Abstract
The objective of this study was to identify the aptitude of sweet potato genotypes derived from botanical seeds and select them for the aptitudes human consumption, ethanol production, and animal feed through separate indices. A row–column incomplete block design was used, restricting relatedness in the draw. A total of 1604 half-sib genotypes resulting from recombination of 55 clones from the germplasm collection of the Federal University of Lavras were evaluated. The accessions UFVJM 58 and UFVJM 61 were used as controls, for a total of 1606 treatments. The aptitude indices corresponded to the mean values of the 10 traits evaluated, with weights assigned to each trait according to the aptitude of interest. The data of all three aptitudes were transformed and standardized. Then, using the Zi index and with 2.5% selection pressure, the most promising genotypes were selected through the best linear unbiased predictor according to the traits evaluated and the aptitude of interest. Sixty genotypes were selected (out of 1604 tested) based on one or more of the three reported aptitudes: 25 showed a single aptitude, whereas 20 showed dual aptitude and 15 showed triple. The data obtained will provide information for breeding programmes of the sweet potato crop. There is great genetic variability for the traits evaluated, facilitating the selection of new genotypes, with the possibility of obtaining new cultivars important for national food sovereignty and making a significant social contribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sweet potato (Ipomoea batatas (L.) Lam.) is cultivated in 111 countries, with a global production of 105.19 million tons in an area of approximately 8.62 million hectares (Faostat 2018). Its main use is for human consumption because it is a source of energy, vitamins, and minerals (Vieira et al. 2015). The plant also has potential as a functional food, given the presence of bioactive compounds such as carotenoids and anthocyanins (Vizzotto et al. 2017; Laurie et al. 2017; Zhang et al. 2016), antioxidants (Vizzotto et al. 2017), and phytonutrients (Sweet Potato 2014; Sun et al. 2019).
The crop has great genetic diversity, which is of great relevance for breeding. This is due to the high recombination potential within the species. King and Bamford (1937) counted the chromosomes of 13 species of Ipomoea and found that only I. batatas had 90 chromosomes, which explains the great diversity. In addition, the crop is strictly allogamous, hexaploid (2n = 6x = 90) and self-incompatible (King and Bamford 1937), which gives it a high degree of heterozygosity in segregating populations. Other species of Ipomoea show less genetic diversity, especially due to the lower number of chromosomes, with Ipomoea trifida (2n = 2x = 30), I. splendor-sylvae (2n = 2x = 30), I. tenuissima (2n = 2x = 30), I. leucanta (2n = 2x = 30) and I. tabascana (2n = 4x = 60) (Sun et al. 2019). Therefore, sweet potato botanical seeds have high potential for use in breeding programmes to obtain new varieties (Folquer 1978). This is because each seed obtained may give rise to a new commercial genotype (Torquato-Tavares et al. 2017). This process of recombination, seed obtainment, and screening is the basis for the selection and breeding of the sweet potato crop, which may involve several stages of polycrosses and recurrent selection (CIP 2009).
In Brazil, sweet potato has a high number of genotypes presumably from crosses along its domestication (Torquato-Tavares et al. 2017). This has resulted in various potential uses for the species, such as ethanol production, forage production for animal feed, and, especially, human consumption (Torquato-Tavares et al. 2017; Ferreira et al. 2019a; Donato et al. 2020). With the exception of its use for human consumption, the potential diverse agronomic aptitudes of the crop have been hardly exploited, even in well-established breeding programmes. Thus, it is important to research these new uses and to disseminate genotypes adapted to different potentialities and regions.
Most sweet potato cultivars were developed mainly for human consumption, due to the high nutritive value of the storage roots of the crop. However, the plant has a high potential for ethanol production and animal feed, which makes it increasingly necessary to identify these new agronomic demands in crop breeding (Gonçalves Neto et al. 2011).
Sweet potato stems and leaves are rich in starch, sugars and vitamins, in addition to having high percentages of crude protein and good digestibility (Monteiro et al. 2007). However, they are commonly discarded after the roots are harvested, generating waste of a strategic food for the animals. In countries such as China and Vietnam, the stems and leaves are used pure or in association with roots as swine feed, either in fresh form or preserved as silage (Monteiro et al. 2007). Viana et al. (2011) highlights that the silage of sweet potato stems and leaves has protein, energy and fermentative contents suitable for animal feed, especially for ruminants. Silages produced from sweet potato stems and leaves have an average of 11.6% crude protein and 63.0% total digestible nutrients (TDN), in addition to low fiber content (Figueiredo et al. 2012; Azevedo et al. 2015), demonstrating the high nutritive value of this food.
As for the production of ethanol, studies indicate that sweet potato may have a higher biofuel yield than common crops such as sugarcane and corn (Viana et al. 2017). In addition, yeast cake from fermentation can also be provided as a component for animal nutrition, as it is a source of protein (Bennett 2015; Pietrosemoli et al. 2016). Another advantage is the lower cost of ethanol produced from sweet potatoes, as the cost of raw materials is generally lower. This indicates the high competitiveness of sweet potato in relation to bioenergetic crops, in addition to ecological, social and sustainable advantages. It is also possible to add value to the ethanol produced with sweet potatoes, using the best quality ethanol (absence of aldehydes and ketones) for the production of noble alcoholic beverages (Pellegrini 2014). Viana et al. (2017) identified promising sweet potato genotypes in research related to ethanol production, with effective yield values of up to 5,930 L ha−1 in 180 days of production and 11,860 L ha−1 year−1, surpassing the potential verified for sugarcane in Brazil. However, it is important to note that, in general, root yields in Brazil are still low, which directly compromises starch production and, consequently, ethanol yield. Therefore, the selection of cultivars for different purposes, such as human consumption, animal feed and ethanol production can provide yields of 25–30 Mg ha−1 in four to five months of cultivation, due to better exploitation of the genetic potential of the species (Andrade Júnior et al. 2009, 2012, 2018; Amaro et al. 2017).
