Abstract
Cowpea is a health-promoting diploid legume species [Vigna unguiculata (L.) Walp., 2n = 2x = 22]. The annual cowpea production is 5.4 million tons of dry seed globally. Despite the fact that cowpea is one of the most drought-tolerant crops, some genotypes with excellent agronomic traits such as high yield under sufficient water supplies have been reported to be highly drought-susceptible, thus still requiring the need for breeding drought-tolerant cowpea genotypes. Therefore, the objectives of this study were to evaluate drought tolerance in cowpea at seedling stage and to identify drought-tolerant cowpea genotypes. In this study, a total of 331 cowpea genotypes were evaluated for drought tolerance at seedling stage. The experiment was conducted in a greenhouse and repeated 3 times. Drought tolerance phenotyping was conducted using a previously described methodology and a total of 11 traits were analyzed. The experiment was validated by the use of drought-tolerant and susceptible controls. Results showed that: (1) a large variation in the evaluated traits for drought tolerance was identified among the 331 cowpea genotypes, (2) a high correlation was found for traits such as plant greenness score and tolerance to trifoliate leaf chlorosis under drought stress (r = 0.8), whereas no linear correlation was found for traits such as tolerance to trifoliate leaf chlorosis and unifoliate leaf SPAD chlorophyll under non-drought stress (r = 0.0), (3) a total of 21 genotypes were found to be drought-tolerant across different traits, and (4) country of origins could impact drought tolerance in cowpea. The top performing genotypes were repeated using an independent experiment to further validate the data. The results from this study would be of interest in breeding programs aiming at improving drought tolerance in cowpea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cowpea is a diploid legume species [Vigna unguiculata (L.) Walp., 2n = 2x = 22], cultivated in various regions where climatic and edaphic conditions are favorable for its production. Cowpea cultivation is prevalent in Africa, Asia, Southern Europe, Oceania, and Central and Latin America. Cowpea is grown for its seeds that provide high quality protein to human consumption. In addition, cowpea seed contains nutrients that can ameliorate human’s diet. Estimates of these nutrients were, in mg per 100-g seed, 6.8 iron, 4.1 zinc, 1.5 manganese, 510.0 phosphorus, and 1430.0 potassium (Frota et al. 2008). Weng et al. (2017) reported that Cowpea seeds contain on average 21.0–26.7% of protein (Weng et al. 2017), and 2.2% lipid (Frota et al. 2008). The lipid in cowpea seeds consists of 30% were saturated fatty acids and 70% were unsaturated fatty acids (Frota et al. 2008). In sub-Saharan Africa, cowpea is widely used as a supplement to fodder for livestock. Cowpea leaves have been shown to enhance feed quality (Olufajo 2012). In addition to being part of the human’s diet, cowpea can also be used as a cover crop. In the United States, cowpea is known as southern pea or blackeye pea. A growing interest in processing cowpea into canned or frozen vegetables has been noticed in the U.S., which provides opportunities to cowpea growers to increase their production (Wilson et al. 2006).
Drought has been a growing threat to agriculture. Drought conditions can cause significant crop yield losses. Drought has been defined as being the results of lack of water supplies that are critical in maintaining proper plant growth and development and in providing reasonable crop yields (Blum and Ebercon 1981). Despite the fact that cowpea is one of the most drought-tolerant legumes, some cultivars that have excellent agronomic traits such as high yield under a normal water irrigation regime are still highly susceptible to drought stress (Ravelombola et al. 2018). The incorporation of drought-tolerant trait into these cultivars would allow for their cultivation in areas where water deficit conditions are prevalent. Doing so will provide cowpea growers with additional production, which will make cowpea production more profitable (Okiror et al. 2008). Moreover, prediction of water shortage still remains challenging despite the significant progress being made in weather forecasting, which has resulted in a poor planning of agricultural activities. Choice of sowing date is one of the critical activities that should be carefully taken into a consideration. However, an unpredicted rainfall shortage occurring few weeks after sowing could lead to severe drought conditions affecting plant seedling, thus leading to plant death (Ajayi et al. 2018). Being provided with genotypes that better withstand drought stress at seedling stage would be an efficient way to address the aforementioned constraints. However, the development of drought-tolerant cultivars requires a good phenotyping strategy and understanding of the genetics of drought tolerance, which has been reported to have a complex mechanism (Golldack et al. 2014).
Drought stress affects all developmental and growth stages of cowpea (Singh et al. 1999; Verbree et al. 2015). Seedling stage is one of the most critical stages to drought stress in cowpea (Agbicodo et al. 2009). Two types of drought tolerance have been described in cowpea. Type I drought-tolerant genotypes can maintain both unifoliate and trifoliate leaves fully green under drought conditions, whereas type II drought-tolerant genotypes can only delay senescence in trifoliate leaves (Mai-Kodomi et al. 1999). A total of 30 cowpea genotypes were tested for their types of drought tolerance and results suggested that type II drought tolerance were more prevalent (Ravelombola et al. 2018). In addition, traits such as leaf chlorosis and leaf SPAD chlorophyll have been demonstrated to be useful in assessing drought tolerance in cowpea (Ravelombola et al. 2018; Singh et al. 1999; Verbree et al. 2015). However, little has been done regarding evaluating cowpea drought tolerance based on these traits using a larger population size. In addition, cowpea has a relatively small genome size (~ 620 Mb) (Lonardi et al. 2019), thus can be used as an excellent model crop to understand the genetics of drought tolerance in legumes. Therefore, the objectives of this study were to evaluate drought tolerance of cowpea at seedling stage and to identify drought-tolerant cowpea genotypes.
