Abstract
Chickpea is the most important pulse crop globally after dry beans. Climate change and increased cropping intensity are forcing chickpea cultivation to relatively higher temperature environments. To assess the genetic variability and identify heat responsive traits, a set of 296 F8–9 recombinant inbred lines (RILs) of the cross ICC 4567 (heat sensitive) × ICC 15614 (heat tolerant) was evaluated under field conditions at ICRISAT, Patancheru, India. The experiment was conducted in an alpha lattice design with three replications during the summer seasons of 2013 and 2014 (heat stress environments, average temperature 35 °C and above), and post-rainy season of 2013 (non-stress environment, max. temperature below 30 °C). A two-fold variation for number of filled pods (FPod), total number of seeds (TS), harvest index (HI), percent pod setting (%PodSet) and grain yield (GY) was observed in the RILs under stress environments compared to non-stress environment. A yield penalty ranging from 22.26% (summer 2013) to 33.30% (summer 2014) was recorded in stress environments. Seed mass measured as 100-seed weight (HSW) was the least affected (6 and 7% reduction) trait, while %PodSet was the most affected (45.86 and 44.31% reduction) trait by high temperatures. Mixed model analysis of variance revealed a high genotypic coefficient of variation (GCV) (23.29–30.22%), phenotypic coefficient of variation (PCV) (25.69–32.44%) along with high heritability (80.89–86.89%) for FPod, TS, %PodSet and GY across the heat stress environments. Correlation studies (r = 0.61–0.97) and principal component analysis (PCA) revealed a strong positive association among the traits GY, FPod, VS and %PodSet under stress environments. Path analysis results showed that TS was the major direct and FPod was the major indirect contributors to GY under heat stress environments. Therefore, the traits that are good indicators of high grain yield under heat stress can be used in indirect selection for developing heat tolerant chickpea cultivars. Moreover, the presence of large genetic variation for heat tolerance in the population may provide an opportunity to use the RILs in future-heat tolerance breeding programme in chickpea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the IPCC reports, an increase of 2–4 °C in temperature is predicted globally by the end of the 21st century (IPCC 2007). It is also estimated that tropical and subtropical parts of the world will be the most affected areas by this rise in temperature (Battisti and Naylor 2009; Lobell and Gourdji 2012). Moreover, due to rise in temperature, a noticeable change in plant phenology and yield was observed across different crop species and also within crop species (Ibáñez et al. 2010; Gaur et al. 2014). Thus, it is an urgent issue to be addressed with regard to heat stress to ensure the food and nutritional security globally.
Chickpea, grown mostly in cool season, is one of the nutrient-rich semi-arid tropical legume crops. Being cultivated in over 60 countries and traded in over 190 countries, chickpea is second after dry beans in the world in terms of production and consumption (FAOSTAT 2014). Abiotic stresses, such as drought, cold, and salinity are constraints for chickpea productivity (Gaur et al. 2007) but recently, heat stress, caused due to increased temperature is also becoming a serious problem to chickpea cultivating areas globally (Gaur et al. 2014).
India is the largest producer of chickpea with about 70% share of the total world chickpea production (FAOSTAT 2014). A noticeable change in chickpea production has been observed in the Indian subcontinent in the last few years. There has been a big shift in chickpea area from cooler northern India to relatively warmer central and southern India (Gaur et al. 2007). Concurrently, due to increased cropping intensity, farmers are growing chickpea in the spring season (15 January onward in southern India) after the harvest of rainy-season crops like corn or rice. As a consequence, the crop is exposed to heat stress during its reproductive phase.
A drastic reduction in yield of chickpea was reported when the crop was exposed to heat stress (35 °C and above) during reproductive phase (Summerfield et al. 1984; Wang et al. 2006; Devasirvatham et al. 2012). Hence, cultivars that can tolerate high temperatures without reduction in yield are needed for sustainable chickpea production.
Heat tolerance is a complex trait. An effective and simple screening method with well-defined traits for selecting heat-tolerant genotypes under field conditions is necessary for breeding heat tolerant cultivars (Devasirvatham et al. 2012). Canci and Toker (2009) studied 377 germplasm lines and 68 accessions of wild Cicer species for genetic variation and identified several heat tolerant genotypes and suggested HI, GY and pods per plant are the traits to be considered for selection. Large genetic variation was reported from the study of Gaur et al. (2010) in a field evaluation of 180 chickpea genotypes at two locations in India. Further, Upadhyaya et al. (2011) too found large variation for heat tolerance in 35 early maturing chickpea lines. Krishnamurthy et al. (2011) observed large genetic variation for heat tolerance in the reference set of chickpea (280 accessions) and found that %PodSet was the most affected trait by heat stress. In another study, evaluation of 167 chickpea genotypes at ICRISAT over two years under heat stress revealed a large genetic variation for heat tolerance (Devasirvatham et al. 2012).