In the selection of sweet potato genotypes for different agronomic aptitudes, the use of selection indices with specific weights for traits considered important for human consumption, animal feed, and ethanol production is a useful and efficient tool for selection (Gonçalves Neto et al. 2011; Camargo et al. 2016). Instead of traditional statistical tools, more refined strategies can maximize the use of indices in selection, increasing their efficiency. Among these tools, techniques that adopt mixed models have stood out, such as best linear unbiased prediction (BLUP), developed by C. R. Henderson in the 1940s (Bernardo 2002). BLUP is a statistical tool of greater precision for selection, showing advantages over the analysis of variance method, especially in the case of experiments with certain degrees of imbalance (Bernardo 2002). In addition, the method provides high efficiency in the classification and ranking of genotypes (Borges et al. 2010).
The objective of this study is to identify different aptitudes (human consumption, ethanol production, animal feed) of sweet potato genotypes obtained from botanical seeds, through the use of selection indices based on mixed models.
Materials and methods
Experimental site and procedure
The experiment was conducted in the experimental area of the Horticulture Sector of the Federal University of Lavras (UFLA), Lavras, Minas Gerais (MG), Brazil, located at 21°14′S latitude, 45°00′W longitude, and 918 m altitude. The soil of the site is classified as a typical Dystroferric Red Latosol (LVdf) (Santos et al. 2013). The climate is Cwa in the Köppen classification, with dry winters and predominant rainfall in the summer, with an average annual total precipitation of 1530 mm and an average annual temperature of 19.4 °C.
Origin of mother plants and treatments
The genotypes were obtained from random intercrossing (polycrosses) of 55 clones from the sweet potato germplasm bank of UFLA. The clones were recombined in the field from February to November 2017 in the municipality of Lavras, MG, Brazil.
From the 32 clones that flowered, 2,000 botanical seeds were obtained. The number of seeds collected per progeny (= half sib family) varied according to the flowering and seed production potential of each progeny. These seeds were taken to the Laboratory of Vegetable Evaluation of the Horticulture sector to break seed-coat dormancy using sulfuric acid. The chemical scarification of the seeds was performed as proposed by Rossel et al. (2008). The seeds were placed in germination boxes distributed equidistantly on Germitest® paper with moisture kept constant with distilled water. These boxes were labelled and stored in Biosystems Organized Development (BOD) incubators for germination, with a photoperiod of 12 h and a temperature of 25 °C, for 7 days. Of the 2,000 selected seeds, 81.35% germinated after treatment with sulfuric acid, originating 1,627 seedlings. After germination, the seedlings were transplanted into 162-cell polyethylene trays containing comercial substrate (Bioplant®) and kept under irrigation in a greenhouse for 30 days.
Plants were then transplanted to 1.0-m-wide × 0.25-m-tall soil beds, spaced 0.25 m between plants and 0.30 m between rows, with identification of the respective half sib family. The objective of this step was the production of stems for subsequent cloning of each genotype (one individual plant derived from a botanical seed). This phase of initial branching is called “A—Clones” by the International Potato Center (CIP 2009). At 90 days after planting, three 0.30 m to 0.40 m segment of stem (with six to eight internodes) of each genotype were collected, and were planted in the ‘clonal evaluation’ stage in mounds.
Clonal evaluation
This stage (“screening” as called by the International Potato Center 2009) was performed to evaluate and characterize the 1627 genotypes obtained (treatments). The experiment was done in the field in the same location as the previous stages (Olericrop Sector of DAG-UFLA).
Initially, the soil of the area was analysed for subsequent fertilization as recommended by CFSEMG (1999). There was no need for liming. The soil was prepared using one subsoiling and two harrowing procedures. Then mounds, spaced 1.0 m apart, were prepared with the aid of a furrower. The fertilization at planting consisted of 700 kg ha−1 of the NPK fertilizer with formula 04-14-08. At planting, 28, 41 and 47.5 kg ha−1 of N, P and K were applied, respectively, and 10 Mg ha−1 of organic compost. After planting, a single top-dressing fertilization was performed with 30 kg ha−1 of nitrogen using 150 kg ha−1 of ammonium sulfate (20% N) in a single dose at 30 days after planting.
Sweet potato stems were planted on April 26, 2018, and harvested on October 29, 2018, 185 days after planting. The spacing used was 0.30 m between plants, and there were three plants per plot. The planting depth of the branch was 0.15 m, and the spacing between rows was 1.0 m, corresponding to a population of 33,333 plants ha−1. Replanting was performed periodically up to the 15th day after planting to maintain the three plants of the experimental unit. Plots that did not survive were replaced by controls, totalling 96 plots of these genotypes. The plants were monitored and showed no need for any pest and disease control measures. Weed management was performed according to crop needs. Sprinkler irrigation was performed using a linear low-density polyethylene hose line with laser-drilled holes.
Experimental design and treatments
The experimental design used was a row–column incomplete block design (IBD), similar to an IBD with Honyaku design (Federer 1956). The regular treatments consisted of 1,604 half-sib sweet potato genotypes from 32 half sib families and two control treatments, UFVJM 58 and UFVJM 61. To quantify the pure experimental error, the controls were evaluated with replications: 49 plots of genotype UFVJM 58 and 47 plots of UFVJM 61 were included, thus totalling of 1700 plots in the experiment. After randomization treatment labels were exchanged to minimize relatedness within blocks.
For this design we established local control in two directions, both in the rows and in the columns. Experiment was laid in a rectangular area with 1700 experimental units arranjed in 17 row blocks (mounds), which measured 90.0 × 1.0 m, containing 100 experimental units each (column blocks). The total area of the experiment was 0.18 ha (90 × 20 m).