Materials and methods
Plant materials
A total of 331 cowpea genotypes were evaluated for drought tolerance in this study (Tables S1–S2). Of which, 36 were breeding lines from the University of Arkansas, Fayetteville, 8 were obtained from the University of California, Riverside and were used to build the first cowpea multiparent advanced generation intercross (MAGIC) population (Huynh et al. 2018), and 287 were Plant Introductions (PIs) from the U.S. Department of Agriculture (USDA) Germplasm Resources Information Network (GRIN) cowpea collection. PIs were provided by the USDA Plant Genetic Resources Conservation Unit at Griffin, GA. These cowpea genotypes originated from more than 32 countries. Seeds from each genotype were planted in the summer of 2018 at the Arkansas Agricultural Experiment Station of the University of Arkansas, Fayetteville, AR. At harvest, one plant was harvested for each genotype. Single-plant derived seeds were cleaned up and carefully sorted prior to conducting the drought tolerance experiment.
Growing conditions and experiment design
Drought tolerance evaluation was conducted in the greenhouse at the Arkansas Agricultural Experiment Station of the University of Arkansas, Fayetteville (Fig. 1). Greenhouse day/night temperatures were 26 °C/21 °C and daylight length was 14 h. The procedures of the drought tolerance evaluation were described previously (Ravelombola et al. 2018; Singh et al. 1999; Verbree et al. 2015). Sterilite propylene boxes (Sterilite corporation, Townsend, MA) with dimensions 88.6 × 42.2 × 15.6 cm were filled up with the soil Sunshine® Mix #1 Natural & Organic (SunGro Horticulture, Agawan, MA) up to 10.5 cm high. Each box was irrigated with 12 L of tap water at 2 days before sowing to attain field capacity.
A total of 10 rows were established within each box with the distance of 7.5 cm between rows. A total of 6 holes were designed within each row. Each genotype was planted within each row and a total of 2 seeds were sown within each hole. Plants were thinned to one plant per hole at emergence. Vigorous and uniform plants were kept. One week after plant emergence, fertilizers were applied by irrigating each row with a 150 mL solution of Miracle-Gro fertilizers (Scotts Miracle-Gro, Detroit, MI). Fertilizers were prepared by dissolving one tablespoon of Miracle-Gro into one gallon of tap water. Irrigation was conducted by watering each row with 150 mL tap water at 3-day interval until the first trifoliate was fully expanded. At this time, irrigation was stopped for one box, which was the drought-stressed box, whereas watering was pursued in another box, which was the well-watered treatment. In order to minimize the environmental effects within the greenhouse, each drought-stressed box was placed next to the well-watered one (Fig. 1). A total of 3 drought-tolerant genotypes (PI293469, PI349674, and PI293568) and 1 drought-susceptible genotype (PI255774) were used to validate the experiments (Ravelombola et al. 2018). These checks were included in the experiment for this study.
Due to space limitations, the experiment was conducted using 3 runs and each run was the replication. Therefore, the experiment was a randomized complete block design (RCBD) with 3 blocks (not a split-plot design since comparing drought and well-watered conditions was not the objective of this study). The experimental unit was one row where each genotype was planted. The factor of interest was the set of 331 cowpea genotypes and each genotype corresponded to one treatment. Soil moisture within boxes was recorded using an HH2 Moisture Meter (Delta-T Devices, Cambridge, UK) every 3 days.
Data measurements
Plant greenness score and recovery rate
Plant greenness score and recovery rate have been previously shown to be accurate parameters for assessing drought tolerance at seedling stage in cowpea (Ravelombola et al. 2018). Plant greenness was recorded when the susceptible genotype was completely dead. Recovery rate corresponded to the number of plants that fully recovered after 1 week of rewatering. Rewatering was conducting when the susceptible genotype were completely dead. Plant greenness was assessed using a previously described scale (1 = plants were completely green, 2 = plants began losing greenness, 3 = signs of chlorosis and necrosis were visible, 4 = chlorosis and necrosis was severe, and 5 = plants were completely dead) (Ravelombola et al. 2018). Data on plant greenness under drought stress were recorded on a per plant basis.
Unifoliate and first trifoliate leaf chlorosis
Evaluating tolerance to unifoliate and first trifoliate leaf chlorosis has been shown to help in determining whether a genotype is type I drought-tolerance or type II drought-tolerance. Type I drought-tolerant cowpea genotypes showed tolerance to both unifoliate and first trifoliate leaf chlorosis, whereas those which were type II drought-tolerant were tolerant to trifoliate leaf chlorosis but susceptible to unifoliate leaf chlorosis (Verbree et al. 2015). For each genotype, the number of plants showing unifoliate chlorosis was evaluated at two different time points. The first one corresponded to the time when the susceptible control had more than 50% of its unifoliate leaves being chlorotic. Unifoliate leaf chlorosis was assessed for the second time when the susceptible control was completely dead. At this time, the number of plants having their first trifoliate leaves being chlorotic was also recorded.
In vivo chlorophyll for unifoliate and first trifoliate leaves
Leaf SPAD chlorophyll on both unifoliate and trifoliate leaves was an objective measurement of both plant greenness and tolerance to unifoliate/first trifoliate leaf chlorosis. Data on leaf SPAD chlorophyll were taken when the susceptible genotype was completely dead and were recorded using a SPAD-502 Plus (Spectrum Technologies, Inc., Plainfield, IL). For each plant, leaf SPAD chlorophyll was taken separately for the unifoliate leaves and trifoliate leaves. For each measurement, one unifoliate leaf was randomly chosen and measurements were taken at three different positions on the leaf surface in order to minimize the edge effect (Ravelombola et al. 2018). For the first trifoliate leaf, one measurement was conducted from each leaf and the average measurement from each first trifoliate leaf (first trifoliate leaves consisted of 3 leaves) was recorded.
