Abstract
Non-random sex allocation in relation to parental, ecological and phenological factors has been investigated in several correlational studies of birds, mostly based on few breeding seasons and relatively small sample sizes, which have led to different results. We investigated sex ratio of nestling barn swallows (Hirundo rustica) in relation to adult sex ratio, laying date, clutch size, colony size and meteorological conditions in a sample of 553 broods (>2200 nestlings) during 10 years. At the population level, nestling sex ratio varied among years and deviated from parity in two years. Sex ratio among adults did not predict offspring sex ratio in the current or the following year. At the within-family level, the proportion of sons increased with laying date in large clutches, did not vary among clutches of intermediate size, and tended to decline with laying date in small clutches. Large colonies harbored more sons. The proportion of males increased with temperature during laying whereas the effects of temperature during the pre- or post-laying periods and that of rainfall were non-significant. These patterns of variation of offspring sex ratio did not differ between years. Thus, we identified several potential causal sources of variation in barn swallow offspring sex ratio, including temporal, phenological and ecological factors. The observation of an association of offspring sex with temperature during laying is novel for birds and may be mediated by effects on maternal steroid hormones profile. The ecological and evolutionary implications of present findings are discussed in the light of adaptive sex allocation theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two main families of evolutionary theories of sex allocation have been developed, which tackle the issue of allocation to the production of sons rather than daughters at different levels (Pen and Weissing 2002).
Fisher (1958) proposed that frequency-dependent selection should favor the evolution of individual reproductive strategies that result in a 1:1 ratio of sexually mature male and female offspring, if production of either sex entails parents with the same investment, or in a sex ratio inversely related to the investment ratio between the two sexes, if one sex is more costly to produce than the other. Fisherian processes have been invoked to interpret the variation in offspring sex ratio in relation to population tertiary (breeding) sex ratio in diverse animal models (Bensch et al. 1999; Ranta et al. 2000). Sex allocation theories operating at the family level, on the other hand, deal with the factors that promote differential allocation to sons and daughters among parents in a population. Whenever parental phenotypic traits (e.g., maternal condition; Trivers and Willard 1973) or extrinsic conditions exist that variably influence the relative reproductive value of sons and daughters, parents should adjust the sex ratio of their offspring according to these factors. In birds, the predictions of sex allocation theory at the family level have been tested for a diverse set of factors. A first broad group of such factors relates to parental phenotypic quality and includes maternal age and ‘condition’ at the time of reproduction (Bradbury and Blakey 1998; Nager et al. 1999; Saino et al. 1999, 2002a; Whittingham and Dunn 2000; Arnold et al. 2003; Thuman et al. 2003) and sexual attractiveness of the parental or genetic father (Burley 1981; Ellegren et al. 1996; Sheldon et al. 1999; Saino et al. 2002a; Pike and Petrie 2005; Rutstein et al. 2005; Whittingham et al. 2005).
The second group includes ecological factors. The proportion of sons has been shown to vary with territory quality and interannual heterogeneity in food abundance (Appleby et al. 1997; Komdeur et al. 1997). Breeding date has been often used as a proxy for ecological conditions (see Hasselquist and Kempenaers 2002 for a review). Social factors such as frequency of helpers in the population have also been shown to predict brood sex ratios (Komdeur et al. 1997; see Hasselquist and Kempenaers 2002).
At the within-family level, offspring sex ratio has been analyzed in relation to laying or hatching order under the hypothesis that mothers adaptively adjust the position of sons and daughters in the laying (and thus also hatching) sequence according to the effects of sex-specific offspring resource requirements and the benefits accrueing to individual offspring from heading the size hierarchy in the brood (Nager et al. 1999; Badyaev et al. 2002; Krebs et al. 2002).
While the number of studies providing convincing evidence of non-random sex allocation in birds has rapidly increased in the last years, the generality and the evolutionary importance of facultative sex allocation strategies is still a debated issue (Hasselquist and Kempenaers 2002; Komdeur and Pen 2002; West and Sheldon 2002). Studies finding significant relationships between offspring sex ratio and parental phenotypic or ecological factors have been paralleled by others showing no significant associations in different species or in different populations of the same species (Rosivall et al. 2004). Most studies reporting ‘positive’ results have provided adaptive interpretations for the observed patterns of sex allocation (see Hasselquist and Kempenaers 2002; Komdeur and Pen 2002; West and Sheldon 2002). However, these interpretations are often hampered by the correlational nature of the majority of the studies, which is enforced by the fact that the specific covariates of sex ratio under scrutiny (e.g., population-level tertiary sex ratio, resource availability, parental age) are hardly amenable to experimental manipulation (but see Le Galliard et al. 2005; Allsop et al. 2006; Warner and Shine 2007). In addition, the generality of the occurrence of sex ratio manipulation has also been questioned based on the inconsistency of predictors of sex allocation within single populations between years (Radford and Blakey 2000). In fact, only relatively few studies have investigated offspring sex ratio over several years (e.g., Budden and Beissinger 2004).
In the present study, we analyze the covariation between the proportion of male and female barn swallow offspring generated over 10 years in a large sample of broods and two classes of variables. First, we investigate the relationships with clutch size, laying date and egg laying order. Second, we analyze the association with diverse ecological factors, including temperature and rainfall during the pre-laying to hatching periods, colony size and breeding sex ratio.