Variables evaluated
The following traits were evaluated in each experimental unit:
-
(a)
Green mass yield (stems + leaves): At the time of harvest, the total green mass of each plot was measured by collecting all aerial part of the plants of the, which were weighed and expressed in Mg ha−1;
-
(b)
Root cluster: At the time of root harvest, according to the arrangement of the tuberized roots in the mound, root clustering was evaluated according to Huamán (1999), in which three evaluators classified the root cluster as either closed (3), open (5), disperse (7), or very disperse (9).
-
(c)
Total root yield: evaluated by weighing all the roots of each plot, with values expressed in Mg ha−1.
-
(d)
General shape of the storage root: was evaluated by assigning scores from 1 to 5 by two evaluators (Azevedo et al. 2002; Huamán 1992): score 1 = root with a fusiform, regular shape, without veins or any type of crack (long elliptic); 2 = root with good shape, close to fusiform, with some veins (elliptic); 3 = root with uneven shape, with veins and a very irregular shape (round elliptic/round + veins); 4 = very large roots, with veins and cracks, commercially undesirable (round elliptic/round + veins + cracks); and 5 = roots totally outside the commercial standards, very irregular and deformed, with many veins and cracks (long irregular/curved/round elliptic/round + veins + cracks).
-
(e)
Resistance to soil insects: evaluated according to Azevedo et al. (2014), in which two evaluators classified the roots as 1 = free from insect damage; 2 = little damage; 3 = damages that impair its appearance for sale; 4 = damage that made it practically unacceptable for sale; and 5 = damage that made it unacceptable for sale.
-
(f)
Predominant colour of the root skin: evaluated as proposed by Huamán (1999), in which two evaluators classified it as 1 = white, 2 = cream, 3 = yellow, 4 = orange, 5 = brownish orange, 6 = pink, 7 = red, 8 = purple red, and 9 = dark purple.
-
(g)
Predominant coloration of the storage root flesh for commerce and industry in general: evaluated as proposed by Huamán (1999), in which two evaluators classified it as 1 = white, 2 = cream, 3 = dark cream, 4 = pale yellow, 5 = dark yellow, 6 = pale orange, 7 = intermediate orange, 8 = dark orange, 9 = strongly pigmented (purple/orange).
-
(h)
Production of beta-carotene and anthocyanins, functional food: evaluated as proposed by Huamán (1999), in which two evaluators classified it as 1 = white, 2 = cream, 3 = dark cream, 4 = pale yellow, 5 = dark yellow, 6 = pale orange, 7 = intermediate orange, 8 = dark orange, 9 = strongly pigmented with anthocyanins (purple).
-
(i)
Intensity of the predominant colour of the storage root flesh: it was evaluated from the scores of two evaluators, with values from 1 to 3, where 1 = pale, 2 = intermediate, and 3 = dark.
-
(j)
Commercial standard: was evaluated by two evaluators, with scores of 1 = worst standard and completely outside the commercial standards; 2 = very large, deformed roots with many defects; 3 = roots with characteristics that would hinder its sale; 4 = roots with good characteristics for sale, with few defects; 5 = roots with excellent condition and characteristics favourable to sale.
At the time of harvest, the plots that did not have tuberized roots or that weighed less than 1.2 kg were discarded. This value represents approximately 13.5 Mg ha−1, which is close to the national average yield (14.07 Mg ha−1 of roots). Roots of purple, orange, or yellow storage root flesh were not discarded because they are biofortified and important in breeding as a functional food due to their bioactive compounds.
Statistical analysis
The data were initially transformed to meet the assumption of normal distribution and to ensure the additivity of the index that would be analysed. For data obtained by scores, the mean of the evaluators was evaluated. The transformation procedure was as follows:
-
(a)
Box-Cox transformation (1964):
$$y_{t} = \frac{{\left( {y^{\lambda } - 1} \right)}}{\lambda }$$(1) -
(b)
Standardization of the variables and resulting indices for each of the aptitudes:
$$z = \frac{{\left( {y_{t} - {\text{mean}}\left( {y_{t} } \right)} \right)}}{{{\text{standard}}\,{\text{dev}}.\,\left( {y_{t} } \right)}}$$(2) -
(c)
Fitting the mixed linear model and obtaining the BLUPs of families and genotypes, according to Henderson (1984):
$$y = Xb + Za + e$$(3)
with the following distributions and structures of means and variances:
where y—vector of observations, yt—vector of transformed observations (for variables that required transformation), λ—power parameter in the transformation (described in Box and Cox, 1964), b—parametric vector of fixed effects, with incidence matrix X, a—parametric vector of random effects, with incidence matrix Z, e—vector of random errors, G—variance–covariance matrix of random effects, R—variance–covariance matrix of random errors, 0—null vector.
For each of the proposed aptitudes (ethanol production, animal feed, and human consumption), a selection index was estimated that corresponded to the weighting of the means of the standardized variables (Zi) by the weights assigned to each trait, as adapted from Gonçalves Neto et al. (2011) for each aptitude (Table 1). Other coefficients were tested for the indices, which were chosen because they yielded better results in terms of dispersion between the BLUP values of the genetic materials and resulted in the selection of different genotypes for each aptitude.
The weightings indicated represented the relative importance of each trait for the aptitude index considered (Gonçalves Neto et al. 2011). Thus, total root yield was the factor with the greatest weighting in the aptitude for biofuel ethanol, followed by root cluster. For human consumption, total root yield is most important, followed by the general commercial standard and visual physical quality. For animal feed, the total root yield and green mass yield are the most important (Gonçalves Neto et al. 2011). The weights were negative for the general shape of storage roots, resistance to soil insects, and colour of the root storage root flesh for sale and industry in general, for which high numerical values on the scales used are considered undesirable.
The best-suited genotypes that were included in the 2.5% selection pressure index for each aptitude were considered suitable. We selected approximately 20–40 potential genotypes for each proposed aptitude.
The IBD was analysed using a mixed model, with random effects of progenies and clones within progenies. The two clones used as controls were the parameter for estimating the pure experimental error. The other tests and inferences were those commonly used for random effects (Bueno and Vencovsky 2000). All data analyses were performed with R statistical software (R Core Team 2018).