Data analysis
ANOVA was conducted to analyze plant greenness score (Score), recovery rate (Recov), number of plants having chlorotic unifoliate leaves (Uni_1: when the susceptible genotype had more than 50% of its unifoliate leaf being chlorotic, Unif: when the susceptible genotype was completely dead), number of plants having chlorotic trifoliate leaves (Tri), unifoliate leaf SPAD chlorophyll under drought stress (C_U_S), unifoliate leaf SPAD chlorophyll under non-drought stress (C_U_NS), relative tolerance index for unifoliate leaf SPAD chlorophyll (RTI_U = 100*(C_U_S/C_U_NS)), trifoliate leaf SPAD chlorophyll under drought stress (C_T_S), trifoliate leaf SPAD chlorophyll under non-drought stress (C_T_NS), and relative tolerance index for trifoliate leaf SPAD chlorophyll (RTI_T = 100*(C_T_S/C_T_NS)).
ANOVA was run using PROC MIXED of SAS® 9.4 (SAS Institute Inc., Cary, NC). Mean separation analysis was done using a protected least significant difference (LSD) procedure at α = 0.05. LSD procedure was defined as LSD = tα/2√2MSError/n, with tα/2 being the critical value from the t-table and having a degree of freedom [df(SSError)] corresponding to the difference between the number of observations and the number of replications, and n being the number of replications. The statistical model for conducting ANOVA was the following.
with µ being the overall mean, Yij being the response from the jth genotype (Gj) (fixed effect) at the ith block (Bi) (random effect), and εij being the random error associated with the ijth observation.
The effects of countries of origin on the different traits evaluated for drought tolerance were assessed using ANOVA. SAS® 9.4 was also used to conduct ANOVA via PROC MIXED. Country of origins was classified into 4 regions (Africa, America, Asia, and Europe_The_MiddleEast). Groups could not be split further due to sample size limitation for some geographical areas. The statistical model for conducting ANOVA was the following.
with µ being the overall mean, Yij being the response from the ith group (Ri) (fixed effect) and εij being the random error associated with the ijth observation.
Data distribution was visualized using the MASS package of R® 3.6.1. Pearson’s correlation coefficients between the traits evaluated for drought tolerance were calculated using JMP Genomics 9 (SAS Institute Inc., Cary, NC). Cluster analysis was conducted using Ward’ method in JMP Genomics 9 (SAS Institute Inc., Cary, NC) (Sahu 2013). The broad sense heritability (H) was estimated using the following formula (Holland 2003).
with σ2G being the total genetic variance, σ2e being the residual variance, and nb being the number of blocks. The estimates for σ2G and σ2e were [EMS(G)-Var(Residual)]/nb and Var(Residual). EMS(G) and Var(Residual) were obtained from the ANOVA table.
Results
Plant greenness score
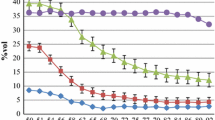
A large variation in plant greenness score was found among the 331 genotypes evaluated for drought tolerance. Plant greenness score under drought stress varied from 1.7 to 5.0, with an average of 3.5 and a standard deviation of 0.6. Plant greenness score was approximately normally distributed as shown in Fig. 2a. Plant greenness was significantly different among the 331 cowpea genotypes (F-value = 2.24, p value < 0.0001) (Table 1). The lower the plant greenness score was, the greener the plant was under drought stress. The genotypes with the lowest plant greenness score were PI664524 (1.7), PI300173 (1.8), PI583550 (2.0), PI582575 (2.0), PI293476 (2.1), PI583251 (2.1), PI293568 (2.1), PI207527 (2.2), PI227829 (2.2), PI293469 (2.2), PI582469 (2.3), PI582697 (2.3), PI194211 (2.4), and PI221730 (2.4) (Table 2), indicating that these genotypes were drought-tolerant based on plant greenness score. The genotypes with the highest plant greenness score were ‘Early Acre’ (4.6), PI582924 (4.6), PI582812 (4.6), PI527563 (4.6), PI582530 (4.6), PI406290 (4.7), PI229796 (4.8), PI583247 (4.9), and PI255774 (5.0) (Table 2), suggesting that these genotypes were susceptible to drought stress based on plant greenness score. For all traits evaluated for drought tolerance, block effect was significant (p values ≤ 0.0059). The broad-sense heritability for plant greenness score was 78.8%.