However, studies were not conducted that involved experimental population bred from two contrasting parents for heat tolerance. A comprehensive approach to understand the nature of genetic parameters for heat tolerance in chickpea may be possible with genetically defined RIL population for heat tolerance as the case in the present study.
The reproductive stage of chickpea is the most sensitive to heat stress (Malhotra and Saxena 1993; Singh et al. 1994). Pod setting and pod filling were severely affected during pod development stage under high-temperature stress (Summerfield et al. 1984; Van Rheenen et al. 1997; Gan et al. 2004).
In previous studies, various traits: number of filled pods, number of seeds, biological yield, harvest index, % pod setting, and 100-seed weight were considered in understanding the heat tolerance in chickpea (Krishnamurthy et al. 2011; Devasirvatham et al. 2012;). The trait seed set percentage or fruit set was considered as one of the key traits for determining the heat tolerance in various studies in other crops such as maize, rice and tomato (Sato et al. 2006; Jagadish et al. 2008; Alam et al. 2017). In the present study, several phenological and agronomical traits were considered for heat tolerance under field condition.
Therefore, this study was conducted to assess the genetic variability under heat stress for yield and yield component traits and selection of secondary traits related to heat stress tolerance in chickpea in an RIL population under field conditions.
Materials and methods
Population development and evaluation
To study the genetic parameters and impact of heat stress on chickpea, two parents ICC 4567 (heat sensitive) and ICC 15614 (heat tolerant) differing in heat tolerance were chosen and crossed to generate an RIL population. A population of 296 RILs was developed using single-seed descent (SSD) method. The parents showed variation in several heat tolerance related traits—grain yield, filled pods, total seeds, pollen viability, pollen germination and pollen tube growth (Devasirvatham et al. 2013). Two heat tolerant checks JG11 and GG2 were also included along with the parents and RILs.
The experiment was carried out at ICRISAT, Patancheru, India (17°30′N; 78°16′E; altitude 549 m) in vertisol soil (fine montmorillonitic isohyperthermic typic pallustert). The F8–9 RIL population was evaluated in two consecutive years during summer season (above 35 °C), (Feb–May, 2013 and summer, Feb–May, 2014) and in one non-heat stress environment (in post-rainy season, Nov–Feb, 2013). Hereafter, the heat stress environment 2013 and heat stress environment 2014 are designated as HSE-2013 and HSE-2014, whereas, non-stress environment as NSE-2013.
In all the environments, the field used for the phenotyping was solarized using polythene mulch during the preceding summer to sanitize the field, especially to get rid of soil-borne diseases. For both non-stress and stress experiments, sowing was done on the ridges with inter- and intra-row spacing of 60 × 10 cm. Each pot consisted of a 2-meter long row. Need-based sprayings of insecticides were provided to control pod borer (Helicoverpa armigera). Experiment plots were maintained weed free by manual weeding. Seeds were treated with the mixture of fungicides 0.5% Benlate® (E.I. DuPont India Ltd., Gurgaon, India) + Thiram® (Sudhama Chemicals Pvt. Ltd., Gujarat, India), before planting.
The RIL population was evaluated in an alpha lattice design (15 × 20) with three replications in all the environments. Sowing for the stress-environments was completed in the first week of February. This exposed the reproductive phase of the RILs to high temperature (> 35 °C). Sowing for the non-stress environment was done on the residual moisture in the last week of November as recommended for normal sowing for chickpea in this region, and provided with essential irrigation. Irrigations were given to heat-stress experiments at regular intervals to avoid the confounding effect of drought stress and make the experiments solely for heat stress. The mean daily temperatures during the reproductive phase of RILs were 37.5/22.33 and 36.7/22.9 °C, in HSE-2013 and HSE-2014, respectively. On the other hand, an optimum temperature of 29.43/15.5 °C for normal growing of chickpea was recorded in NSE-2013.
Variables measured
Heat tolerance is a complex trait. Several indirect traits are used to define the heat tolerance factor in plants. In chickpea, number of filled pods per plot (FPod), total number of seeds per plot (TS), grain yield (GY), harvest index (HI), biomass (BM), 100-seed weight (HSW) and per cent pod setting (%PodSet), were found to be associated with heat tolerance in chickpea (Krishnamurthy et al. 2011; Devasirvatham et al. 2013). These seven traits along with days to 50% flowering (DF50) and visual score on podding behaviour (VS) were recorded in the RIL population. The data for FPod, TS, GY, BM and HI were taken from a continuous patch of half-meter (0.5 m) long from the 2-meter plot. Visual scoring on podding behaviour (VS) at maturity and per cent pod setting (%PodSet) were recorded from whole plot. For visual scoring, score-1 was considered most sensitive (least number of pods) whereas; score-5 was taken as most tolerant (maximum number of pods) under heat stress. Under non-stress environment, there was no difference in podding. Hence, no visual score data were recorded in this environment.