Results and discussion
Considering the criterion of initially discarding genotypes with data from the least promising plots at the time of harvest, 858 genotypes (53.42%) of the 1606 evaluated were discarded, as were the 23 genotypes that did not set. This removal of genotypes with lower productivity is important because it selects the most favorable for crop breeding. On the other hand, maintaining genotypes with purple, orange, and yellow storage root flesh allows the establishment of improvements for the production of biofortified and functional foods. These roots have bioactive compounds essential to human health, such as pro-vitamin A and anthocyanin compounds (Vizzotto et al. 2017), and have the potential for use in new selection or breeding programmes targeting biofortification.
A total of 842 remaining plots, including 96 of the controls, all were effectively evaluated for the 10 traits studied. The mean root yield of the 746 genotypes evaluated in the experiment, excluding the controls, was 27.71 Mg ha−1. This value was approximately double the national average for Brazil and the world, which is 14.07 Mg ha−1 and 12.26 Mg ha−1 for roots, respectively (Andrade Júnior et al. 2018; Faostat 2018). These data show the agronomic potential of these experimental genotypes for the breeding of the crop.
Selection at the genotype level (individual derived from botanical seed)
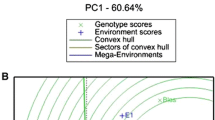
It was possible to identify, among the 748 genotypes effectively evaluated, those most promising for selection. Given the many promising genotypes, the application of a selection pressure of 2.5% for each aptitude allowed the identification of 60 genotypes recommended for the three proposed aptitudes overall (Fig. 1A, B, C).
In the BLUP graph of the effect of the selection index, the genotypes are shown by zooming in the original graph, corresponding only to the best genotypes selected for each aptitude of interest. The value “0” on the ordinate axis (y) of the graph indicates the mean of the genetic values derived from the traits of the best genotypes selected based on the established index. The genotypes shown in the graphs are therefore those selectable within each proposed aptitude.
The 60 selectable genotypes belonged to a total of 16 of the 32 half sib families (Table 2). This reinforces the potential of these families as sources of the best genotypes for each aptitude of interest, once again showing their potential for future breeding programmes. In the selection for ethanol production, 37 half-sib genotypes and the two controls (UFVJM 58 and UFVJM 61) were indicated for that aptitude (Fig. 1A and Table 2). For the animal feed, 36 and again the two controls were indicated (Fig. 1B and Table 2). For human consumption, 38 genotypes plus one control (UFVJM 58) were indicated (Fig. 1C and Table 2). Within the group of 60 selectable genotypes, some had multiple aptitudes (Table 2).
The sweet potato genotypes derived from botanical seeds showed great variation in the 10 traits analysed. The total root yield of the 748 genotypes ranged from 125.78 Mg ha−1 to 0.56 Mg ha−1, and the green mass yield was between 204.44 and 1.11 Mg ha−1, which defined the aptitude of the selected genotypes (Table 2).
The selection of the 60 genotypes took into account the chance of each one presenting the most favourable weights of the 10 traits for each proposed aptitude. Thus, the selected genotype has a combination in the optimal linear standardization that meets the weights for the reported aptitude. Therefore, some genotypes were not selected because they had unfavourable traits that, when combined, led to their ineptitude, even with favourable results for some highly important traits. This occurs because these traits show negative correlations or no correlation (Gonçalves Neto et al. 2012). That is, a trait can negatively influence another trait of interest, leading to a sum of unfavourable factors and impairing its selection.
The fact that the UFVJM 61 and UFVJM 58 controls were repeated 47 and 49 times, respectively, resulted in greater accuracy in the measurement of their means, yielding a better and more reliable determination of the genetic effect on the index. This may have contributed to the fact that they presented indices above the standardized mean with greater confidence for the selection of the three aptitudes at the genotype level. Conversely, the experimental genotypes, because they had only one replicate, showed standardized means with longer prediction intervals (Fig. 1A, B, C). Even so, it was possible to identify the most promising genotypes in BLUP by their genetic value, given the variability, number of individuals tested, and degree of relatedness included in the selection.
Considering the genotypes with more than one aptitude (multiple aptitude), six genotypes with aptitude for ethanol production and animal feed, eleven with aptitude for animal feed and human consumption, five with aptitude for human consumption and ethanol production, and sixteen with triple aptitude were selected (Table 2). Regarding the genotypes that showed a single aptitude, five were indicated for animal feeding, seven for human consumption, and 12 for ethanol production; ethanol production had more genotypes due to its less demanding weights (Table 2). The selection of genotypes with more than one aptitude is good for producers because it provides diversified and sustainable production, meaning better use of the plant and greater profitability.
Andrade Júnior et al. (2018), studying the quantitative and qualitative potential of 39 sweet potato accessions from the germplasm bank of UFVJM and nine commercial cultivars without the use of selection indices, found 6 accessions of the germplasm bank of UFVJM and two commercial accessions with potential aptitudes similar to the ones we found. It is noteworthy that most of the genotypes selected by Andrade Júnior et al. (2018) are related to the mother plants used in this study, once again demonstrating their potential. The clones derived from UFVJM (1, 14, 18, and 58) were selected as superior genotypes in both studies.
The mean root yield of the 60 selected genotypes evaluated in this study was 30.6 Mg ha−1, and the green mass yield was 45.41 Mg ha−1. The mean total root yield and green mass of the controls (UFVJM 58 and UFVJM 61) were 31.42 Mg ha−1 and 47.08 Mg ha−1, respectively (Table 3). The similar performance of the genotypes and controls reinforces the potential of these selectable genotypes, given that the controls were already selected by an advanced breeding programme.
It is noteworthy, however, that 21 of the 60 selected genotypes showed root yields above the mean of the best control (> 32.97 Mg ha−1) and that 10 of these 60 also had green mass yields above the mean of the best control (> 59.95 Mg ha−1) (Table 3). This demonstrates the superiority of these genotypes over the control in absolute values and their potential for selection for different aptitudes (Table 2).