Distributions of phenotypic trait values for drought tolerance in a total of 331 cowpea genotypes. For multicolor histograms, red histograms represented traits evaluated under drought stress, whereas blue histograms displayed traits evaluated under non-drought stress. a Plant greenness score, b recovery rate, c average number of plants having chlorotic unifoliate leaves when more than half of the plants of the susceptible control have chlorotic unifoliate leaves, d average number of plants having chlorotic unifoliate leaves when the susceptible control was completely dead, e average number of plants having chlorotic trifoliate leaves, f unifoliate leaf SPAD chlorophyll under drought stress (red) and under non-drought stress (blue), g relative tolerance index for unifoliate leaf SPAD chlorophyll under drought stress, h Trifoliate leaf SPAD chlorophyll under drought stress (red) and under non-drought stress (blue), and i relative tolerance index for trifoliate leaf SPAD chlorophyll under drought stress. (Color figure online)
Recovery rate
The average number of fully recovered plants varied from 0.0 to 3.3, with an average of 0.3 and a standard deviation of 0.6. The distribution of the average number of fully-recovered plants was right-skewed (Fig. 2b). A log2 transformation was applied prior to conducting ANOVA. A significant genotype effect on the average number of fully recovered plants was identified (F-value = 3.82, p value < 0.0001) (Table 2). The genotypes with the highest plants that were fully recovered after 1 week of rewatering were PI406293 (3.3), PI339587 (2.7), PI293582 (2.3), PI390421 (2.3), 09-481 (2.3), PI662992 (2.3), 09_1090 (2.3), PI664524 (2.0), PI75962 (2.0), PI339600 (2.0), 09-749 (2.0), PI608035 (2.0), PI610533 (2.0), 09-655 (2.0), and PI271256 (2.0) (Table 2), indicating that these genotypes have the ability to survive when water supplies become available after some time of drought stress. However, a large number of genotypes did not recover. For example, the genotypes PI503326 (0), PI666251 (0), PI189374 (0), PI255774 (0), ‘Epic Select.4’ (0) (Table 2) fail to recover after rewatering. The broad-sense heritability for recovery rate was 73.8%.
Unifoliate leaf chlorosis 1 (Uni_1)
Tolerance to unifoliate chlorosis was first assessed when the susceptible control, PI255774, had more than 50% of its unifoliate leaves being chlorotic. The average number of plants having chlorotic unifoliate leaves (Uni_1) varied from 0.0 to 6.0, with an average of 2.5 and a standard deviation of 1.5. Uni_1 was approximately normally distributed (Fig. 2c). Uni_1 was significantly different among the 331 cowpea genotypes evaluated for drought tolerance (F-value = 2.34, p value < 0.0001) (Table 1). The genotypes that were the most tolerant to unifoliate chlorosis were PI152196 (0), PI152197 (0), PI167284 (0), PI180014 (0), PI190191 (0), PI194213 (0), PI582942 (0), PI583200 (0), PI583203 (0), PI583251 (0), PI583550 (0), PI662993 (0), PI292897 (0), Suvita_2 (0), IT84S_2246 (0), and PI75962 (0) (Table 2). The ones that were the most susceptible to unifoliate chlorosis were PI255774 (5.3), PI293545 (5.3), PI582354 (5.3), PI582468 (5.3), PI582541 (5.3), PI582727 (5.3), PI582850 (5.3), PI582926 (5.3), PI583247 (5.3), PI582815 (5.7), PI582810 (6.0), and PI349674 (6.3) (Table 3). The broad-sense heritability for Uni_1 was 80.1%.
Unifoliate leaf chlorosis 2 (Uni_f)
Tolerance to unifoliate chlorosis was re-evaluated when the susceptible control, PI255774, was completely dead. The average number of plants having unifoliate chlorotic leaves (Uni_f) ranged between 2.0 and 6.0, with an average of 5.6 and a standard deviation of 0.6. The distribution of Uni_f was left-skewed (Fig. 2d). A log2 transformation was applied before running ANOVA. A significant difference in Uni_f was found among the cowpea genotypes (F-value = 1.58, p value < 0.0001) (Table 1). The genotypes that were the most tolerant to unifoliate leaf chlorosis were PI664524 (2.0), PI582942 (3.0), PI598335 (3.0), PI293568 (3.3), PI194213 (3.7), PI583200 (3.7), PI583203 (3.7), PI583251 (3.7), PI292897 (3.7), PI583209 (3.7), and PI300173 (3.7) (Table 2). A large number of genotypes were susceptible to unifoliate leaf chlorosis. For example, the genotypes PI250416 (6.0), ‘Empire’ (6.0), ‘Empress’ (6.0), ‘Epic Select.4’ (6.0), and ‘Excel’ (6.0) (Table 2) were susceptible to unifoliate leaf chlorosis. The broad-sense heritability for Uni_f was 63.5%.
First trifoliate leaf chlorosis
A large variation in tolerance to first trifoliate leaf chlorosis was identified among the different cowpea genotypes evaluated for drought tolerance. The average number of plants having chlorotic first trifoliate leaves (Tri) varied from 0.0 to 6.0, with an average of 4.5 and a standard deviation of 1.4. Tri was left-skewed distributed (Fig. 2e). A log2 transformation was done prior carrying out ANOVA. A significant difference in Tri among the 331 cowpea genotypes was identified (F-value = 2.42, p value < 0.0001) (Table 1). The genotypes that were highly tolerant to first trifoliate leaf chlorosis were PI293476 (0), PI583550 (0), PI664524 (0.3), PI583251 (0.3), PI194211 (0.3), PI662993 (0.3), PI207527 (0.7), PI293568 (0.7), PI582575 (0.7), PI194213 (1.0), PI227827 (1.0), PI293470 (1.0), PI293582 (1.0), IT00K_1263 (1.0), PI194210 (1.0), and PI194209 (1.0) (Table 2). A large number of genotypes were susceptible to first trifoliate leaf chlorosis. For example, PI491193 (6.0), ‘Early Scarlet’ (6.0), ‘Elegance’ (6.0), ‘Empress’ (6.0), and ‘Epic Select.4’ (6.0) (Table 2) were highly susceptible to first trifoliate leaf chlorosis.