Statistical analyses
The analysis of variance (ANOVA) was performed using GenStat 17th Edition (VSN International, Hemel, Hempstead, UK) for individual environments using mixed model analysis. For each trait and environment, the analysis was performed considering entry, and block nested with replication as random effects, and replication as fixed effect. In order to pool the data across environments and to make the error variances homogeneous, individual variances were estimated and modelled for the error distribution using residual maximum likelihood (ReML) procedure. Z value and F value were calculated for random effects and fixed effects, respectively in these analyses. Broad sense heritability was estimated (Falconer et al. 1996) as
and pooled broad sense heritability was calculated (Hill et al.2012) as
where, H2 is broad sense heritability, Vg is genotypic variance, Vge is G × E interaction variance, Ve is residual variance, ne is number of environments, and nr is number of replications.
Pearson correlation analysis and linear regressions were fitted using Microsoft Excel 2016 (Microsoft Corp., 1985, Redmond, Washington, USA). Associations among the traits were determined by principal component analyses (PCA) and Path Analysis using R version 3.0.2_ 2013 (R Project for Statistical Computing, http://www.r-project.org/).
Results
Genetic variation in RIL population
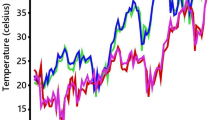
The range of day/night temperatures was recorded as 33.7/23.1–39.8/25 °C, and 27/21.4–39.0/22.8 °C, respectively, in HSE-2013 and HSE-2014 whereas it was 26.9/12.6–32.7/12.5 °C in the NSE-2013 (Fig. 1). During the reproductive phases of HSE-2013 and HSE-2014, the average day/night temperature was 37.52/22.50 and 36.73/22.99 °C, respectively. The non-stress environment recorded an average temperature of 29.63/15.49 °C. High temperatures (> 35 °C) during the reproductive stage of the crop in HSE-2013 and HSE-2014 provided optimal conditions for heat tolerance screening.
Daily maximum and minimum temperatures (oC) during the late sown crop growing period (stress season) in 2013 and 2014. (34/19 °C is the threshold temperature for the maximum and minimum temperatures for chickpea yield, respectively. The maximum day temperatures were 39.8 and 39.0 °C, and maximum night temperatures were 24.9 and 27.2 °C in heat stress environments 2013, and 2014, respectively. Crop growing period was 2nd week of February to 3rd week of May.)
Predicted means of parents for all the seven traits except BM in both the heat stress environments as well as in pooled environments significantly (p < 0.001) differed. As expected, the difference of the predicted means of parents was non-significant for GY, BM, HI and %PodSet in the non-stress environment (Table 1). The yield reduction of heat tolerant parent ICC 15614 under heat stress environments was 10.45 and 22.04%, in HSE-2013 and HSE-2014, respectively, whereas the loss was much higher in heat-sensitive parent ICC 4567 in HSE-2013 and HSE-2014 (41.77 and 57.01%, respectively) in comparison to NSE-2013. The population means for all the traits of the heat stress environments were found to be lower than the population mean of non-stress environment except DF50 in HSE-2014. For instance, in NSE-2013, the population mean of GY was 73.45 g, whereas 57.1 g (22.26% reduction) and 49.09 g (33.30% reduction) were observed for heat stress environments during 2013 and 2014, respectively (Table 1). Similarly, 29.44 and 41.61% reduction for FPod; and 45.86 and 44.31% reduction for %PodSet were observed in HSE-2013 and HSE-2014, respectively, as compared to NSE-2013. Reductions of 8.8 and 7.56% were observed in seed weight in HSE-2013 and HSE-2014, respectively in contrast to NSE-2013.The range of all traits in both the heat stress environments was very high (Table 1). A similar trend in range for all the traits was observed in pooled environment of HSE-2013 and HSE-2014. As expected, the range of all the traits in non-stress environment was comparatively low.
A highly significant (P < 0.001) genetic variance was observed for all the traits in two heat stress environments (Table 2). Pooled analysis of two heat stress environments also revealed a highly significant genetic and G × E interaction variance (Table 2). The genotypic and phenotypic coefficient of variation (GCV and PCV) estimates were very high in case of FPod, TS, GY and %PodSet (23.29–30.22%), moderate for HSW (14.46–14.61%) and low (3.51 and 4.53%) for DF50 in HSE-2013 and HSE-2014, respectively (Table 1). GCV and PCV values for VS (low to moderate), BM and HI (moderate to high) were not consistent across the heat stress environments (Table 1). High GCV and PCV values were also observed for the traits FPod, TS, GY, %PodSet in pooled over years for both the heat stress environments. As anticipated, all the traits showed low to moderate (4.25–16.29%) degree of GCV and PCV estimates in the NSE-2013 (Table 1).