According to Schweinberger et al. (2016), for each ton of roots processed, an estimated 160.03 L of ethanol can be produced. Considering that the mean root yield of the five best genotypes selected for ethanol production, which were more productive than the best control, ranged from 46.422 to 91.111 Mg ha−1, we estimated a mean yield between 7334.60 and 14.395, 54 L ha−1 of ethanol for these best genotypes. According to Gonçalves Neto et al. (2011), sugarcane, with a high stalk yield (> 100 Mg ha−1 of stalks) and an ethanol yield of 90 ethanol L of per Mg of raw sugarcane, reaches an ethanol yield of 9,000 L ha−1 in a 12-month cycle (BNDES 2008). Taking into account the cycle of sweet potato genotypes evaluated in this study (5 to 6 months), there is a high potential for ethanol production (14,669.20 L to 28,791.10 L year−1 in two putative harvests over 12 months.
The genotypes selected for animal feeding have important favourable traits. Among the 38 selectable genotypes, the five best genotypes showed green mass yields between 100.0 and 166.67 Mg ha−1 (Table 3). Gonçalves Neto et al. (2011) and Andrade Júnior et al. (2018) found mean shoot dry mass values ranging from 16.32% to 20.30%. Therefore, a minimum dry mass yield of 17.32 Mg ha−1 was estimated, considering the average of the five best genotypes for branch yield and selected for animal feed in this study. In addition, similar results have been observed for the maize crop, which has high dry mass yield in the silage, approximately 20 to 25 Mg ha−1 dry mass per hectare (Fernandes et al. 2016; Neumann et al. 2017), but with a high technological level compared to that used in the sweet potato crop. In addition, sweet potato can still be cultivated in at least two cycles per year, at a lower production cost, surpassing the maize dry mass yield in some regions. When considering maize silage without the ear, the dry mass yield in the silage reaches 6.83 Mg ha−1 (Costa et al. 2000). Thus, sweet potato has the potential for higher dry mass yield per ha per year, and it can be cultivated throughout the year (Ferreira et al. 2019b).
Regarding the crude protein (CP) content in the dry mass, Figueiredo et al. (2012) reported a value of 11.59%. Our result of 17.32 Mg ha−1 of dry mass of stems and leaves was greater than that found for total forage dry mass in six grasses of the genus Brachiaria (Brachiaria brizantha cv. Marandu, cv. Xaraés, cv. Piatã, cv. MG4, cv. Paiaguás and Brachiaria hybrid Convert HD 364), whose highest value was 8.41 Mg ha−1 (Gobbi et al. 2018), evaluated during the same dry season. In addition to the supply of fresh stems and leaves, the inclusion of roots as a source of carbohydrates together with the aereal parts, a source of CP, also makes the crop a promising option for the production of hay (Donato et al. 2020) and silage (Gonçalves Neto et al. 2011; Andrade Júnior et al. 2018).
In addition, this estimated mean shoot dry mass yield (17.32 Mg ha−1), in a cycle of up to 6 months, represented a CP yield in the dry mass of 2,007.39 kg ha−1, considering the 11.59% CP proposed by Figueiredo et al. (2012). This was equivalent to 4181.22 kg of soybean meal, considering the 48% of CP in soybean meal (Guimarães et al. 2015). As stated by Lovatelli (2014), approximately 780 to 800 kg of soybean meal is produced from the processing of one ton of soybean. Therefore, for 4181.22 kg ha−1 of soybean meal, 5292.69 kg of raw soybean, or 88.21 bags ha−1 is required. This reinforces the potential of sweet potato branches for animal feed, as the CP present in stems and leaves is higher than that provided by soybean, whose average yield in Brazil is close to 54 bags ha−1 and has a higher cost.
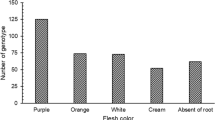
Among the 39 genotypes selected for human consumption, 11 genotypes showed an excellent or good general shape of the storage root, which is a requirement for the human consumption market (CEAGESP 2014). The consumer wants fusiform sweet potatoes, regular and without veins or cracks, similar to what was observed for these 11 genotypes (grades 1 and 2). In addition, 32 genotypes showed no or little damage by soil insects, which is a good visual quality indicator. Furthermore, 13 genotypes of the selected genotypes showed excellent or good commercial standards, which indicates their quality and general acceptance for sale for human consumption compared to those already commercialized. Among the storage root flesh colour, four white genotypes were observed, one of which was intense; 24 cream, three dark cream, three pale yellow, one of which was intense; one intermediate orange; and three strongly pigmented by anthocyanins (purple).
Regarding root cluster, 14 of the 60 genotypes selected during harvest had a closed cluster (score close to 3). Roots that are more clustered are easier to grow in mounds without harming the roots, in addition to accelerating and facilitating mechanical and manual harvesting, increasing the efficiency, quality, and yield of the sweet potato harvesting process (CIP 2001) and avoiding root defects at the time of harvest. Of the selected genotypes, 26 presented open root clusters, 10 disperse roots, and 10 very disperse roots. These disperse and very disperse genotypes showed greater difficulty in manual harvesting of storage roots from the soil, in addition to greater yield losses due to cuts that depreciate the root (Table 3). However, genotypes of this kind can be used for other purposes, such as for ethanol production and animal feed. The preferred scores are close to 3 because this means roots clustered in the neck of the plant and facilitate harvesting, which may vary according to their aptitude. The configuration of root cluster is linked to the length of the root stalk that attaches to the stem (Huamán 1992), which is mainly associated with genetic factors. When the root stalk is absent or short, the root cluster is closed. On the other hand, when this root stalk is elongated, the root cluster is dispersed.