Unifoliate leaf SPAD chlorophyll
Unifoliate leaf SPAD chlorophyll (C_U_NS) was evaluated for plants under non-drought stress conditions. A large variation in C_U_NS was identified among the cowpea genotypes. C_U_NS ranged between 18.5 and 54.5, with an average of 34.4 and a standard deviation of 4.2. C_U_NS was approximately normally distributed (Fig. 2f). A significant variation in C_U_NS was found among the 331 cowpea genotypes evaluated for drought tolerance (F-value = 1.8, p value < 0.0001) (Table 1). The genotypes IT84S_2246 (54.5), IT93K_503_1 (53.8), PI582863 (46.6), IT89KD_288 (45.3), and Suvita_2 (44.7) had the highest C_U_NS, whereas PI583202 (26.2), PI583513 (25.4), PI663148 (25.4), PI583551 (25.2), and PI583240 (18.5) had the lowest C_U_NS (Table 3). The broad-sense heritability for C_U_NS was 70.5%.
A large variation in unifoliate leaf SPAD chlorophyll (C_U_S) was found among the 331 cowpea genotypes under drought stress. C_U_S varied from 5.1 to 53.7, with an average of 24.4 and a standard deviation of 7.3. The distribution of C_U_S was approximately normal (Fig. 2f). A large variation in C_U_S was identified among the cowpea genotypes (F-value = 2.33, p value < 0.0001) (Table 1). The genotypes with the highest C_U_S were IT84S_2246 (53.7), IT93K_503_1 (48.0), PI583200 (47.0), Suvita_2 (44.4), and ‘EpicSelect.4’ (41.1) (Table 3), indicating that these genotypes were drought-tolerant based on unifoliate leaf SPAD chlorophyll under stress. The genotypes with the lowest C_U_S were PI582468 (10.1), PI293545 (9.2), PI582815 (7.7), PI582850 (7.2), and PI582810 (5.1) (Table 3), suggesting that these genotypes were susceptible to drought conditions based on unifoliate leaf SPAD chlorophyll. The broad-sense heritability for C_U_S was 79.9%.
Relative tolerance index for unifoliate leaf SPAD chlorophyll (RTI_C_U) was computed in order to assess the relative effect of drought stress on unifoliate leaf SPAD chlorophyll. A large variation in RTI_C_U was found among the 331 cowpea genotypes. RTI_C_U varied from 19.7 to 183.1, with a mean of 72.7 and a standard deviation of 20.7. RTI_C_U was approximately normally distributed (Fig. 2g). A significant difference in RTI_C_U was found among the cowpea genotypes (F-value = 1.81, p value < 0.0001) (Table 1). The genotypes with the highest RTI_C_U were PI583240 (183.1), PI663148 (136.8), PI293500 (122.2), IT00K_1263 (118.4), and PI200867 (113.7) (Table 3), whereas those with the lowest RTI_C_U were PI293545 (27.1), AR_BE_1 (26.1), PI582850 (23.3), PI582815 (21.1), and PI582810 (19.7) (Table 3). The broad-sense heritability for RTI_C_U was 70.8%.
Trifoliate leaf SPAD chlorophyll
SPAD chlorophyll on the first trifoliate leaf (C_T_NS) was also analyzed for the plants under non-drought stress conditions. A large variation in C_T_NS was found among the cowpea genotypes evaluated for drought tolerance. C_T_NS ranged between 26.7 and 54.7, with an average of 38.3 and a standard deviation of 4.2. The distribution of C_T_NS was approximately normal (Fig. 2h). The effect of the genotype on C_T_NS was significant (F-value = 1.96, p value < 0.0001) (Table 1). The genotypes having the highest C_T_NS were IT84S_2246 (54.7), IT93K_503_1 (53.3), IT89KD_288 (51.9), PI582863 (50.9), and PI582789 (49.3), whereas those with the lowest C_T_NS were PI582566 (29.4), PI583274 (28.9), PI663011 (28.2), PI583551 (27.6), and PI583197 (26.7) (Table 3). The broad-sense heritability for C_T_NS was 74.2%.
Data on SPAD chlorophyll on the first trifoliate leaf (C_T_S) was also investigated under water stress. C_T_S varied from 22.0 to 57.7, with an average of 37.0 and a standard deviation of 5.0. C_T_S was approximately normally distributed (Fig. 2h). A large variation in C_T_S was identified among the 331 cowpea genotypes (F-value = 686.13, p value < 0.0001) (Table 1). The genotypes with the highest C_T_S were IT84S_2246 (57.7), IT93K_503_1 (55.5), PI390421 (52.4), IT89KD_288 (50.3), and Suvita_2 (48.7) (Table 3), indicating that these genotypes had a good tolerance to trifoliate leaf chlorosis. The genotypes with the lowest C_T_S were PI582572 (25.3), PI582571 (24.6), PI582421 (24.3), PI582570 (24.1), and PI582567 (22.0) (Table 3), suggesting that these genotypes were susceptible to trifoliate leaf chlorosis under drought stress. The broad-sense heritability for C_T_S was 70.9%.
Relative tolerance index was calculated to assess the relative effect of drought stress on trifoliate leaf SPAD chlorophyll (RTI_C_T). A large variation in RTI_C_T was identified among the cowpea genotypes. RTI_C_T varied from 61.8 to 414.2, with an average of 98.3 and a standard deviation of 13.6. RTI_C_T was approximately normally distributed (Fig. 2i). A significant difference in RTI_C_T was found among the cowpea genotypes (F-value = 1.24, p value = 0.0113) (Table 1). The genotypes with the highest RTI_C_T were PI583551 (141.2), PI583550 (131.7), PI293584 (128.8), PI354860 (126.0), and PI354854 (125.9) (Table 3), indicating that these genotypes were drought-tolerant based on RTI_C_T. The genotypes PI582810 (71.2), PI582571 (68.6), PI582573 (68.4), PI582421 (63.6), and PI582567 (61.8) (Table 3) had the lowest RTI_C_T, suggesting that these genotypes were the most susceptible based on RTI_C_T. The broad-sense heritability for RTI_C_T was 41.7%.