For some of the variables we considered, we could formulate explicit predictions about the effect on the sex of the offspring. In the barn swallow population we have been studying since 1993, nestling sex ratio has been shown to be close to 1:1 on average (but see below), whereas the sex ratio among the adults has been male-biased on average during the set of years when our population has been studied (see Saino et al. 2003; our unpublished data), suggesting female-biased annual mortality after fledging. Since the phenotypic quality of barn swallow offspring is known to decline with increasing brood (and thus clutch) size as well as late in the breeding season (Saino et al. 1997), we predicted that the proportion of males should increase with breeding date and with clutch size because parents are expected to adaptively invest more in the rare sex (i.e., females) particularly when extrinsic conditions enhance offspring phenotypic quality. We also speculated that the combined negative effects of date and clutch size on offspring quality should result in an increasing proportion of males late in the season particularly in large clutches. Under the assumption that females, being the under-represented sex in the breeding population, are more valuable than males, we predicted that the last eggs in a clutch would have relatively more chances of carrying a son than a daughter compared to the first eggs because late-hatching nestlings are at a disadvantage in sib-sib competition and are therefore in poorer condition (Ferrari et al. 2006).
Ecological conditions during breeding may influence optimal maternal sex allocation, via an effect on maternal condition, which can affect the amount of reserves that are delivered to the eggs. If sons and daughters are differentially susceptible to variation in egg quality, sex of the offspring may covary with temperature and rainfall during ovulation and laying. In the barn swallow, it has been shown that high air temperature result in increased egg mass and yolk antioxidants (Saino et al. 2004a), possibly because high air temperatures enhance availability of insect prey during the phase of rapid yolk development (Johnson 2000) or during the period of deposition of the albumen. Similarly, rainfall might also influence maternal condition and thus egg quality via potentially contrasting effects on foraging success and production of insect populations. However, the sex that suffers more from reduced egg quality cannot be univocally predicted based on current knowledge of our model species.
The effect of colony size could also not be predicted because of insufficient knowledge of the causal relationships between maternal physiology and offspring sex, and the potentially contrasting effects of coloniality on maternal physiology. On one hand, large colony size may affect the frequency of territorial disputes between breeding pairs and increase the level of social stress (Møller 1994). High levels of corticosterone, the main hormone involved in the stress response in birds, have been shown to be associated with a high proportion of daughters in the peacock (Pavo cristatus) (Pike and Petrie 2005, 2006). On the other hand, in large colonies, high rates of territorial aggressive interactions may translate into higher testosterone levels in the barn swallow (Galeotti et al. 1997). Experimentally increased testosterone levels, in turn, have been shown to result in a higher proportion of sons in the starling (Sturnus unicolor) (Veiga et al. 2004).
Finally, Fisherian theory of sex allocation predicts that parents should invest more in the rare sex. Thus, adult barn swallow may skew the sex ratio of their offspring towards the rarer sex based on current tertiary sex ratio. In addition, because of temporal autocorrelation of tertiary sex ratios in consecutive breeding seasons, it should pay adults to invest more in the production of the sex which is currently relatively rare because it will also be rare when current offspring will start to reproduce the following breeding season. We therefore predicted that tertiary sex ratio in one year negatively predicted the sex ratio of the offspring in that year, but also in the following year.
Materials and methods
This study was carried during 10 breeding seasons over the 12-years period 1994–2005 at a total of 29 colonies (=farms) located in the Po Valley east and south-east of Milano, mainly within the boundaries of the Parco Regionale Adda Sud in a climatically homogeneous, intensively cultivated lowland (see Ambrosini et al. 2002 for a detailed description of the study area). The data on offspring sex for most of the broods from years 1994 to 2000 considered in the present study had been presented in a previous study (Saino et al. 2002a), where, however, no analysis of the effect of ecological, temporal or laying order effects had been presented. The present study is thus based on a three-fold larger sample size, includes 4 additional years, and investigates relationships that had not been analyzed in the previous study. Given the large size of the sample of broods, in some years we could not identify the parents of most of the broods and we could therefore not analyze the covariation between sex ratio and parental age or e.g., maternal condition. The data considered in the present study refer only to unmanipulated broods.
In all study years, nests were visited every day to record breeding events. For the purposes of the present study, we considered the date when the first egg was laid and clutch size (i.e., the number of eggs at clutch completion). In the study years following 1999, the eggs were marked according to laying order. In a subsample of these nests at least part of the hatchlings could be assigned to their original egg by frequently visiting the nest around the predicted day of hatching. When nestlings were 7–12-days old, depending on the year, they were subjected to a blood sampling by puncturing the ulnar vein to extract genetic material for molecular sex determination (see below).
Correct identification of sex was verified first by sexing 40 adult barn swallows (20 putative males and 20 females) whose sex had been assigned according to the following criteria (see also Møller 1994): singing behavior (only males sing), outermost tail feathers length (only males have extremely long tails; only individuals that were presumably males with very long tails and females with very short tails were considered); egg incubation behavior (only females incubate in European barn swallow populations); incubation patch (only females develop an incubation patch); cloacal protuberance (which is more pronounced in males). All molecular sexing confirmed sex identification based on morphology and behavior. Furthermore, we re-sexed 40 nestlings (20 putative males and 20 putative females) that had been sexed by A. Pilastro (see Saino et al. 2002a), and invariably obtained the previously assigned sex. We are therefore confident that the genetic sex of the nestlings was correctly identified. For the purposes of the present study we considered only clutches ranging between 3 and 7 eggs because clutches of apparently two eggs are likely to result from predation of first or last laid eggs. For all eggs, and thus nestlings in a brood, laying date was considered as the day of laying of the first egg.