Heritability and other genetic parameters
All the traits, except BM in the HSE-2014, showed heritability in the range of 72.01–91.25% in both the heat stress environments. The heritability of GY was 82.18 and 80.89%, respectively for HSE-2013 and HSE-2014 (Table 1). The heritability of BM was higher (83.18%) in HSE-2013 than HSE-2014 (49.84%). On the other hand, VS, FPod, TS, BM, GY, HI and %PodSet showed heritability in the range of (47.60–65.95%) in the non-stress environment of 2013. It is clear that the heritability for these traits in the non-stress environment was much lower than the heritability in both the stress environments. The heritability of HSW and DF50 was very high and consistent across the environments (93.45–97.51% and 82.59–87.82%, respectively) (Table 1).
In both the HSEs, genetic advance (GAM) as in Table 1, was observed very high for all the traits except DF50 (6.78%) and VS (16.09%) in HSE-2013; and DF50 (7.61%) and BM (15.47%) in HSE-2014. The GA for all the traits varied from 26.5 to 58.07% in HSE- 2013 and from 25.51% to 57.99% in HSE-2014 (Table 1). Further, GA of FPod, TS, HSW, %PodSet and GY was consistent across the HSEs. In contrast, the majority of the traits showed moderate genetic advance (14.6–20.01%) in NSE-2013. Among all the traits, HSW showed a high genetic advance (25.78%) in NSE-2013. (Table 1).
Trait associations
Usefulness of independent secondary traits in the selection process can be assumed by their significant association with a dependent trait like GY. In this present study, grain yield was positively and significantly associated with all the traits except DF50 and HSW in both the heat stress environments (Table 3). The traits-VS (r = 0.66**and r = 0.73**), FPod (r = 0.88**and r = 0.90**), TS (r = 0.89** and r = 0.89**), %PodSet (r = 0.63**and r = 0.50**) showed high correlation with GY whereas, moderate to high correlation for BM (r = 0.74** and r = 0.57**) and HI (r = 0.32** and r = 0.84**) in HSE-2013 and HSE-2014, respectively. In contrast, a negative (but low in magnitude) correlation value was found for DF50 (r = − 0.20** and − 0.26**) and HSW (r = − 0.12* and − 0.08 ns) in HSE-2013 and HSE-2014, respectively (Table 3).
Among the secondary traits FPod had positive association with TS (r = 0.97**, r = 0.96**), %PodSet (r = 0.72**, r = 0.59**), BM (r = 0.70**, r = 0.40**) and HI (r = 0.22**, r = 0.84**) in HSE-2013 and HSE-2014, respectively (Table 3). Similarly, TS was found to be positively associated with %PodSet (r = 0.73**, r = 0.60**), BM (r = 0.68**, r = 0.38**) and HI (r = 0.25**, r = 0.84**) in the two heat stress environments, respectively.
Regression analysis
Regression study revealed the contribution of independent traits like DF50, VS, FPod, TS, BM, HI, HSW & %PodSet to the variation in response trait like GY. Linear regression of FPod and TS on GY validated that both the traits have a very high contribution to the total yield variation in both the heat stress environments. FPod accounted for 78 and 79% of total yield variation, respectively, in HSE-2013 and HSE-2014, while for TS it was 79 and 81% in both the environments (Fig. 2; Supplementary Table 1). VS and %PodSet were also found to have a contribution in the yield variation. In HSE-2013 and HSE-2014, VS contributed 38 and 52% variation in yield whereas %PodSet showed 40 and 26% contribution towards yield variation, respectively. Like other analyses, the results of regression analysis for BM and HI with GY in both the heat stress environments were inconsistent. There was a yield contribution of 54% in HSE-2013 and 32% in HSE-2014 for BM, and only 10% contribution in HSE-2013 for HI but it was 70% in HSE-2014 (Fig. 2; Supplementary Table 1). There was a negligible contribution for HSW and DF50 for yield in the two heat stress environments. The analysis revealed only 2 and 0.7% contribution of HSW for yield variation, respectively, in HSE-2013 and HSE-2014. Similarly, DF50 also showed only 4% and 8% yield variation in HSE-2013 and HSE-2014, respectively (Fig. 2; Supplementary Table 1).
Across the heat stress environments (pooled analysis), similar kind of trend was visible for linear regression. FPod was the highest contributor in total yield variation with 79% closely followed by TS (77%) (Supplementary Table 1). VS and %PodSet were found to have individual contributions of 62 and 48% towards yield variation, respectively. BM and HI had good contribution with 61 and 57%, respectively. HSW was the least contributor with 3% and DF50 had only 8% contribution in yield variation (Supplementary Table 1).