Drought tolerance and geographical locations
The effect of geographical locations on traits evaluated for drought tolerance was assessed. Results showed that geographical location differences were significant for traits such as plant greenness score (F-value = 5.94, p value = 0.0005), recovery rate (F-value = 4.09, p value = 0.0068), average number of plants having chlorotic unifoliate leaves when the susceptible control had more than 50% of its unifoliate leaves being chlorotic (F-value = 11.39, p value < 0.0001), average number of plants having chlorotic first trifoliate leaves (F-value = 9.7, p value < 0.0001), unifoliate leaf SPAD chlorophyll (F-value = 4.65, p value = 0.0032), relative tolerance index for unifoliate leaf SPAD chlorophyll (F-value = 7.33, p value < 0.0001), and relative tolerance index for trifoliate leaf SPAD chlorophyll (F-value = 6.53, p value = 0.0002) (Table 4) (Fig. 3). Genotypes from America and Asia had the lowest plant greenness score, thus more drought-tolerant (Table 5). Interestingly, genotypes from Africa had the highest plant greenness score, which was not expected. Genotypes from America and Asia recovered the best after rewatering. Despite the fact that genotypes from America and Asia were equally recovered after rewatering, those from America had large variation in terms of recovery rate (Fig. 3b). Results suggested that genotypes from America and Asia had the highest unifoliate leaf SPAD chlorophyll, thus being more drought-tolerant based on this trait. However, genotypes from Europe and the Middle East had the lowest unifoliate leaf SPAD chlorophyll (Table 5). Relative tolerance index was the highest for genotypes from Asia and America and was the lowest for those from Europe and the Middle East. In addition, genotypes from Africa, Europe, and the Middle East had more plants with unifoliate leaf chlorosis than those from America and Asia under drought stress (Table 5). Most of the genotypes from Africa were more susceptible to trifoliate leaf chlorosis than those from other regions under water deficit conditions. In addition, the genotypes from Asia were the best in terms relative tolerance index for trifoliate leaf SPAD chlorophyll, then followed by the genotypes from America, and the genotypes from Africa, Europe, and the Middle East ranked last in terms of trifoliate leaf SPAD chlorophyll (Table 5).
Boxplots showing the variation of the traits evaluated for drought tolerance for each geographical area (origin). The x-axis represented the geographical where Afr = Africa (n = 100), Am = America (n = 77), As = Asia (n = 32), and E_ME = Europe and the Middle East (n = 17). Genotypes without information on the origin were not included in the analysis. Below each x-axis are shown the p values obtained from the ANOVA. The y-axis displayed the different traits values. a Plant greenness score, b recovery rate, c average number of plants having chlorotic unifoliate leaves when more than half of the plants of the susceptible control have chlorotic unifoliate leaves, d average number of plants having chlorotic unifoliate leaves when the susceptible control was completely dead, e average number of plants having chlorotic trifoliate leaves, f unifoliate leaf SPAD chlorophyll under non-drought stress, g unifoliate leaf SPAD chlorophyll under drought stress, h relative tolerance index for unifoliate leaf SPAD chlorophyll under drought stress, i trifoliate leaf SPAD chlorophyll under non-drought stress, j trifoliate leaf SPAD chlorophyll under drought stress, and k relative tolerance index for trifoliate leaf SPAD chlorophyll under drought stress
No significant geographical location effects were identified for the average number of plants having chlorotic unifoliate leaves when the susceptible control was completely dead (F-value = 0.78, p value = 0.5076), unifoliate leaf SPAD chlorophyll under non-drought stress (F-value = 1.21, p value = 0.3039), trifoliate leaf SPAD chlorophyll under non-drought stress (F-value = 2.28, p value = 0.078), and trifoliate leaf SPAD chlorophyll under drought stress (F-value = 1.46, p value = 0.2241) (Table 5).
Correlation analysis and genotype ranking across traits
Correlation between traits analyzed for drought tolerance was investigated. Plant greenness score was highly correlated with tolerance to trifoliate leaf chlorosis (r = 0.8), but was moderately correlated with unifoliate leaf chlorosis (r = 0.4–0.5), unifoliate leaf SPAD chlorophyll under drought stress (r = − 0.5), relative tolerance index for unifoliate leaf SPAD chlorophyll (r = -0.4), trifoliate leaf SPAD chlorophyll under drought stress (r = -0.4), and relative tolerance index for trifoliate leaf SPAD chlorophyll (r = -0.4) (Table 6). A high correlation was identified between unifoliate leaf SPAD chlorophyll under non-drought stress and trifoliate leaf SPAD chlorophyll under non-drought stress (r = 0.7), unifoliate leaf SPAD chlorophyll under drought stress and trifoliate leaf SPAD chlorophyll under drought stress (r = 0.6), and trifoliate leaf SPAD chlorophyll and relative tolerance index for trifoliate leaf SPAD chlorophyll (r = 0.6) (Table 6). However, trifoliate leaf SPAD chlorophyll under non-drought stress was not correlated with unifoliate leaf chlorosis under drought stress (r = 0.0) and trifoliate leaf chlorosis under drought stress (r = 0.0) (Table 6).