Sexing methods
Sexing procedure of nestlings from 1994 to 2000 was based on methods originally devised by Griffiths et al. (1998) (see also Saino et al. 1999, 2002a). Amplification of DNA extracted from samples from 2001 to 2005 was carried out in a total volume of 20 μl with the following final reaction conditions: 50 mM KCl; 10 mM Tris–HCl pH 9 (25°C); 0.1 % Triton X100; 2 mM MgCl2; 0.2 mM of each dNTP; 200 ng of each primer and 1.25 units of Taq Polymerase (Promega, Madison, WI, USA). Between 50 and 100 ng of genomic DNA were used as template. Polymerase Chain Reaction (PCR) was performed in a T1 Thermocycler (Biometra, Goettingen, Germany) under the following conditions: an initial denaturing step at 94°C for 7 min was followed by 30 cycles of 48°C for 30 s, 72°C for 30 s and 94°C for 1 min. The program concluded with a final cycle of 48°C for 30 s and 72°C and 72°C for 5 min. PCR products were separated by electrophoresis for 45 min at 7–10 V/cm in a 2.5% agarose gel stained with ethidium bromide. PCR products were visualized under UV light; a single band identified a male and two different bands identified a female. A 100 base pair ladder with a range 100–2642 bp (Roche, Mannheim, Germany) was used as size marker. A male and a female adult barn swallows were used a reference in every elecrophoresis.
Ecological variables
Colony size was expressed as the number of pairs breeding in the colony (=farm). Mean daily air temperature (°C) data recorded 2 m above ground level and daily rainfall data (mm) were obtained for the Montanaso Lombardo wheather station owned by the Italian Ministero delle Politiche Agricole e Foreste (www.ucea.it). The maximum distance between the colonies and the weather station was 25 km and the maximum difference in elevation was 25 m. These data were not available online for spring/summer 1994 at the time when the present analyses were conducted and sex ratio data for 1994 have therefore been excluded from the analyses of the effect of temperature and rainfall. After an exploratory analysis of the effect of temperature on a day-by-day basis (see Results), for each clutch we computed the mean of the mean daily temperatures during the: (1) ten days preceding laying of the first egg; (2) the days of laying (3 to 7 days depending on clutch size); (3) the first seven days of incubation; (4) the subsequent seven days, i.e., approximately until the day when the last egg in each clutch hatched. Rainfall data were organized in the same way as those of temperature. Temperature and rainfall data were not recorded by the Montanaso wheather station on some days. Mean temperatures and rainfall were calculated only when meteorological data were available for at least half of the days included in a particular phase of the breeding cycle. Missing meteorological data led to the exlcusion of a maximum of 19 nestlings for temperature and 46 nestlings for rainfall.
Data on tertiary sex ratio were obtained by capturing the large majority of the adults at some of the colonies (Saino et al. 2002b, 2004b). The size of the sample of adult birds captured ranged from 262 to 735 per year. Adults were marked individually with color and/or plastic bands and sexed according to standard methods. Adult sex ratios showed high positive annual autocorrelation (our unpublished data).
Statistical analyses
We used generalized linear mixed model analyses of variance to test the effect of predictor variables on offspring sex, which was scored as 0 = female or 1 = male. In these analyses we assumed a binomial error distribution and a logit link-function. Analyses were run using the GLIMMIX macro for SAS (Version 8), which uses iteratively reweighted likelihoods to fit generalized linear mixed models . The observed variances were consistent with the assumed distribution since the extra-dispersion parameter φ had values close to 1 in all analyses (Littell et al. 1996). In all models, nest of origin was included as a random effect. Year was included as a fixed effect because we aimed at testing the interannual variation in the frequency of male and female offspring over the specific period we considered and we also aimed at testing the effect of the interaction between year and breeding date on offspring sex. Clutch size was also included as a fixed factor and ecological variables as continuous covariates.
In some cases, the models were overparametrized and the convergence criterion of the iterative GLIMMIX procedure was not met. In these cases we simplified the model by removing higher order interactions first. However, we also adopted a step-down procedure to simplify the models by excluding non-significant terms (Crawley 1993), whereby at each step of the procedure the term with the smallest effect size was excluded from the model. Interactions were excluded before the relevant main effects. If not otherwise specified, parameter estimates are given with their standard error in parentheses.
Results
The sample included 553 broods from 10 breeding seasons in the 12-years period 1994–2005. We sexed 2264 nestlings, 1185 of which were male and 1079 were female (proportion of males = 0.523). Average clutch size was 4.88 (0.03, N = 553). Therefore, 83.9% of the eggs generated a nestling that could be sexed. Since in the barn swallow approximately 10% of the eggs fail to hatch (Saino et al. 2004a), approximately 6% of the eggs generated a nestling that could not be sexed. The mean within-brood sex ratio was 0.528 (0.012) and the mean within-brood proportion of eggs that generated a sexed offspring was 0.843 (0.008).
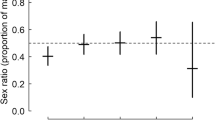
The annual offspring population sex ratio did not deviate from 0.50, except in 1997 and 2004 (binomial P = 0.050 and <0.001, respectively; P always >0.080 in the remaining years) (Fig. 1). Overall, we found a slight excess of sons when data from all years were pooled (binomial P = 0.027). This was largely due to the effect of 2004, when the sex ratio was male-biased and the sample was large. However, the number of males did not significantly exceed the number of females at pairwise comparisons within years (sign test: P = 0.34). In fact, sex ratio was <0.50 in 6 out of 10 study years. The chances of nestlings being male varied among years (F = 3.29, df = 9, 499, P = 0.0007; Fig. 1). The proportion of males was significantly larger in 2004 than in 1999 (P = 0.003) or 2002 (P = 0.014) at Tukey tests, whereas the differences were non-significant between the other pairs of years.