Path analysis
Results from path analysis showed that TS was the major direct contributor to grain yield in both the heat stress environments (0.91 and 0.62, respectively) as well as in pooled years (0.53) (Table 4). FPod was found to have the highest positive indirect effect on GY through TS (0.88 and 0.60 in HSE-2013 and HSE-2014, respectively). On the other side, FPod showed negative direct effect on GY in HSE-2013 (− 0.12) and low positive direct effect in HSE-2014 (0.05) and in pooled years (0.12). %PodSet had a low negative direct effect on GY (− 0.01, − 0.03, − 0.02 in HSE-2013, HSE-2014 and pooled years, respectively). However, its contribution towards GY was via TS with high and positive indirect effects (0.66 in HSE-2013, 0.38 in HSE-2014 and 0.42 in pooled years) (Table 4). Though HSW was found to have a high direct effect on yield but the overall effect on GY was negative. BM and HI had too high and positive direct effect on GY (Table 4). It was a negative direct effect of DF50 on GY in all three environments. In brief, TS contributed directly to GY and most of the other traits contributed to GY indirectly through TS.
Principal component analysis
Principal component analysis (PCA) was performed based on the predicted means (BLUPs) for the two heat stress environments and pooled over years. The results from PCA analysis revealed that the first two principal components explained 67.70 and 72.86% of the total phenotypic variability in HSE-2013 and HSE-2014, respectively (Supplementary Table 2; Fig. 3). In HSE-2013, the PC1 explained 52.96% for the first axis and PC2 explained 14.74% for the second whereas, in HSE-2014, 56.38 and 16.48%, respectively. FPod, TS, GY, VS, and %PodSet were the main contributing traits in PC1 for both HSEs. In the HSE-2013, BM contributed − 0.36 in PC1 and − 0.40 in PC2, and whereas, in the second stress environment (2014) it contributed more in PC2 and in a different direction (0.61) (Supplementary Table 2). In HSE-2013, HI contributed 0.85 in PC2 and in PC1 in different direction (− 0.06) whereas, in HSE-2014 it was − 0.40 in PC1 and − 0.04 in PC2 (Supplementary Table 2).
Biplots based on PCA showing the relationship of secondary traits with Grain Yield (GY) in HSE-2013 and HSE-2014 at ICRISAT- India. DF50, Days to 50% flowering; VS, Visual score; FPod, Number of filled pods per plot; TS, Number of seeds per plot; HSW, 100-seed weight; BM, Biomass; GY, Grain yield; HI, Harvest Index; %PodSet, Percentage pod setting
Discussion
The heat tolerant (ICC 15614) and the heat sensitive parent (ICC 4567) used in the development of RIL population used in this study were earlier studied for different traits related to heat stress by Devasirvatham et al. (2013). They studied these genotypes along with other 165 chickpea genotypes for two years both in controlled and field conditions. Based on physiological traits (in vivo and in vitro pollen germination, pollen tube growth, pollen fertility) and yield component traits (grain yield, biomass, pod setting), ICC 4567 and ICC 15614 were found to be heat sensitive and heat tolerant, respectively. In this study, RILs developed from a cross between ICC 4567 (heat sensitive) and ICC 15614 (heat tolerant) were evaluated in both non-stress and heat stress environments to see the effects of heat stress on the expression of various traits. It was found in earlier studies that a temperature higher than 35 °C during reproductive phase adversely affects growth, development, and yield in chickpea (Basu et al. 2009; Krishnamurthy et al. 2011; Devasirvatham et al. 2012; Gaur et al. 2014). A 50% reduction in pod set was observed at 35 °C for chickpea genotypes (Devasirvatham et al. 2013). Thus, the late sowings in February 2013 and 2014 provided perfect conditions to expose RILs to heat stress (37.5/22.50 °C and 36.73/22.99 °C, in HSE-2013 and HSE-2014, respectively) during their reproductive phase. On the other hand, for NSE-2013 (timely sown) an average maximum temperature of < 30 °C temperature was suitable for the timely sown crop which was suggested by Berger et al. (2011). As shown in Fig. 1, both the heat stress trials were exposed to high temperature (above day/night temperature of 35/20 °C), therefore, both the heat stress environments were ideal for screening RILs for heat tolerance.
The average percentage reduction of HSW, FPod and GY in HSEs with a comparison to NSE-2013 was in accordance with the findings of Gaur et al. (2007) where a reduction of 13, 43 and 51% was reported for HSW, FPod and GY, respectively. The reduction in %PodSet (50%) in HSEs was similar to the results of Devasirvatham et al. (2013). In addition, %PodSet was found to be the most affected trait in our study which is similar to the findings of Krishnamurthy et al. (2011). Furthermore, HSW was the least affected trait across the HSEs which was also reported by Gaur et al. (2007) and Canci and Toker (2009).