Genotype ranking across traits was analyzed in order to identify the genotypes that were drought-tolerant and drought-susceptible based on multiple traits. Genotypes were ranked for all traits (Table S3) and genotypes that overlapped between highly correlated traits were chosen. Highly correlated traits were score (overall greenness score), tri (average number of plants with chlorotic first trifoliate leaves when the susceptible control was completely dead), and uni_1 (average number of plants with chlorotic unifoliate leaves when the susceptible control, PI255774, had more than 50% chlorotic unifoliate leaves). In fact, if some traits were highly correlated, ranking should be also consistent across traits. Therefore, the genotypes with the highest overall plant greenness and whose ranking was almost consistent across other highly correlated traits were PI664524, PI300173, PI583550, PI293476, PI583251, PI207527, PI227829, PI293469, PI194211, PI194213, PI291140, PI292892, IT84S_2246, PI194208, PI152197, PI354864, PI583209, PI598335, PI662993, and PI293500 (Table 7), indicating that these genotypes could be highly drought-tolerant. Of these genotypes, 8 were from America, 3, were from the Africa, 1 from Asia, and 1 from the Middle East. A similar approach was used to identify the most susceptible genotypes based on traits that were highly correlated. Results suggested that the genotypes PI255774, PI583247, PI582924, PI582530, PI582810, PI503326, PI582566, PI582468, ‘Early Scarlet’, and PI582850 were highly susceptible to drought stress (Table 7). A cluster analysis approach was used to further validate our results where the drought-tolerant genotypes were successfully separated from the drought-susceptible ones (Fig. 4) (Fig. S1). The top 10 drought-tolerant genotypes and the susceptible control were repeated to further validate the results (Fig. 1d).
Discussion
Drought stress has resulted in significant crop yield losses worldwide (Cairns et al. 2013). The use of drought-tolerant crop cultivars could mitigate the effects of drought stress. Cultivar development requires an extensive phenotyping, which will contribute towards the identification of drought-tolerant lines. Drought stress occurring at seedling stage could be detrimental to cowpea production (Verbree et al. 2015). In this study, we have evaluated a total of 331 cowpea genotypes for their tolerance to drought stress at seedling stage. We found that the 3 genotypes that were reported to be drought-tolerant in our previous study (Ravelombola et al. 2018) ranked among the top 20 genotypes that were best performing in terms of plant greenness score in this current study, indicating that our experiments were robust. In addition, the 8 founders that were used to develop the first MAGIC cowpea population were included in the panel. Results showed that 2 founders, IT84S_2246 and IT00K_1263, were highly drought-tolerant. Drought field phenotyping on this MAGIC cowpea population was conducted by Huynh et al. (2018), and results suggested that the 2 aforementioned founders were also drought-tolerant under field conditions. However, Huynh et al. (2018) found a significant variation across locations and years when screening drought tolerance under field conditions. We suggest that the top genotypes that were proven to be drought-tolerant at seedling stage should be retested under field conditions for future projects. The process of screening a large number of genotypes in a greenhouse setup and selecting the top ones for field screening would save a lot of resources in a breeding program. Doing so will allow cowpea breeders to develop a large number of populations, each with significant size, and stack a significant number of alleles of interest. The macro greenhouse/field drought tolerance screening would be a powerful tool that could be used in plant breeding. This study is a first step towards establishing a macro greenhouse/field drought tolerance screening in cowpea.
Cowpea drought tolerance phenotyping using the ‘wooden box’ technique has been proven to be effective (Ravelombola et al. 2018; Verbree et al. 2015). Cowpea genotypes that are tolerant to unifoliate chlorosis and/or trifoliate chlorosis were well-differentiated using this technique (Fig. 1b). In addition to leaf chlorosis under drought stress, plant greenness score has also been used to assess drought tolerance in cowpea. Plant greenness score has been shown to help identify wilting status of cowpea plants under drought stress. Drought-tolerant genotypes were slow-wilting, whereas those that were more drought-susceptible were fast-wilting (Ravelombola et al. 2018; Verbree et al. 2015).
Drought tolerance has been reported to be a complex mechanism in crop (Golldack et al. 2014). Singh et al. (1999) suggested that drought tolerance should be investigated separately for different growth and developmental stages of cowpea, and at each stage, different parameters such as tolerance to trifoliate leaf chlorosis or unifoliate leaf chlorosis should also be interpreted separately. We support the statement of Singh et al. (1999) since the Pearson’s correlation coefficient between trifoliate leaf chlorosis and unifoliate leaf chlorosis was 0.4–0.5. In addition, the broad-sense heritability between traits was different, suggesting that the genetics mechanism underlying the different traits analyzed in this study could be different, especially for the traits that were not correlated at all. Mai-Kodomi et al. (1999) coined type I drought-tolerant cowpea the genotypes that have both unifoliate and trifoliate leaves fully green under drought stress, and type II drought-tolerance the genotypes that were only able to delay senescence at the trifoliate leaf level. In this study, type II drought-tolerant genotypes were prevalent. In addition, we found that geographical locations could impact drought tolerance in cowpea. Similar results were obtained for salt tolerant-related traits in cowpea (Ravelombola et al. 2017). Results suggested that cowpea genotypes from Asia were likely drought-tolerant that those from other geographical areas. Earlier reports were still conflicting regarding the center of origin of cowpea genotypes (Singh et al. 1999). Previous investigations stated that cowpea could have been first domesticated from either East Africa or India. In this study, most of the genotypes that were grouped into Asia were from India, which could suggest some relationship between drought tolerance and possible origin of domestication.