There was no significant interannual trend of variation in the sex ratio of the offspring in the population (correlation coefficient between year and sex ratio: r = −0.150, N = 10, df = 8, P = 0.68), and a similar result was obtained when mean within-brood sex ratio was considered (r = −0.203, N = 10, df = 8, P = 0.57). There was no temporal autocorrelation of offspring sex ratios between consecutive years (P > 0.70, Box–Ljung tests for analyses of population sex ratio and mean brood sex ratios).
Sex ratio of the adults (tertiary sex ratio) did not predict offspring sex ratio during the current year (r = 0.14, N = 10, df = 8, P = 0.71) or in the following breeding seasons (r = 0.19, N = 10, df = 8, P = 0.60).
Sex allocation in relation to clutch size, laying date and laying order
The frequency distribution of the clutches according to their size was as follows (number of broods in parenthesis): 3 (28); 4 (108); 5 (308); 6 (92), 7 (7). We analyzed the independent and combined effects of year, clutch size and laying date on offspring sex in mixed model analyses of variance. Only two-ways interactions where included in the initial model before stepdown selection of predictors because of overparametrization (see Methods). The effects of the interactions between year and laying date or clutch size on sex of the offspring were non-significant (Table 1). After step-down selection (Table 1), the factor year retained a highly significant effect on sex (see also above). In addition, the effect of laying date varied according to the size of the clutch (Table 1). This relationship was highly significant for clutches of 6 eggs, with the positive sign of the coefficient, implying that the proportion of males increased late in the season (Fig. 2). The slope of the relationship for clutches of seven eggs was also positive and similar to that for 6 eggs, albeit non-significant possibly because of the small sample size. In fact, clutches of 7 eggs were very rare (1.3%, see above). The slopes of the relationship for clutches of 5 or 4 eggs were similar and very close to 0, whereas for clutches of 3 the slope was negative (Table 1). Mean laying date computed across years was May 8th (±15.9 days SD) in the present sample. In the period comprised between −2 SD and +2SD of the mean laying date, the proportion of males could be predicted to increase from 0.324 to 0.835 in clutches of 6, based on a linear mixed model analysis of variance of nestlings from these clutches with nest as a random factor. In the same time interval, the probability of nestlings being males in the clutches of 7 was predicted to increase from 0.370 to 0.947, whereas this probability declined from 0.800 to 0.386 in clutches of 3 eggs. When we tested for an overall variation in sex ratio in relation to laying date and clutch size by excluding the interaction term from the model, no significant effects emerged (details not show). An analysis where clutch size was included as a covariate rather than as a factor consistently showed a significant interaction between the effects of laying date and clutch size (F = 5.32, df = 1, 596, P = 0.021); the positive coefficient associated with the interaction term (0.009 (0.004 SE)) indicates that a unit increase in date had a larger positive effect on the proportion of males in large compared to small broods.
Proportion of males in individual broods originating from clutches of different sizes in relation to the date of laying of the first egg of the clutch. Regression lines are fitted by GLM analyses with a binomial error distribution and a logit link function with brood as a random effect and laying date as a covariate. Full circles indicate two overlapping data points and open squares indicate 3 overlapping data points. In the graph for clutches of 6 and 7 eggs, triangles refer to clutches of 7 eggs (open symbol: 1 datum; filled symbol: two overlapping data). The line fitted to data for 7-eggs clutches is dashed
Laying order of the original egg could be identified for 875 nestlings from 219 nests. The effect of laying order on sex was non-significant (F = 0.34, df = 1, 739, P = 0.561) in a mixed model analysis of variance including the same terms as the minimum adequate model presented in Table 1.
Brood sex ratio in relation to meteorological conditions during breeding, colony size and tertiary sex ratio
We first aimed at testing the effect of ambient temperature and rainfall on offspring sex. As noted above, we had no a priori expectations on what period relative to egg laying had greater chances to influence the probability of an egg containing a male or a female. We therefore first ran separate mixed model analyses of variance in which temperature or rainfall in each day from day −10 (0 = date of laying of the first egg) to day +20 (which corresponds to the day of hatching of the largest clutches) were included as covariates together with the same terms retained in the minimum adequate model presented in Table 1, and investigated the size of the effect as reflected by their t-value. These exploratory analyses showed that the largest effects of temperature occurred during laying and shortly after clutch completion (Fig. 3). However, no clear pattern of temporal variation in the intensity of the effect of rainfall emerged (details not shown). We therefore decided to compute mean temperatures in four different stages of the breeding cycle, i.e., (1) prelaying; (2) laying; (3) first half of the incubation period; (4) second half of the incubation period (see Methods). For homogeneity, the same decision was adopted for rainfall data. Sex of the offspring was then analysed in relation to weather conditions in four different generalized mixed models in which we always included the same terms as in the minimum adequate model in Table 1 plus temperature in period 1; temperatures in periods 2–4; rainfall in period 1; rainfall in periods 2–4. All meteorological variables could not be simultaneously included in the same model because of overparametrization.
