stage was significantly higher than that for other fungi (Tukey–Kramer test, Fig. 2). The dominance within a leaf roll of Penicillium at the egg stage (mean ± SE) was 4.3 ± 0.3 in leaf rolls constructed in the laboratory and 1.7 ± 0.6 in leaf rolls collected in the field. The dominance within a leaf roll of Penicillium in leaf rolls at the larval stage was 3.3 ± 0.9 (mean ± SE), which was highest among the fungal species, but not significantly different from that of Cladosporium sp. 1 (3.3 ± 0.9) and hyphomycete sp. 1 (0.8 ± 0.8; Tukey–Kramer test; Fig. 2).
Mean ± SE of dominance within a leaf roll of each fungal species isolated from three types of leaf rolls of E. lespedezae. (a) Leaf rolls at the egg stage collected in the field (n = 10). (b) Leaf rolls at the egg stage constructed in the laboratory (n = 10). (c) Leaf rolls at the larval stage constructed in the laboratory (n = 4). Different letters indicate significant differences between fungal combinations (Tukey–Kramer test; P < 0.05)
Decomposition ability of mycangial fungi
Lignin content decreased only slightly after 3 months (Table 3). The final lignin content did not differ among inoculation (P. sp. 1, P. sp. 2, and uninoculated) or sterilization (untreated and surface-sterilized) treatments, nor was the inoculation × sterilization interaction significant (two-way ANOVA; Table 4). The decomposition ability of P. sp. 1, P. sp. 2, epiphytes, and endophytes were similarly low. Among gas-sterilized leaf pieces, those inoculated with P. sp. 1 or P. sp. 2 had lower lignin content than uninoculated leaf pieces (mean ± SE, 411.15 ± 16.52 mg/g), although the difference was not significant (one-way ANOVA, df = 2, F = 6.02, P > 0.05; Table 3).
Some polysaccharide appeared to be decomposed by both endophytes and epiphytes (Table 3). Among the gas-sterilized leaf pieces, those inoculated with P. sp. 1 or P. sp. 2 had a slightly lower polysaccharide content than those uninoculated leaf pieces (Table 3), suggesting that P. sp. 1 and P. sp. 2 decomposed only small amounts of polysaccharide. Among the unsterilized and surface-sterilized leaf pieces, those inoculated with P. sp. 1 or P. sp. 2 did not show greatly different polysaccharide content from uninoculated leaf pieces, suggesting that the Penicillium fungi did not decompose polysaccharides more than epiphytes and endophytes.
Although soluble sugar was consumed dramatically in unsterilized leaf pieces, the decrease in soluble sugars was not so dramatic in gas-sterilized and surface-sterilized leaf pieces (Table 3). Among the unsterilized and surface-sterilized leaf pieces, those inoculated with P. sp. 1 or P. sp. 2 did not show greatly different soluble sugar content than uninoculated leaf pieces. Among the gas-sterilized leaf pieces, those inoculated with P. sp. 1 or P. sp. 2 had a slightly higher soluble sugar content than uninoculated leaf pieces, suggesting that some of the soluble sugars that disappeared were not decomposed by fungi.
No distinct changes in nitrogen content were detected in the three treatments (Table 3). The presence of Penicillium species did not affect the nitrogen content.
Discussion
Two Penicillium species were isolated from both mycangia of females and leaf rolls of E. lespedezae. Either P. sp. 1 or P. sp. 2 was isolated from 80% of females, and they were the most dominant fungal species in leaf rolls, especially at the egg stage, similar to results for E. splendidus (Takabe 2004). These results suggest that Penicillium fungi potentially affect the performance of early-instar larvae. The number of insect samples used in this study for isolation of fungi was small, and there is a possibility that another fungal species is found from mycangia of E. lespedezae. At least, however, it is evident that mycangial fungi of E. lespedezae have not been selected into one species within a population. Isolation of more than two fungal species from a symbiotic insect species was also reported in bark/ambrosia beetles (Paine et al. 1997) and fungus-growing termites (Aanen et al. 2002). In these cases, several fungal species may be maintained within an insect species because these fungi have similar function to symbiotic insects and fitness of insects does not differ among individuals with different fungal species.
While only Penicillium fungi dominated on the leaf roll at the egg stage, Cladosporium sp. 1 and hyphomycete sp. 1 were isolated as frequently as Penicillium fungi from leaf rolls at the larval stage. This differences in the species of fungi and frequency of isolation with age of leaf rolls may be explained by the change in chemical component of the leaf roll through decomposition by fungi. It was reported that fungal species on freshly fallen leaves were different from those on decomposing leaves (Osono 2002). How Penicillium fungi influence the fungal succession on leaf rolls and how fungal succession influence the larval survival will be needed to study to reveal the role of mycangial fungi.