The drought-tolerant genotypes that were identified in this study could be used as parents to develop drought-tolerant cultivars. In addition, the drought-tolerant genotypes could be crossed with the susceptible ones to develop mapping populations for drought tolerance-related studies in cowpea, which is required for developing molecular markers that are used in marker-assisted selection (MAS).
Conclusions
In this study, a total of 331 cowpea genotypes were evaluated for drought tolerance at seedling stage based on different traits. A large variation in the evaluated traits for drought tolerance was found among the 331 cowpea genotypes. A high correlation was found for traits such as plant greenness score and tolerance to trifoliate leaf chlorosis under drought stress (r = 0.8), whereas no linear correlation was found for traits such as trifoliate leaf chlorosis and unifoliate leaf SPAD chlorophyll under non-drought stress (r = 0.0). The genotypes PI583550, PI583251, PI194213, IT84S_2246, PI152197, PI662993, PI664524, PI227829, PI293469, PI291140, PI292892, PI194208, PI354864, PI583209, PI300173, PI293476, PI207527, PI194211, PI582465, and PI293500 were found to be drought-tolerant across different traits. The results from this study could be used in breeding programs aiming at improving drought tolerance in cowpea.
References
Agbicodo EM, Fatokun CA, Muranaka S, Visser RGF, van der Linden CG (2009) Breeding drought tolerant cowpea: constraints, accomplishments, and future prospects. Euphytica 167:353–370
Ajayi AT, Gbadamosi AE, Olumekun VO (2018) Screening for drought tolerance in cowpea (Vigna unguiculata L. Walp) at seedling stage under screen house condition. Int J Biotechnol 11(1):1–9
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21(1):43–47
Cairns JE, Crossa J, Zaidi PH, Grudloyma P, Sanchez C, Araus JL, Thaitad S, Makumbi D, Magorokosho C, Banziger M, Menkir A (2013) Identification of drought, heat, and combined drought and heat tolerant donors in maize. Crop Sci 53(4):1335–1346
Frota KMG, Soares RAM, Arêas JAG (2008) Chemical composition of cowpea (Vigna unguiculata L. Walp), BRS-Milênio cultivar. Food Sci Technol 28(2):470–476
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151
Holland J (2003) Estimating and interpreting heritability for plant breeding: an update. Plant Breed Rev 22:9–112
Huynh BL, Ehlers JD, Huang BE, Muñoz-Amatriaín M, Lonardi S, Santos JR, Ndeve A, Batieno BJ, Boukar O, Cisse N, Drabo I (2018) A multi-parent advanced generation inter-cross (MAGIC) population for genetic analysis and improvement of cowpea (Vigna unguiculata L. Walp.). Plant J 93(6):1129–1142
Lonardi S, Muñoz-Amatriaín M, Liang Q, Shu S, Wanamaker SI, Lo S, Tanskanen J, Schulman AH, Zhu T, Luo MC, Alhakami H (2019) The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J 98(5):767–782
Mai-Kodomi Y, Singh BB, Myers O (1999) Two mechanisms of drought tolerance in cowpea. Indian J Genet Plant Breed 59(3):309–316
Okiror SO, Onyilagha JC, Dunbar T (2008) Investigating the potentials of four cowpea (southern pea) cultivars for fresh seed production. Int J Appl Agric Res 3(1):67–74
Olufajo O (2012) Agronomic performance of improved cowpea varieties under natural infestation with Alectra vogelii (Benth.) in the northern Guinea savannah of Nigeria. Agric Trop Subtrop 45(2):66–71
Ravelombola W, Shi A, Weng Y, Clark J, Motes D, Chen P, Srivastava V (2017) Evaluation of salt tolerance at germination stage in cowpea [Vigna unguiculata (L.) Walp.]. HortScience 52(9):1168–1176
Ravelombola W, Shi A, Qin J, Weng Y, Bhattarai G, Zia B, Zhou W, Mou B (2018) Investigation on various aboveground traits to identify drought tolerance in cowpea seedlings. HortScience 53(12):1757–1765
Sahu PK (2013) Research methodology: a guide for researchers in agricultural science, social science and other related fields. Springer, New York
Singh BB, Mai-Kodomi Y, Terao T (1999) A simple screening method for drought tolerance in cowpea. Indian J Genet Plant Breed 59(2):211–220
Verbree DA, Singh BB, Payne WA (2015) Genetics and heritability of shoot drought tolerance in cowpea seedlings. Crop Sci 55(1):146–153
Weng Y, Shi A, Ravelombola WS, Yang W, Qin J, Motes D, Moseley DO, Chen P (2017) A rapid method for measuring seed protein content in cowpea (Vigna unguiculata (L.) Walp)). Am J Plant Sci 8(10):2387–2396
Wilson C, Liu X, Lesch SM, Suarez DL (2006) Growth response of major US cowpea cultivars. I. Biomass accumulation and salt tolerance. HortScience 41:225–230
Acknowledgements
This work is supported, in part, by the USDA National Institute of Food and Agriculture Hatch project accession number 1002423 and 1017337, and also by USDA Crop Germplasm Committees program for Vigna germplasm evaluation with Agreement Number/FAIN: 58-6046-9-004, Project Number: 6046-21000-012-13S, and Accession No.: 436920.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1.
Diversity of cowpea genotypes based on drought tolerance-related traits (PPTX 197 kb)
Rights and permissions
About this article
Cite this article
Ravelombola, W., Shi, A., Chen, S. et al. Evaluation of cowpea for drought tolerance at seedling stage. Euphytica 216, 123 (2020). https://doi.org/10.1007/s10681-020-02660-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02660-4