Effect size (t-values) for the effect of mean daily temperature on the likelihood of an egg containing male or female embryo, as obtained from separate mixed model analyses of variance in which temperature in each day from day −10 (0 = date of laying of the first egg) to day +20 (which corresponds to the day of hatching of the largest clutches) was included as a covariate. The days of laying are indicated by the sequence of up to six eggs
Temperature during the prelaying period did not predict sex (F = 0.01, df = 1, 485, P = 0.939). High temperatures during the laying and early incubation periods were associated with relatively high frequency of males among the offspring (Figs. 3 and 4). Only the effect of temperature during the laying period, however, was statistically significant (F = 7.08, df = 1, 508, P = 0.008, coefficient = 0.080 (0.030); first week of incubation: F = 2.08, df = 1, 471, P = 0.149, coefficient = 0.041 (0.029); second week: F = 1.67, df = 1, 506, P = 0.197, coefficient = 0.043 (0.033)). Analyses on rainfall data before laying or during laying and incubation showed no significant effect on offspring sex (details not shown). Population offspring sex ratio was not predicted by mean air temperature during the breeding season (r = −0.34, N = 9, df = 7, P = 0.37), and the same was the case when the concomitant effect of annual mean laying date was controlled for in partial correlation analyses.
In an analysis including the terms retained in the stepdown model presented in Table 1, the chances that nestlings were male showed a marginally significant increase with colony size (F = 4.03, df = 1, 521, P = 0.045, coefficient = 0.008 (0.004)).
Discussion
In this long-term correlational study of offspring sex ratio of a large sample of barn swallow broods we found that the proportion of sons: (1) varied among years but was unrelated to current tertiary sex ratio or tertiary sex ratio in the previous year; (2) increased with laying date in large but not in medium-sized or small clutches; (3) increased with temperature during laying (but was unaffected by temperature in other periods of the breeding cycle and by rainfall) and with colony size. These patterns were consistent between years.
In the present study we did not make a specific attempt to investigate whether the observed variation in offspring sex ratio arose at the time of ovulation and meiosis, or during incubation or shortly after hatching (before blood sampling) via sex-related mortality. A long-term study of embryo sex ratio based on egg collection from several hundreds of nests would be ethically unacceptable. Sampling blood in the first days after hatching is technically difficult and may result in extensive mortality, with relatively small benefits compared to sampling blood few days later. In fact, post-hatching mortality is low in this population and is mainly due to complete loss of broods following predation and accidental reasons that are likely to be unrelated to offspring sex. Partial brood mortality for starvation and parasitism, and thus unrelated to predation, death of either parent or accidental reasons (e.g., nest falling) accounts for only less than 5% of hatched eggs (our unpublished data), and is particularly low during the late nestling period. Thus, we consider our data to accurately reflect offspring sex ratio around the time of fledging but we will not make any strong claims about when sex ratio variation is established, although we are convinced that given the very low nestling mortality, sex ratio at fledging reflects that at at hatching. Admittedly, we have no clue to whether mortality differs between the sexes before hatching. We will therefore discuss some of the proximate mechanisms that may be operating in influencing sex ratio, and interpret the findings in an evolutionary perspective.
Annual secondary sex ratio variation in relation to tertiary sex ratio
Fisher’s model of sex allocation predicts that parents adjust their offspring sex ratio favoring the sex that is currently less frequent in the breeding population. In species where generations overlap and interbreed, however, the chances that an individual will acquire a mate will also depend on the sex ratio of the previous generations. We had thus also speculated that tertiary sex ratio should predict offspring sex ratio in the population the next year because of strong temporal autocorrelation of the tertiary sex ratio between consecutive years (see Methods). Thus, we tested the correlations between offspring sex ratio in year i and tertiary sex ratio both in year i−1 and i, while predicting a negative relationship. However, the data did not support either of these predictions and weak, positive, statistically non-significant relationships were observed.
For breeding adults, assessing the tertiary sex ratio in the current population, and adjusting offspring sex ratio consequently, may be difficult and particularly so for individuals that arrive from migration and thus start breeding early. This is because early-arriving individuals will have their first clutch when a large fraction of the population is still not present at the breeding grounds (see Saino et al. 2002b, 2004b). In addition, tertiary sex ratio may vary locally, thus making the local tertiary sex ratio a poor indicator of population sex ratio. Finally, the overall effect of sex ratio adjustment by older individuals at the population level based on local (colony) tertiary sex ratio during the previous year may be weak and obscured by sex allocation decisions adopted by immigrating yearlings, which should not be influenced by tertiary sex ratio during the previous year. However, extremely high breeding phylopatry, low post-natal dispersal distances, and onset of breeding at the age of 1 year in this species (Møller 1994) should facilitate adaptive adjustment of sex allocation in relation to fluctuations in local tertiary sex ratio.
Offspring sex ratio varied significantly between consecutive years, but without a clear temporal trend. Deviation of offspring sex ratio from parity also varied markedly among years. It has been shown in some studies that maternal condition may influence sex allocation and thus explain variation in secondary sex ratio at the family level but also at population level among years (e.g., Nager et al. 1999; Badyaev et al. 2002). The relationship between brood sex ratio and maternal condition could not be tested in the absence of a reliable indicator of maternal condition (e.g., body mass corrected for body size; Whittingham et al. 2005) for all the broods. Preliminary analyses of the effect of ecological conditions in the winter quarters of Italian barn swallows, as reflected by the Normalized Difference Vegetation Index, suggest no carryover effects on offspring sex ratio the following spring (our unpublised data; Saino et al. 2004b). In addition, air temperature, which was found to predict among-families variation in offspring sex ratio, did not predict interannual variation.
In summary, present results do not support the hypothesis that annual offspring sex ratio at the population level is adjusted according to a mechanism of differential allocation to the underrepresented sex in the breeding population or is influenced by major ecological factors during breeding or wintering.