The mycangial fungi seldom decomposed lignin and polysaccharides compared to other epiphytes and endophytes of leaf rolls. Further, soluble sugar contents of leaves were not enhanced by mycangial fungi. These suggest that the role of mycangial fungi is not to improve the nutritional value of leaf rolls by decomposing indigestible lignin and polysaccharides. As is the case in some leaf-cutting ants (Abril and Bucher 2002), the symbiosis of the weevil with the mycangial fungi differs from other insect-fungus symbioses in which the fungi contribute to decomposition of lignin or cellulose (Martin and Martin 1978; Kukor and Martin 1983; Watanabe et al. 1998).
Another considerable contribution of the fungus to the fungus-insect symbiosis is to provide antimicrobial activity against pathogenic or toxic microbes (Evans 1989; Kendrick 2000; Martin et al. 2005). An antimicrobial effect of mycangial fungi against harmful fungi has been reported in bark beetles (Bridges and Perry 1985). Because Penicillium species were reported to produce antifungal or antibacterial compounds (Domsch et al. 1993; Yamaji et al. 1999; Kendrick 2000), P. sp. 1 and P. sp. 2 may improve their survival rate by protecting larvae from harmful fungi. Further, Penicillium species were reported not to have harmful effect on fly larvae although other sympatric fungi such as Aspergillus and Alternaria species inhibited larval growth (Rohlfs et al. 2005). This harmless nature of Penicillium for insect larvae may be one of the reasons that they are selected as mycangial fungi of Euops weevils.
Although the main role of the mycangial fungi was not clear, our results suggest that the mycangial fungi do not accelerate the decomposition of lignin or polysaccharides, despite the expected advantages. Further studies of the antagonistic relationships between mycangial fungi and other microbes in leaf rolls will be needed to reveal the benefits gained by the weevil from the symbiosis with mycangial fungi.
References
Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ (2002) The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA 23:14887–14892
Abe T, Higashi M (1991) Cellulose centered perspective on terrestrial community structure. Oikos 60:127–133
Abril AB, Bucher EH (2002) Evidence that the fungus cultured by leaf-cutting ants does not metabolize cellulose. Ecol Lett 5:325–328
Bacci M Jr, Anversa MM, Pagnocca FC (1995) Cellulose degradation by Leucocoprinus gongylophorus, the fungus cultured by the leaf-cutting ant Atta sexdens rubropilosa. Antonie von Leeuwenhoek 67:385–386
Barras SJ, Hodges JD (1969) Carbohydrates in inner bark of Pinus taeda as affected by Dendroctonus frontalis and associated microorganisms. Can Entomol 101:489–493
Batra LR (1979) Insect–fungus symbiosis. Allenheld & Osmun, Montclair, NJ
Batra LR, Batra SWT (1979) Termite–fungus mutualism. In: Batra LR (ed) Insect–fungus symbiosis. Allenheld & Osmun, Montclair, NJ, pp 77–116
Bridges JR (1983) Mycangial fungi of Dendroctonous frontalis (Coleoptera: Scolytidae) and their relationship to beetle population trends. Environ Entomol 12:858–861
Bridges JR, Perry TJ (1985) Effects of mycangial fungi on gallery construction and distribution of bluestain in southern pine beetle-infested pine bolts. J Entomol Sci 20(2):271–275
D’Ettorre P, Mora P, Dibangou V, Rouland C, Errard C (2002) The role of the symbiotic fungus in the digestive metabolism of two species of fungus-growing ants. J Comp Physiol B 172:169–176
Domsch KH, Gams W, Anderson T-H (1993) Compendium of soil fungi, vol 1. IHW-Verlag, Eching
Evans HC (1989) Mycopathogens of insects of epigeal and aerial habitats. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds) Insect–fungus interactions. Academic Press, London, pp 205–238
Gardes M, Bruns TD (1993) ITS primer with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rust. Mol Ecol 21:113–118
Haack RA, Slansky F Jr (1987) Nutritional ecology of wood-feeding Coleoptera, Lepidoptera, and Hymenoptera. In: Slansky F Jr, Rodriguez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 449–486
Hochuli DF (1996) The ecology of plant/insect interactions: implications of digestive strategy for feeding by phytophagous insects. Oikos 75:133–141
Hölldbler B, Wilson EO (1990) The ants. Belknap, Cambridge
Hyodo F, Inoue T, Azuma J-I, Tayasu I, Abe T (2000) Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol Biochem 32:653–658
Iwamoto S, Tokumasu S, Suyama Y, Kakishima M (2002) Molecular phylogeny of four selected species of the strictly anamorphic genus Thysanophora using nuclear ribosomal DNA sequences. Mycoscience 43:169–180
Kendrick B (2000) The fifth kingdom, 3rd edn. Focus Publishing, USA