Among the 60 genotypes selected, 11 presented an excellent root shape and were classified as ideal for human consumption because they had a fusiform shape, which is required for this market (Azevedo et al. 2002). (Table 3). Furthermore, 12 of them were free of insect damage (Table 3). This factor is of paramount importance for root quality in postharvest yield and processing, shelf life, and commercial acceptance (Azevedo et al. 2014).
In 15 of the 60 selectable genotypes, an optimal commercial standard was identified (Table 3). These genotypes did not present residues, defects, or roots unsuitable for sale, as they had maximum yields between the total harvest and the commercial harvest, with a uniform pattern among the roots that was good for human consumption. This favours their commercialization, providing economic return to the producer by increasing the market acceptance of the genotype and showing a higher harvest yield. The genotypes met the CEAGESP standards (2014) for quality, purity, uniformity, and absence of defects, putting them in the noble category.
In the case of skin colour, the importance gradient was described by the sequence from highest to lowest desired score, corresponding to 1—white; > 2—purple; > 3—cream; > 4—pink; > 5—reddish purple; > 6—red; > 7—yellow; > 8—orange; > 9—brownish orange. Among the 60 selectable genotypes, one with white skin, two cream, 26 yellow, 17 pink, 11 purple red, and 3 dark purple were identified. The colours white, purple, cream, pink, and reddish purple, in decreasing order of importance, are usually preferred by the consumer market and considered as the market standard by CEAGESP (2014) for skin colour (Table 3).
Regarding storage root flesh colour, among the 60 selectable genotypes, 10 had a white storage root flesh colour, six of them intense (score 3); 37 were cream-coloured, one of them intense and suitable for traditional human consumption; three dark cream, one of them intense; four pale yellow, one intense; one orange of intermediate intensity; and five purple that were strongly pigmented by anthocyanins (intense purple), with cream and white colour being the most preferred for general sale (Table 3). The closer the storage root flesh colour intensity score is to 3, the better for human consumption and functional feeding.
Scores close to 1.0 for storage root flesh colour (white and cream) are current standards for human consumption in the Brazilian market, while scores close to 9 with more intensity indicate the presence of bioactive and functional compounds such as antioxidants and pro-vitamin A (purple, orange, and yellow) (Sun et al. 2019). Thus, in addition to standard genotypes suitable for human consumption, promising genotypes for use as functional foods were also identified, i.e., those with orange, yellow, and purple storage root flesh colour, some with high intensity, as observed in seven selected genotypes (Table 3). These genotypes are valuable for human nutrition due to their nutritional composition as functional foods given the strong relationship between internal colour and the presence of functional bioactive compounds (Vizzotto et al. 2017; Laurie et al. 2017; Zhang et al. 2016), antioxidants (Vizzotto et al. 2017), and phytonutrients (Sweet Potato 2014). Selecting for sweet potato genotypes as done here is highly relevant for the establishment of new crop breeding programmes for biofortification of its roots.
Conclusions
The use of mixed models with selection indices was efficient for the selection of sweet potato genotypes for different aptitudes. A total of 37 genotypes plus two controls (UFVJM 61 and UFVJM 58) suitable for ethanol production, 36 genotypes plus again two controls (UFVJM 61 and UFVJM58) suitable for animal feeding, and 38 genotypes plus one control (UFVJM58) suitable for human consumption were selected.
Genotypes 556, 765, 1221, 1231, 1319, and the control UFVJM 61 are considered suitable for both ethanol production and animal feed.
Genotypes 220, 301, 455, 736, and 1311 are considered suitable for both human consumption and ethanol production.
Genotypes 49, 181, 314, 660, 663, 716, 1000, 1164, 1238, 1258, and 1538 are considered suitable for both animal feed and human consumption.
Genotypes 161, 221, 225, 238, 450, 540, 655, 759, 791, 799, 1008, 1234, 1252, 1299, 1562, and the control UFVJM 58, are considered suitable for human consumption, animal feed and ethanol production.
Genotypes 290, 829, 1210, 1288, and 1436 are suitable for animal feed only.
Genotypes 11, 107, 354, 546, 574, 695, 884, 987, 1005, 1011, 1235, and 1565 are suitable for ethanol production only.
Genotypes 106, 144, 404, 622, 648, 992, and 1554 are suitable for human consumption only.
References
Amaro GB, Fernandes FR, Silva GO, Mello AFS, Castro LAS (2017) Desempenho de cultivares de batata-doce na região do Alto Paranaíba-MG. Hort Bras 35:286–291. https://doi.org/10.1590/S0102-053620170221
Andrade Júnior VC, Viana DJS, Fernandes JSC, Figueiredo JÁ, Nunes UR, Neiva IP (2009) Selection of sweet potato clones for the region Alto Vale do Jequitinhonha. Hort Bras 27:389–393. https://doi.org/10.1590/S0102-05362009000300024
Andrade Júnior VC, Viana DJS, Pinto NAVD, Ribeiro KG, Pereira RC, Neiva IP, Azevedo AM, Andrade PCR (2012) Características produtivas e qualitativas de ramas e raízes de batata-doce. Hort Bras 30:584–589. https://doi.org/10.1590/S0102-05362012000400004