The mixed model analysis of variance (ANOVA) revealed a significant variation among the RILs for all the traits across the environments suggesting that a good amount of variation existed for the studied traits. Parents differed significantly for all the traits except biomass (BM) in both the heat stress environments. This was also observed by Devasirvatham et al. (2013) under similar field conditions. The significant difference between parents for heat-tolerance related traits under heat stress environments validated the experimental conditions for evaluation of RILs for heat tolerance. In contrast, no difference between the parents for GY, HI, BM and %PodSet in the non-stress environment indicated that both heat sensitive and heat tolerant parents have equal yield potential in absence of heat stress. In the normal sowing environment (NSE-2013), due to the large seed size of sensitive parent, there was a significant difference between parents for the traits- FPod and TS but not for the grain yield. Thus, the population developed from these parents was ideal for studying effects of heat stress on different traits.
A prior knowledge on the relative magnitudes of genetic, genotype × environment interaction and environmental variance can be helpful for designing a heat tolerance breeding programme. In chickpea, limited information is available on genetic parameters for heat tolerance. Analysis of variance showed higher genetic components than the residual components in HSE-2013 and HSE-2014, and opposite in NSE-2013.The large genetic variation under heat stress environments and low genetic variation in the non-stress environment for RILs might be the reason for this. The highly significant genetic and genotype × environment interaction for the pooled analysis (HSE-2013 and HSE-2014) is an indication of the fact that in spite of highly interactive with environments, the genotypic difference among RILs was highly significant and consistent across the heat stress environments (Upadhyaya et al. 2011). However, the genetic and genotype × environment interaction variance components of biomass (BM) though statistically significant, were not consistent. It indicates seasonal variability for BM in different seasons was largely due to the interaction effects of genotype and environment rather than genotypic differences.
Estimation of the GCV and PCV components helps to assess the magnitude of genetic variation present in a population for the trait per se. A very high GCV and PCV values of FPod, TS, %PodSet, and GY under heat stress environments and moderate under non-stress environment indicate large effect of heat stress on the RILs for creating variation among them. This also affirms the fact that each RIL behaved differently under stress i.e. their tolerance level to high temperature is different from each other. However, the GCV and PCV value for HSW in both heat stress and non-stress environment was moderate (similar magnitude) indicating very low influence of heat stress on HSW. Moreover, a consistently high heritability (94.72% in HSE-2013 and 97. 51% in HSE-2014), indicates that the trait was stable across environments with relatively less G × E interactions (Serraj et al. 2004). The inconsistent GCV and PCV values for BM and HI across heat stress environments showed that these traits were highly influenced by environment.
Heritability information helps to know the extent of genetic expression under given environment. In this study, the broad sense heritability for yield and yield component traits was higher in magnitude in heat-stress environments. It may be because these traits expressed more distinctly in heat stress environment, as depicted by a wide range of variability in the RILs. This wide variability further led to larger genotypic variability and broad sense heritability under heat stress environment. Higher magnitude of heritability (70–90%) was also reported in earlier studies in chickpea (Vadez et al. 2012, Varshney et al. 2014) and other crops (Pinto et al. 2010, Paliwal et al. 2012) under abiotic stress environmental conditions. The presence of high heritability values for yield and yield contributing traits in both the HSEs indicates selection will be more effective under heat stress environment. (Krishnamurthy et al. 2011). The high heritability coupled with high genetic advance indicates the influence of additive genes affecting these traits.
Association studies
Positive and strong association between FPod, TS, VS and %PodSet with grain yield revealed the importance of these characters in determining yield under heat stress environment. Correlation study showed a low but negative correlation of DF50 with GY (Mallu et al. 2015). It appears that early maturing lines could escape the adverse effects of heat stress on the yield. On the other side, HSW showed no significant correlation with GY. In addition, the result showed the negative associations of trait pairs like HSW vs. TS and HSW vs. FPod. Thus, it can be assumed that the yield is the outcome of an increased number of filled pods and seeds rather than the mass of seed. Increase in seed size leads to a reduction in number of seeds per plant as well as grain yield.
In addition, the path analysis results showed that TS had maximum direct effect on GY. On the other hand, FPod had low negative/positive direct effect on GY, but high positive correlation with GY. The positive correlation was because of its high positive indirect effects through TS (Hassan et al. 2005). Similarly, VS and %PodSet also contributed positively and indirectly towards GY through TS. Thus, the combination of these traits is important to create a selection index for heat tolerant genotypes in chickpea.