Family sex ratio in relation to clutch size, laying date and laying order
The results of the analyses of the effects of clutch size and laying date partly confirmed the predictions based on previous knowldege of the same population of barn swallows. Male-biased tertiary sex ratio recorded on average in our study population since 1993 (e.g., Saino et al. 2003) together with the observation of even secondary sex ratios and the decline in offspring quality in relation to brood (and thus clutch) size and laying date (Saino et al. 1997), led us to speculate that the proportion of females should decrease with laying date and clucth size. Opposite to our expectation, the proportion of males was unaffected by laying date and clutch size. However, the results confirmed the prediction of a larger positive effect of laying date on the proportion of males in large compared to small clutches. This pattern of multiplicative effects of laying date and clutch size may reflect an adaptive strategy of sex allocation because some sons will fail to acquire a mate in this socially monogamous species with a predominantly male-biased breeding sex ratio. Producing more males particularly in late, large clutches will thus mainly reduce the phenotypic quality and viability of the less valuable sex.
Based on a large sample, we found no variation of offspring sex in relation to laying order. Existing evidence for variation in the proportion of sons along the laying sequence varies considerably between species (Badyaev et al. 2002; Arnold et al. 2003; Chicon et al. 2003). The barn swallows seem to adopt a ‘brood survival’ strategy, as mortality due to limiting parental care appears minimal in our study population (Ferrari et al. 2006). While late laid eggs hatch relatively late, the disadvantage for late hatched nestlings seems not to be reflected into higher mortality risk (Ferrari et al. 2006). Differential sex allocation strategies in relation to laying order may thus have not evolved in the barn swallow because of weak selection differential on early compared to late hatched nestlings.
Family sex ratio in relation meteorological conditions and colony size
Male offspring were more frequent when eggs had been laid in relatively warm days. The statistically significant effect of temperature during egg laying as well as the non-significant effect at other stages of the breeding cycle were observed while controlling for the effects of laying date. Thus, variation in sex ratio with temperature does not reflect seasonal variation in sex ratio. In fact, the simple effect of laying date on sex ratio was found not to be significant. We had speculated that temperature could affect sex allocation by influencing food availability and thus allocation of resources available to egg formation, based on a previous study where we could show that temperature positively affects egg size and quality (Saino et al. 2004a). However, in the absence of information about the fitness functions of sons and daughters in relation to egg size and quality, we could not formulate explicit predictions about which sex would be advantageous to produce under specific meteorological conditions.
In birds, ovarian follicles enter a phase of rapid increase (the Rapid Yolk Development, RYD, phase) starting several days before the ovarian follicle matures and ovulation occurs (Johnson 2000). During the RYD, yolk material is accumulated. Shortly (0.5–4 h) before ovulation, when meiosis I occurs (Romanoff 1960; Johnson 1996), one of the sex chromosomes (either W or Z) is retained by the oocyte while the other migrates to the polar body, thus ultimately determining the sex of the zygote. The egg is then laid after deposition of the albumen and formation of the eggshell in different parts of the oviduct approximately one day after ovulation. Thus, the sex of the embryo is genetically determined one day or so before laying.
Recent studies have shown experimentally that maternal hormonal profile may affect sex determination. Eggs laid by poultry hens injected with a high dose of progesterone, the main follicular hormone secreted during meiosis, were less likely to carry a male embryo compared to eggs from control females or females injected with a small dose of the hormone (Correa et al. 2005). Pike and Petrie (2005, 2006) have found that naturally or experimentally elevated maternal corticosterone levels in peafowl are associated with a high proportion of daughters. Some studies have shown that ambient temperature can have short term effects on the hormonal profile of birds, and particularly on the concentration of progesterone and testosterone, which have been identified in manipulative studies as candidate participants of the physiological machinery controlling sex of ovulated eggs (see above). In captive turkeys, high ambient temperatures result in reduced levels of progesterone and testosterone (Rozenboim et al. 2004). Temperature may have an effect mediated by ecological factors (e.g., food availability) which, in turn, have the potential to interfere with maternal hormonal profile. For example, low temperatures may depress food availability, thus influencing metabolic pathways which are partly regulated by glucocorticosteroids. Circulating corticosterone concentrations are known to vary with ambient temperature and also air pressure (Frigerio et al. 2004; Wolfenson et al. 2001), possibly because of the involvment of corticosterone in metabolism and control of energetic homeostasis (Norris 1997; Remage-Healey and Romero 2001). It should be noted, however, that these putative effects of temperature on sex chromosome segregation, either direct or indirect, should operate via short-term influences on maternal physiology, given that no seasonal trend in offspring sex ratio existed and air temperatures markedly vary during the barn swallow breeding season. Hence, long-term mechanisms of physiological adjustment of hormone levels with varying temperatures may prevent clear seasonal variation of offspring sex ratio. Conversely, short-term variation in temperatures may perturbate maternal physiology, thus ultimately affecting the ‘sex’ of maternal gametes. This mechanism is different from that envisaged by Göth and Booth (2005) where the observed association between brood sex ratio and temperature was inferred to be due to an effect of temperature during incubation on embryo mortality.
These mechanisms mediating the effect of temperature on offspring sex may simply reflect the consequences of non-adaptive inability of maternal physiology to buffer the consequences of short-term variation in extrinsic factors. Small variation in sex ratios could thus be non-adaptive and mainly reflect the consequences of variation in maternal physiology, which impose only marginal fitness costs and are therefore exposed to weak negative selection. The weak effects of extrinsic factors on sex ratios in fact corroborates the idea that the variation in sex ratios we documented mainly arises because of e.g., non-adaptive maternal effects in the presence of weak contrasting selection.