Andrade Júnior VC, Elsayed A, Azevedo A, Santos E, Ferreira M (2018) Potencial quantitativo e qualitativo de genótipos batata-doce. Rev Sci Agrar 19:28–35. https://doi.org/10.5380/rsa.v19i1.50158
Azevedo SM, Maluf WR, Silveira MA, Freitas JA (2002) Reação de clones de batata-doce aos insetos de solo. Ciênc Agrotec 26:545–549
Azevedo AM, Andrade Júnior VC, Viana DJS, Elsayed A, Pedrosa CE, Neiva IP, Figueiredo JA (2014) Influence of harvest time and cultivation sites on the productivity and quality of sweet potato. Hortic Bras 32:21–27. https://doi.org/10.1590/S0102-05362014000100004
Azevedo MA, Andrade Júnior VC, Figueiredo JA, Pedrosa CE, Viana DJS, Lemos VT, Neiva IP (2015) Divergência genética e importância de caracteres em genótipos de batata-doce visando a produção de silagem. Rev Bras Ciênc Agrar 10:479–484. https://doi.org/10.5039/agraria.v10i3a5165
Bennett BC (2015) Subsistence farming in Southern Florida (1840–1940): the importance of sweet potato-in memory of Daniel F Austin. Econ Bot 169:185–198. https://doi.org/10.1007/s12231-015-9312-0
Bernardo R (2002) Breeding for quantitative traits in plant. Stemma Press, Woodbury
BNDES - Banco Nacional do Desenvolvimento. BIOETANOL de cana-de-açúcar: energia para o desenvolvimento sustentável. Rio de Janeiro: BNDES, 2008. 316 p. Accessed 27 Mar 2019 Disponível em: http://www.bioetanoldecana.org/pt/download/bioetanol.pdf
Borges V, Ferreira PV, Soares L, Santos GM, Santos AMM (2010) Seleção de clones de batata-doce pelo procedimento REML/BLUP. Acta Sci Agron 32:643–649. https://doi.org/10.4025/actasciagron.v32i4.4837
Box G, Cox D (1964) An analysis of transformations. J R Stat Soc 26:211–252. https://doi.org/10.1111/j.2517-6161.1964.tb00553.x
Bueno Filho JSS, Vencovsky R (2000) Alternativas de análise de ensaios em látice no melhoramento vegetal. Pesqui Agropecu Bras 35:259–269. https://doi.org/10.1590/S0100-204X2000000200004
Camargo LKP, Resende JTV, Mógor AF, Camargo CK, Kurchaidt SM (2016) Uso de índice de seleção na identificação de genótipos de batata doce com diferentes aptidões. Horticul Bras 34:514–519. https://doi.org/10.1590/s0102-053620160410
CEAGESP (2014) N-842. Normas de Classificação—Programa brasileiro para a modernização da horticultura.V. 1, n. 1 (2003-),—São Paulo: PBMH, 2003-V.12,. n.2, jul.\ dez. 2014. 08 p.: il.: 30 cm. ISNN 2237-6666. Accessed 22 Jun 2019 Disponível em: http://www.ceagesp.gov.br/wp-content/uploads/2015/07/batata_doce.pdf
CFSEMG—Comissão de Fertilidade do Solo do Estado de Minas Gerais (1999) Recomendações para o uso de corretivos e fertilizantes em Minas Gerais: 5a Aproximação. Editora UFV, Viçosa
CIP (2001) Centro Internacional De La Papa. Sweet potato facts., Lima. Accessed 23 Jun 2019 Disponível em: http://www.cipotato.org/market/Sweetpfacts/swtpfact.htm
CIP (2009) Centro Internacional De La Papa. Plant breeding and farmer participation. chapter 13, Selection methods Part 5: Breeding clonally propagated crops. Cross Ref View Record in Scopus. CIP, International Potato Center (Annual Report—Embracing the SDGs in, 2015 : CIP, International Potato Center (Annual Report–Embracing the SDGs in 2015), CIP
Costa C, Creste CR, Arrigoni MD, Silveira AC, Magalhães RGJ, Bicudo SJ (2000) Potencial para ensilagem, composição química e qualidade da silagem de milho com diferentes proporções de espigas. Acta Sci Anim Sci 22:835–841. https://doi.org/10.4025/actascianimsci.v22i0.3231
Donato LMS, Andrade Júnior VC, Brito OG, Fialho CMT, Silva AJM, Azevedo AM (2020) Uso de ramas de batata-doce para produção de feno. Ciênc Anim Bras 21:e53493. https://doi.org/10.1590/1809-6891v21e-53493
Faostat. Food and Agriculture Organization of the United Nations Statistics Division: Sweet Potatoes. Accessed 17 Apr 2018 Disponível em: http://www.fao.org/faostat/en/#data/QC/visualize
Federer WT (1956) Augmented (or hoonuiaku) designs. HSPA 55:191–208
Fernandes GF, Evangelista AF, Borges LS (2016) Potencial de espécies forrageiras para produção de silagem: revisão de literatura. Nutritime 13:4652–4656
Ferreira MAM, Andrade Júnior VC, Oliveira AJM, Ferreira EA, Brito OG, Silva LR (2019a) Physiological characterization of plant growth in sweet potato. Hort Bras 37:112–118. https://doi.org/10.1590/s0102-053620190118
Ferreira EA, Pereira GAM, Alves DP, Agazzi LRA, Silva DV, Andrade Júnior VC (2019b) Capacidades fisiológicas e produção de matéria seca de acessos de batata-doce batata-doce. Cult Agron 28:493–504. https://doi.org/10.32929/2446-8355.2019v28n4p493-504
Figueiredo JA, Andrade Júnior VC, Pereira RC, Ribeiro KG, Viana DJS, Neiva IP (2012) Avaliação de silagens de ramas de batata-doce. Horticul Bras 30:708–712. https://doi.org/10.1590/S0102-05362012000400024
Folquer F (1978) La batata (camote): estudo de la planta y su producción comercial. Editorial Hemisfério Sur, Buenos Aires
Gobbi K, Lugão S, Bett V, Abrahão J, Tacaiama A (2018) Massa de forragem e características morfológicas de gramíneas do gênero Brachiaria na região do Arenito Caiuá/PR. Bol Ind Anim 75:1–9. https://doi.org/10.17523/bia.2018.v75.e1407
Gonçalves Neto AC, Maluf WR, Gomes LAA, Gonçalves RJS, Silva VF, Lasmar A (2011) Aptidões de genótipos de batata-doce para consumo humano, produção de etanol e alimentação animal. Pesqui Agropecu Bras 46:1513–1520. https://doi.org/10.17523/bia.2018.v75.e1407