The PCA analysis gives information about the cluster of traits explaining maximum variability in the population under given environment. In the present study, GY, FPod, TS, VS and %PodSet traits were clustered together and contributed to maximum variability for yield under two stress environments consistently. Hence, phenotypic selection for these traits will be successful for the screening of chickpea genotypes under heat stress. In the previous studies, BM and HI were found to be good selection criteria for heat tolerance in chickpea (Canci and Toker 2009; Krishnamurthy et al. 2011). Like other analyses in this study, in PCA analysis too, BM and HI showed inconsistent results over the two heat stress environments. Hence, it is better not to include these traits as selection criteria for heat-tolerance in chickpea. Further, the contribution DF50 and HSW towards two main principal components was negligible. This response was also evident from the bi-plot since the vectors corresponding to DF50 and HSW are of shortest magnitude. In both the heat stress environments, vectors of the traits GY, TS, FPod, %PodSet and VS were very close (r = cosθ = + 1) whereas, in non-stress environment BM is closer than the other traits (Fig. 3). HSW in both the heat stress environments showed close to zero or weak negative correlation.
Conclusions
This study revealed the presence of large genetic variation in yield and yield-related traits in the RILs population evaluated under heat stress environments. Among the quantitative traits, seed size (HSW) was the least affected and %PodSet was the most affected trait by heat stress. Results suggest that the influence of environment on genotypes was significantly higher under heat stress condition. From the results of correlation and regression, it is evident that FPod, TS, %PodSet and VS are associated with GY under heat stress condition and can be used as indirect selection criteria for developing heat tolerant genotypes under field conditions. TS and FPod are the most preferred traits as TS had maximum positive direct contribution and FPod had maximum indirect contribution towards yield under stress. Finally, the large genetic variation found in this population can be exploited for future heat tolerance breeding programme in chickpea.
Abbreviations
- %PodSet:
-
Pod setting percentage
- ANOVA:
-
Analysis of variance
- BM:
-
Biomass
- BLUP:
-
Best linear unbiased prediction
- DF50:
-
Days to 50% flowering
- FPod:
-
Number of filled pods per plot
- G × E:
-
Genotype × Environment
- GCV:
-
Genotypic coefficient of Variation
- GY:
-
Grain yield
- HI:
-
Harvest index
- HSE:
-
Heat stress environment
- HSW:
-
100-seed weight
- ICRISAT:
-
International crops research institute for the semi-arid tropics
- NSE:
-
Non-stress environment
- PCV:
-
Phenotypic coefficient of variation
- ReML:
-
Residual maximum likelihood
- RIL:
-
Recombinant inbred line
- TS:
-
Total number of seeds per plot
- VS:
-
Visual scoring
References
Alam MA, Seetharam K, Zaidi PH, Dinesh A, Vinayan MT, Nath UK (2017) Dissecting heat stress tolerance in tropical maize (Zea mays L.). F Crop Res 204:110–119
Basu PS, Ali M, Chaturvedi SK (2009) Terminal heat stress adversely affects chickpea productivity in northern India—Strategies to improve thermo tolerance in the crop under climate change, In: ISPRS Arch., XXXVIII-8/W3 Workshop Proceedings Impact of Climate Change on Agriculture, pp 23–25
Battisti DS, Naylor RL (2009) Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323(5911):240–244
Berger JD, Milroy SP, Turner NC, Siddique KH, Imtiaz M, Malhotra R (2011) Chickpea evolution has selected for contrasting phenological mechanisms among different habitats. Euphytica 180(1):1–15
Canci H, Toker C (2009) Evaluation of yield criteria for drought and heat resistance in chickpea (Cicer arietinum L.). J Agron Crop Sci 195(1):47–54
Devasirvatham V (2012) The basis of chickpea heat tolerance under semi-arid environments. The University of Sydney, Camperdown
Devasirvatham V, Gaur PM, Mallikarjuna N, Raju TN, Trethowan RM, Tan DK (2013) Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. F Crop Res 142:9–19
Falconer DS, Mackay TFC, Frankham R (1996) Introduction to quantitative genetics. Trends Genet 12(7):280
Food and Agriculture Organization (FAO) (2014): Food and Agricultural Organization of the United Nation, FAO Statistical Database. In http://faostat3.fao.org/download/Q/QC/E
Gan Y, Angadi SV, Cutforth H, Potts D, Angadi VV, McDonald CL (2004) Canola and mustard response to short periods of temperature and water stress at different developmental stages. Can J Plant Sci 84(3):697–704
Gaur PM, Srinivasan S, Gowda CLL, Rao BV (2007) Rapid generation advancement in chickpea. J SAT Agric Res 3(1):1–3