Kukor JJ, Martin MM (1983) Acquisition of digestive enzymes by siricid woodwasps from their fungal symbiont. Science 220:1161–1163
Lee KE, Wood TG (1971) Termites and soil. Academic Press, London
Littledyke M, Cherrett JM (1976) Direct ingestion of plant sap from cut leaves by the leaf-cutting ants Atta cephalotes (L.) and Acromyrmex octospinosus (Reich) (Formicidae, Attini). Bull Entomol Res 66:205–217
Madden JL (1988) Sirex in Australasia. In: Berryman AA (ed) Dynamics of forest insect populations: patterns, causes, implications. Plenum, New York, pp 407–429
Martin MM (1991) The evolution of cellulose digestion in insects. Philos Trans R Soc Lond B 333:281–288
Martin MM, Martin JS (1978) Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis: the role of acquired digestive enzymes. Science 199:1453–1455
Martin MM, Weber NA (1969) The cellulose-utilizing capability of the fungus cultured by the Attine ant Atta colombica tonsipes. Ann Entomol Soc Am 62:1386–1387
Martin T, Oliveira L, Garcia P (2005) Larval mortality factors of Spodoptera littoralis in the Azores. BioControl 50:761–770
Maynard AL, Loosli BS, Harold FH, Warner RG (1979) Animal nutrition. McGrow-Hill Book Company, New York
Miura K, Kudo M (1970) An agar-medium for aquatic hyphomycetes. Trans Mycol Soc Jpn 11:116–118 (in Japanese)
Morgan FD (1968) Bionomics of siricidae. Annu Rev Entomol 13:239–256
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Osono T (2002) Phyllosphere fungi on leaf litter of Fagus crenata: occurrence, colonization, and succession. Can J Bot 80:460–469
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
Peterson SW (2000) Phylogenetic analysis of Penicillium species based on ITS and lsu-rDNA nucleotide sequences. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Hardwood, Amsterdam, pp 163–178
Quinlan RJ, Cherrett JM (1979) The role of fungus in the diet of the leaf-cutting ant. Ecol Entomol 4:151–160
Richard F-J, Mora P, Errard C, Rouland C (2005) Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J Comp Physiol B 175:297–303
Riedel A (2002) Taxonomy, phylogeny, and zoogeography of the weevil genus Euops (Insecta: Coleoptera: Curcurionoidea) in the Papuan region. PhD thesis, Ludwig Maximilians University, Munich
Rohlfs M, Obmann B, Peterson R (2005) Competition with filamentous fungi and its implication for a gregarious lifestyle in insects living on ephemeral resources. Ecol Entomol 30:556–563
Sakurai K (1985) An attelabid weevil (Euops splendida) cultivates fungi. J Ethol 3:151–156
Sawada Y, Morimoto K (1986) The mycetangia and the mode of the fungus transmission in the weevil genus Euops (Coleoptera: Attelabidae). Sci Bull Fac Agr Kyusyu Univ 40(4):197–205 (in Japanese with English summary)
Schoonhoven LM, Jermy T, van Loon JJA (1997) Insect–plant biology. Chapman & Hall, London
Silva A, Bacci M Jr, Siqueira CG, Bueno OC, Pagnocca FC, Hebling MJA (2003) Survival of Atta sexdens workers on different food sources. J Insect Physiol 49:307–313
Siqueira CG, Bacci M Jr, Pagnocca FC, Bueno OC, Hebling MJA (1998) Metabolism of plant polysaccharides by Leucoagaricus gongylophorus, the symbiotic fungun of the leaf-cutting ant Atta sexdens L. Appl Environ Microb 64:4820–4822
Suzuki K, Uehara C (1997) Cradle structure and formation process in the subfamilies Apoderinae and Attelabinae (Coleoptera, Attelabidae) from Japan. Bull Hoshizaki Green Found 1:99–204
Takabe N (2004) Host use of Euops splendidus and dynamics of its mutualistic fungi through development of the beetle. Master’s thesis, Nagoya University (in Japanese)
Talmadge KW, Keegstra K, Bauer WD, Albersheim P (1973) The structure of plant cell wall. Plant Physiol 51:158–173
Watanabe H, Noda H, Tokuda G, Lo N (1998) A cellulase gene of termite origin. Nature 394:330–331
Wheeler Q, Blackwell M (1984) Fungus–insect relationships. Columbia University Press, New York
Wilding N, Collins NM, Hammond PM, Webber JF (1989) Insect–fungus interactions. Academic Press, London
Yamaji K, Fukushi Y, Hashidoko Y, Yoshida T, Tahara S (1999) Characterization of antifungal metabolites produced by Penicillium species isolated from seeds of Picea glehnii. J Chem Ecol 25:1643–1645
Acknowledgements
We thank Naoki Takabe and Hisashi Kajimura for providing important information regarding the mycangial fungi of Euops; Seiji Tokumasu for identification of the fungi; and Takuo Hishi for helpful advice on the statistical analyses. This study is supported by JSPS Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, C., Fukasawa, Y., Hirose, D. et al. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol 22, 711–722 (2008). https://doi.org/10.1007/s10682-007-9196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-007-9196-2