Gonçalves Neto AC, Maluf WR, Gomes LAA, Maciel GM, Ferreira RPD, Carvalho RC (2012) Correlação entre caracteres e estimação de parâmetros populacionais para batata-doce. Horticul Bras 30:713–719. https://doi.org/10.1590/S0102-05362012000400025
Guimarães TP, Peron HJMC, Silva DB, Moreira KKG, Neves Neto JT, Silva BMN, Santos FC (2015) Exigências Proteicas para bovinos de corte. Multi Sci J 1:90–99. https://doi.org/10.33837/msj.v1i1.43
Henderson CR (1984) Aplications of linear models in animal breeding. University of Guelph, Guelph
Huamán Z (1992) Systematic botany and morphology of the sweet potato plant. CIP Tech Info Bull 25:1–25
Huamán Z (1999) Sweet potato germplasm management training manual. International Potato Center (CIP), Lima
King JR, Bamford R (1937) The chromosome number in Ipomea and related genera. J Hered 28:279–282. https://doi.org/10.1093/oxfordjournals.jhered.a104385
Laurie SM, Faber M, Claasen N (2017) Incorporating orange-fleshed sweet potato into the food system as a strategy for improved nutrition: the context of South Africa. Food Res Inter 104:77–85. https://doi.org/10.1016/j.foodres.2017.09.016
Lovatelli C (2014) Biodiesel: impactos positivos do aumento da mistura. Agroanalysis 34:30–31
Monteiro AB, Massaroto JA, Gasparino CF, Silva RR, Gomes LAA, Maluf WR, Silva Filho JC (2007) Silagens de cultivares e clones de batata doce para alimentação animal visando sustentabilidade da produção agrícola familiar. Rev Bras De Agroecologia 2:978–981
Neumann M, Leão GF, Coelho MG, Figueira DN, Spada CA, Perussolo LF (2017) Aspectos produtivos, nutricionais e bioeconômicos de híbridos de milho para produção de silagem. Arch Zootec 66:51–57. https://doi.org/10.21071/az.v66i253.2125
Pellegrini C (2014) The shochu handbook: an introduction to Japan’s indigenous distilled drink. Telemachus Press, Dublin
Pietrosemoli S, Moron-Fuenmayor OE, Paez A, Villamide MJ (2016) Effect of including sweet potato (Ipomoea batatas Lam) meal in finishing pig diets on growth performance, carcass traits and pork quality. Anim Sci J 87:1281–1290. https://doi.org/10.1111/asj.12546
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rossel G, Espinoza C, Javier M, Tay D (2008) Directrizes de regeneração: batata-doce. In: Dulloo ME, Thormann I, Jorge MA, Hanson J (eds) Crop specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme (SGRP), Rome
Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JÁ, Cunha TJF, Oliveira JB (2013) Sistema brasileiro de classificação de solos. Embrapa, Brasília
Schweinberger CM, Putti TR, Susin GB, Trierweiler JO, Trierweiler LF (2016) Ethanol production from sweet potato: the effect of ripening, comparison of two heating methods, and cost analysis. Can J Chem Eng 94:716–724. https://doi.org/10.1002/cjce.22441
Silveira MA (2008) Batata-doce: uma nova alternativa para a produção de etanol. Instituto Euvaldo Lodi, Brasília
Sun Y, Pan Z, Yang C, Jia Z, Guo X (2019) Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea batatas) storage roots. Molecules 24:1–13. https://doi.org/10.3390/molecules24244476
SWEET POTATO. In-depth nutrient profile (2014) Disponível em: Accessed 8 Sept 2018 http://www.whfoods.com/genpage.phptname=nutrientprofile&dbid=128
Torquato-Tavares A, Nascimento IR, Pascual-Reyes ID, Santana WR, Silveira MA (2017) Potential for sweet potato (Ipomoea batatas (L.) Lam.) single crosses to improve ethanol production. Rev Chapingo Ser Hortic 23:59–74. https://doi.org/10.5154/r.rchsh.2016.05.013
Viana DJS, Andrade Júnior VC, Ribeiro KG, Pinto NAVD, Neiva IP, Figueiredo JA, Lemos VT, Pedrosa CE, Azevedo AM (2011) Potencial de silagens de ramas de batata-doce para alimentação animal. Cienc Rural 41:1466–1471. https://doi.org/10.1590/S0103-84782011000800027
Viana DJS, Pinto SSS, Dias KPF, Nelson DL, Pantoja LA, Pinto NAVD, Andrade Júnior VC, Santos AS (2017) Evaluation of productivity of sweet potato genotypes for first and second generation bioethanol production. Afr J Biotechnol 16:2289–2298. https://doi.org/10.5897/AJB2017.16083
Vieira AD, Miranda VC, Alves AF, Tavares AT, Momenté VG (2015) Agronomic evaluation of clones of sweet potato with potential for ethanol production. Appl Res Agrotec 8:69–74. https://doi.org/10.5935/PAeT.V8.N1.08
Vizzotto M, Pereira ES, Vinholes JR, Munhoz PC, Ferri NML, Castro LAS, Krolow ACR (2017) Physicochemical and antioxidant capacity analysis of coloreds weet potato genotypes: in natura and thermally processed. Cienc Rural 47:1–8. https://doi.org/10.1590/0103-8478cr20151385
Zhang X, Yang Y, Wu Z, Weng P (2016) The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J Agric Food Chem 64:2582–2590. https://doi.org/10.1021/acs.jafc.6b00586
Acknowledgements
The authors thank CNPq and FAPEMIG for the financial resources and scholarships provided for the project. The present study was conducted with the support of the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES)—Funding Code 001.
Funding
The present study was conducted with the support of the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES)—Funding Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the present research did not involve any experimentation on humans or animals.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Silva, J.C., de Andrade Júnior, V.C., de Sousa Bueno Filho, J.S. et al. Mixed model-based indices for selection of sweet potato genotypes for different agronomic aptitudes. Euphytica 218, 86 (2022). https://doi.org/10.1007/s10681-022-03033-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-022-03033-9