Gaur PM, Chaturvedi SK, Tripathi S, Gowda CLL, Krishnamurthy L, Vadez V, Mallikarjuna N, Varshney RK (2010) Improving heat tolerance in chickpea to increase its resilience to climate change. In: Proceeding of the 5th International food legumes research conference and 7th European conference on grain legume, Antalya, pp 26–30
Gaur PM, Jukanti AK, Samineni S, Chaturvedi SK, Basu PS, Babbar A, Jayalakshmi V, Nayyar H, Devasirvatham V, Mallikarjuna N, Krishnamurthy L (2014) Climate change and heat stress tolerance in chickpea. In: Tuteja N, Gill SS (eds) Climate change and plant abiotic stress tolerance. Wiley - VCH Verlag GmbH &Co, Weinheim, pp 837–856
Hassan M, Atta BM, Shah TM, Haq MA, Syed H, Alam SS (2005) Correlation and path coefficient studies in induced mutants of chickpea (Cicer arietinum L.). Pakistan J Bot 37(2):293
Hill J, Becker HC, Tigerstedt PM (2012) Quantitative and ecological aspects of plant breeding. Springer Science & Business Media, Berlin
Ibáñez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Lee SD, Kobori H, Silander JA (2010) Forecasting phenology under global warming. Philos Trans R Soc Lond B Biol Sci 365(1555):3247–3260
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva
Jagadish SV, Craufurd PQ, Wheeler TR (2008) Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci 48(3):1140–1146
Krishnamurthy L, Gaur PM, Basu PS, Chaturvedi SK, Tripathi S, Vadez V, Rathore A, Varshney RK, Gowda CLL (2011) Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet Resour 9(1):59–69
Lobell DB, Gourdji SM (2012) The influence of climate change on global crop productivity. Plant Physiol 160(4):1686–1697
Malhotra RS, Saxena MC (1993) Screening for cold and heat tolerance in cool season food legumes. In: Singh KB, Saxena MC (eds) Breeding for stress tolerance in cool season food legumes. Wiley, Chichester, pp 227–244
Mallu TS, Nyende AB, Rao NG, Odeny DA, Mwangi SG (2015) Assessment of interrelationship among agronomic and yield characters of chickpea. Int J Agric Crop Sci 8(2):128–135
Paliwal R, Röder MS, Kumar U, Srivastava JP, Joshi AK (2012) QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor Appl Genet 125(3):561–575
Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas JJ, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet 121(6):1001–1021
Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, Ikeda H (2006) Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann Bot 97(5):731–738
Serraj R, Krishnamurthy L, Kashiwagi J, Kumar J, Chandra S, Crouch JH (2004) Variation in root traits of chickpea (Cicer arietinum L.) grown under terminal drought. F Crop Res 88(2):115–127
Singh KB, Malhotra RS, Halila MH, Knights EJ, Verma MM (1994) Current status and future strategy in breeding chickpea for resistance to biotic and abiotic stresses. Euphytica 73(1–2):137–149
Summerfield RJ, Hadley P, Roberts EH, Minchin FR, Rawsthorne S (1984) Sensitivity of chickpeas (Cicer arietinum) to hot temperatures during the reproductive period. Exp Agric 20(1):77–93
Upadhyaya HD, Dronavalli N, Gowda CLL, Singh S (2011) Identification and evaluation of chickpea germplasm for tolerance to heat stress. Crop Sci 51(5):2079–2094
Vadez V, Krishnamurthy L, Thudi M, Anuradha C, Colmer TD, Turner NC, Siddique KH, Gaur PM, Varshney RK (2012) Assessment of ICCV 2 × JG 62 chickpea progenies shows sensitivity of reproduction to salt stress and reveals QTL for seed yield and yield components. Mol Breed 30(1):9–21
van Rheenen HA, Singh O, Saxena NP (1997) Using evaluation techniques for photoperiod and thermo-insensitivity in pulses improvement. Recent Advantages in Pulses Research. Indian Society of Pulses Research and Development, IIPR, Kanpur, India, pp 443–458
Varshney RK, Thudi M, Nayak SN, Gaur PM, Kashiwagi J, Krishnamurthy L, Jaganathan D, Koppolu J, Bohra A, Tripathi S, Rathore A (2014) Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor Appl Genet 127(2):445–462
Wang J, Gan YT, Clarke F, McDonald CL (2006) Response of chickpea yield to high temperature stress during reproductive development. Crop Sci 46(5):2171–2178
Acknowledgements
National Food Security Mission (NFSM), Govt. of India; and Tropical Legumes II (TL II) project of Bill and Melinda Gates Foundation (BMGF) for financial support and Department of Science and Technology (DST), Govt. of India, for a fellowship to PJP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, P.J., Samineni, S., Sajja, S.B. et al. Capturing genetic variability and selection of traits for heat tolerance in a chickpea recombinant inbred line (RIL) population under field conditions. Euphytica 214, 27 (2018). https://doi.org/10.1007/s10681-018-2112-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2112-8