Alternatively, they may represent an adaptive mechanism that matches relative egg quality to offspring sex. A study of the same population of barn swallows has shown that large eggs are laid during spells of good weather (Saino et al. 2004a). Much of the variation in egg size may be due to egg albumen content (Ferrari et al. 2006). The albumen is produced by the mother during the hours preceding laying. In an experiment where albumen was subtracted from the eggs, nestlings hatched from experimental eggs were found to be smaller than those originating from control eggs throughout the nestling period (Ferrari et al. 2006). If relatively low temperatures reduce egg mass (Saino et al. 2004a) via an effect on albumen deposition, females may benefit from adjusting the sex of the offspring by producing more of the sex which will suffer less from reduced egg albumen content. However, whether individuals less resistant to reduced or albumen mass tend to be male or female is unknown.
The association between offspring sex and temperature in unlikely to be due to adaptive adjustment of offspring sex based on yolk quality because temperature during the RYD phase (i.e., during several days before the start of laying) did not predict offspring sex. In theory, temperature may differentially affect viability of the offspring either sex shortly after laying, before incubation starts. In addition, the possibility exists that sex-related mortality occurs during incubation as a consequence of temperature variation. The latter hypothesis seems unlikley to be correct, given that variation in temperature during incubation did not predict offspring sex.
In conclusion, we found that barn swallow offspring sex ratio varied according to year, the combined effects of laying date and clucth size, and a major ecological factor such as ambient temperature during laying. Some of these patterns of variation can be reconciled with adaptive interpretations of parental sex allocation strategies, within the heuristic limits imposed by the correlational nature inherent in this kind of long-term, large-scale field studies. However, offspring sex ratio was unrelated to tertiary sex ratio, lending no support to the prediction based on theory of frequency-dependent sex allocation. In general, we found that the patterns of the significant relationships between offspring sex and phenological or ecological variables did not vary among years. While variation in progeny sex ratio in relation to temporal and phenological variables has been documented in other studies of birds, to the best of our knowledge this is the first observation of an association between ambient temperature during laying and offspring sex. Homeothermy of birds may have led researchers to overlook the potential consequences of this factor for progeny sex ratio. Ambient temperature may be an important determinant of sex allocation either via a non-adaptive response of maternal physiology to short-term environmental changes or as part of an adaptive sex allocation strategy whereby parents decide on offspring sex based on the quality of the eggs as ultimately influenced by temperature.
References
Ambrosini R, Bolzern AM, Canova L, Arieni S, Møller AP, Saino N (2002) The distribution and colony size of barn swallows in relation to agricultural land use. J Appl Ecol 39:524–534
Allsop DJ, Warner DA, Langkilde T, Du W, Shine R (2006) Do operational sex ratios influence sex allocation in viviparous lizards with temperature-dependent sex determination? J Evol Biol 19:1175–1182
Appleby BM, Petty SJ, MacDonald DW (1997) Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco)? Proc R Soc Lond B 264:1111–1116
Arnold KE, Griffiths R, Stevens DJ, Orr KJ, Adam A, Houston DC (2003) Subtle manipulation of egg sex ratio in birds. Proc R Soc Lond B 270:S216–S219
Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318
Bensch S, Westerdahl H, Hansson B, Hasselquist D (1999) Do females adjust the sex of their offspring in relation to the breeding sex ratio? J Evol Biol 12:1104–1109
Bradbury RB, Blakey JK (1998) Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc R Soc Lond B 265:895–899
Budden AE, Beissinger SR (2004) Against the odds? Nestling sex ratio variation in green-rumped parrotlets. Behav Ecol 15:607–613
Burley NT (1981) Sex ratio manipulation and selection for attractiveness. Science 211:721–722
Chicon M, Dubiec A, Stoczko M (2003) Laying order and offspring sex in blue tits (Parus caeruleus). J Avian Biol 34:355–359
Correa SM, Adkins-Regan E, Johnson PA (2005) High progesterone during avian meiosis biases sex ratios towards females. Biol Lett 1:215–218
Crawley MJ (1993) GLIM for ecologists. Blackwell Scientific, Oxford
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proc Natl Acad Science, USA 93:11723–11728
Ferrari RP, Martinelli R, Saino N (2006) Differential effects of egg albumen content on barn swallow nestlings in relation to hatch order. J Evol Biol 19:981–993
Fisher RA (1958) The genetical theory of natural selection, 2nd edn. Dover Publications Inc., New York
Frigerio D, Dittami J, Mostl E, Kotrschal K (2004) Excreted corticosterone metabolites co-vary with ambient temperature and air pressure in male greylag geese (Anser anser). Gen Comp Endocrinol 137:29–36
Galeotti P, Saino N, Sacchi R, Møller AP (1997) Song correlates with social context, testosterone and body condition in male barn swallows. Anim Behav 53:687–700
Göth A, Booth DT (2005) Temperature-dependent sex ratio in a bird. Biol. Lett. 1:31–33
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hasselquist D, Kempenaers B (2002) Parental care and adaptive brood sex ratio manipulation in birds. Phil Trans R Soc Lond B 357:363–372
Krebs EA, Green DJ, Double MC, Griffiths R (2002) Laying date and laying sequence influence the sex ratio of crimson rosella broods. Behav Ecol Sociobiol 51:447–454
Komdeur J, Daan S, Tinbergen J, Mateman AC (1997) Extreme adaptive modification in sex ratio of the Seychelles warbler’s eggs. Nature 385:522–525
Komdeur J, Pen I (2002) Adaptive sex allocation in birds: the complexities of linking theory and practice. Phil Trans R Soc Lond B 357:373–380
Johnson AL (1996) The avian ovarian hierarchy: a balance between follicle differentiation and atresia. Poultry Avian Biol Rev 7:99–110
Johnson AL (2000) Reproduction in the female. In: Whitlow CG (ed) Avian Physiology. Academic Press, London, pp 569–596
Le Galliard JF, Fitze PS, Cote J, Massot M, Clobert J (2005) Female common lizards (Lacerta vivipara) do not adjust their sex-biased investment in relation to the adult sex ratio. J Evol Biol 18:1455–1463
Littell RC, Milliken GA, Stroup WW, Wolfinger RS (1996) SAS system for mixed models. SAS Institute Inc., Cary, North Carolina
Møller AP (1994) Sexual selection and the barn swallow. Oxford University Press, Oxford
Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Norris DO (1997) Vertebrate Endocrinology. Academic Press, Boston
Pen I, Weissing FJ (2002) Optimal sex allocation: steps towards a mechanistic theory. In: Hardy ICW (ed) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge, pp 26–45
Pike TW, Petrie M (2005) Offspring sex ratio is related to paternal train elaboration and yolk corticosterone in peafowl. Biol Lett 1:204–207
Pike TW, Petrie M (2006) Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc R Soc Lond B 273:1093–1098
Radford AN, Blakey JK (2000) Is variation in brood sex ratios adaptive in the great tit (Parus major)? Behav Ecol 11:294–298
Ranta E, Lummaa V, Kaitala V, Merila J (2000) Spatial dynamics of adaptive sex ratios. Ecol Lett 3:30–34
Remage-Healey L, Romero M (2001) Corticosterone and insulin interact to regulate glucose and trygliceride levels during stress in a bird. Am J Physiol Regul Integr Comp Physiol 281:R994–R1003
Romanoff AJ (1960) The avian embryo. MacMillian, New York
Rosivall B, Torok J, Hasselquist D, Bensch S (2004) Brood sex ratio adjustment in collared flycatchers (Ficedula albicollis): results differ between populations. Behav Ecol Sociobiol 56:346–351
Rozenboim I, Mobarky N, Heiblum R, Chaiseha Y, Chang SW, Biran I, Rosenstrauch A, Sklan D, El Halawani ME (2004) The role of prolactin in reproductive failure associated with heat stress in the domestic turkey. Biol Repr 71:1208–1213
Rutstein AN, Gorman HE, Arnold KE, Gilbert L, Orr KJ, Adam A, Neger R, Graves JA (2005) Sex allocation in response to paternal attractiveness in the zebra finch. Behav Ecol 16:763–769
Saino N, Ambrosini R, Martinelli R, Calza S, Møller AP, Pilastro A (2002a) Offspring sexual dimorphism and sex-allocation in relation to parental age and paternal ornamentation in the barn swallow. Mol Ecol 11:1533–1544
Saino N, Ambrosini R, Martinelli R, Møller AP (2002b) Mate fidelity, senescence in breeding performance, and reproductive trade-offs in the barn swallow. J Anim Ecol 71:309–319
Saino N, Calza S, Møller AP (1997) Immunocompetence of nestling barn swallows (Hirundo rustica) in relation to brood size and parental effort. J Anim Ecol 66:827–836
Saino N, Ellegren H, Møller AP (1999) No evidence for adjustment of sex allocation in relation to paternal ornamentation and paternity in barn swallows. Mol Ecol 8:399–406
Saino N, Romano M, Ambrosini R, Ferrari RP, Møller AP (2004a) Timing of reproduction and egg quality covary with temperature in the insectivorous barn swallow (Hirundo rustica). Funct Ecol 18:50–57
Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP (2003). Maternal antibodies but not carotenoids in barn swallow egges covary with embryo sex. J Evol Biol 16:516–522
Saino N, Szèp T, Romano M, Rubolini D, Møller AP (2004b) Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol Lett 7:21–25
Sheldon BC, Andersson S, Griffith SC, Ornborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Thuman KA, Widemo F, Griffith SC (2003) Condition-dependent sex allocation in a lek-breeding wader, the ruff (Philomachus pugnax). Mol Ecol 12:213–218
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 177:90–92
Veiga JP, Viñuela J, Cordero PJ, Aparicio JM, Polo V (2004) Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm Behav 46:47–53
Warner DA, Shine R (2007) Reproducing lizards modify sex allocation in response to operational sex ratios. Biol Lett 3:47–50
West SA, Sheldon BC (2002) Constraints in the evolution of sex ratio adjustment. Science, 295:1685–1688
Whittingham LA, Dunn PO (2000) Offspring sex ratios in tree swallows: females in better condition produce more sons. Mol Ecol 9:1123–1129
Whittingham LA, Dunn PO, Nooker JK (2005) Maternal influences on brood sex ratios: an experimental study on tree swallows. Proc R Soc Lond B 272:1775–1780
Wolfenson D, Bechrach D, Maman M, Graber Y, Rozenboim I (2001) Evaporative cooling of ventral regions of the skin in heat-stressed laying hens. Poultry Sci 80:958–964
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saino, N., Martinelli, R. & Romano, M. Ecological and phenological covariates of offspring sex ratio in barn swallows. Evol Ecol 22, 659–674 (2008). https://doi.org/10.1007/s10682-007-9189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-007-9189-1