Abstract
The cabbage root fly Delia radicum L. (Diptera: Anthomyiidae) is one of the major pests of many Brassica crops in the temperate areas of Europe and North America. At present, turnip (B. rapa ssp. rapa L.) varieties resistant to the pest does not exist. With the aim to fill this gap, a no-choice tolerance test of 56 accessions among turnips, turnip tops and turnip greens was performed under controlled conditions by introducing D. radicum eggs. Plant survival, leaf and root conditions, pupae number and weight significantly varied among plant accessions. Ten putatively resistant and ten susceptible accessions (control group) were selected from this first screening, transplanted in the field and exposed to natural infestation to detect antibiosis and antixenosis mechanisms. Both in the laboratory and in the field, pupae number significantly varied within accessions and between resistant and susceptible group, although pupal weight did not, indicating the absence of antibiosis effect on this early stage. In the field, the number of galleries was significantly lower in the resistant group in comparison with the control. Resistant accessions had smaller size, and a smaller, white and mostly buried root. Within the resistant and susceptible accessions, larger plants harboured more pupae, however purple roots were those most preferred, and the hosted pupae weighed most. Three accessions from the resistant group (MBGBR0178, MBGBR0570 and MBGBR0371) stand out for resistance to D. radicum possibly through antixenosis mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turnip (Brassica rapa L.) is one of the oldest cultivated vegetables that has been used for human consumption in temperate Europe since 2500–2000 BC. According to the studies based on morphology, geographic distribution, isozymes and molecular data, Europe should be one primary centre of origin for oil and turnip types, whereas East Asia should be another primary centre for Indian oil types and Chinese leafy vegetables (Gómez-Campo and Prakash 1999; Vogl-Lukasser et al. 2007). In the Iberian Peninsula, this plant constitutes, together with cabbage (B. oleracea L. capitata), kale (B. oleracea L. acephala) and leaf rape (B. napus L. var. pabularia), an important supply of vegetables during the winter. In the coldest regions of Portugal and NW Spain the edible parts of B. rapa subsp. rapa include turnip greens (young leaves harvested in the vegetative period), turnip tops (fructiferous stems with the flower buds and the surrounding leaves) and turnips (roots), used for culinary profit as well as for winter fresh fodder (Padilla et al. 2005). The leaves are characterized by a particular bitter and pungent taste, which has been related to the degradation product of some glucosinolates such as gluconapin, glucobrassicanapin (Padilla et al. 2007) and progoitrin (Francisco et al. 2009a). The genetics and the phylogenetic relationship among cultivated types of B. rapa subsp. rapa have been studied (Zhao et al. 2005; Takuno et al. 2007; Soengas et al. 2011). Also, the variation of phytochemical contents (Krumbein et al. 2005; Padilla et al. 2007; Francisco et al. 2009b, 2012; Schreiner et al. 2011; Cartea et al. 2012; Thiruvengadam and Chung 2015), the agronomic characteristics (Padilla et al. 2005; Francisco et al. 2011a), and the sensorial traits (Francisco et al. 2009a, 2010) are well known. In addition, the nutritional properties have been also investigated because turnips, turnip tops and turnip greens are a good source of health-promoting bioactive compounds such as glucosinolates, vitamin A, vitamin C, vitamin K, flavonoids, hydroxycinnamic acids, folate and calcium (Fernandes et al. 2007; Francisco et al. 2010, 2011a, b). Resistance to diseases such as black rot (Xanthomonas campestris pv. campestris (Pammel) Dowson) has been assessed among turnip accessions (Lema et al. 2015). However, the plant–insect interactions are still poorly known.
The cabbage root fly, Delia radicum L. (Diptera: Anthomyiidae), is one of the major pests of many Brassica crops in the temperate areas of Europe and North America, and represents the most dangerous phytophagous for turnip (B. rapa ssp. rapa) and swede (B. napus var. napobrassica (L.) Reichb.) crops. This species is monovoltine in northern Europe and multivoltine in central Europe and U.S.A. (Biron et al. 2002). After emergence in spring, flies feed during 4–6 days on protein and carbohydrate of flowering fruit trees before mating. Females lay white, oblong, 1 mm long, grouped eggs around the base of young plants or in the soil surface. The eggs hatch into white maggots after about 4–6 days at 15–20 °C. The larvae feed for about 3 weeks on the roots and stems of the cabbage plants. Finally, they form reddish-brown pupae which develop in the soil and hatch into adult flies after approximately 20 days. Two pauses in development may take place: the first in summer (quiescence) when the ground is above 22 °C; the second in winter (diapause), beginning in September–October (Dreves et al. 2006). Direct damage to the plant, as a result of feeding by D. radicum larvae on root tissue, and indirect damage, by facilitating the entry of secondary root pathogens, reduce both yield and quality of the vegetables and eventually induce plant death (Griffith 1986). The most common feeding symptoms of cabbage maggot are plant yellowing, stunting and slow growth.
Preventive management practices to decrease D. radicum populations include: crop covering with mesh netting or exclusion fences (Bomford et al. 2000), trap cropping (Rousse et al. 2003), “push–pull strategy” (Kergunteuil et al. 2015), the avoidance of sowing/planting during the main egg-laying periods, tillage regime and row spacing (Dosdall et al. 1998). The biological control of the cabbage root fly population is exerted by generalist feeding epigeal predators, which consume immature stages of D. radicum. Among them, ground beetles (Coleoptera: Carabidae) and rove beetles (Coleoptera: Staphylinidae) are particularly effective (Eyre et al. 2009; Hummel et al. 2012). Several parasitoids (Hemachandra et al. 2007), entomopathogenic fungi (Bruck et al. 2005) and nematodes (Georgis et al. 2006) attacking D. radicum larvae and pupae have been also described. However, these control measures are not effective enough and, finally, the control of D. radicum mainly depends on the application of insecticides, such as the organophosphates trichlorfon and diazinon, which rarely provide 100% control (Cartea et al. 2010a). Actually, the use of chemicals is limited because they are hazardous to the environment, and also because pupae developing below-ground may escape the application (Joseph and Zarate 2015). In contrast, host plant resistance to insect herbivores may be a useful approach for Brassica vegetables (Felkl et al. 2005; Cartea et al. 2010b). High levels of resistance to the cabbage root fly have been reported especially in wild Brassica species (e.g. B. incana, B. spinescens and B. fruticulosa) (Jensen et al. 2002; Felkl et al. 2005; Shuhang et al. 2016), whereas moderate resistance have been described within cultivated forms of B. oleracea (Ellis et al. 1999; Jyoti et al. 2001). Nevertheless, this level has not been considered high enough for a practical exploitation in breeding programmes. Previous studies on the resistance of B. rapa to D. radicum found that turnips were always the most susceptible plants in comparisons with other brassicaceous species such as B. napus L., B. juncea L., and Sinapis alba L. (Dosdall et al. 1994) and B. carinata L. and B. nigra L. (Dosdall et al. 2000). To our knowledge, no information is available on the existence of strongly resistant varieties of B. rapa subsp. rapa against the cabbage root fly.
Plant resistance is the heritable ability of plants to escape the herbivore enemies (Mitchell et al. 2016). It is generally categorized into three functional categories: antibiosis, antixenosis and tolerance (Smith 1989, 2005). Antixenosis (non-preference) describes the extent to which the plant will be able to escape herbivores due to its morphological and biochemical characteristics; antibiosis refers to the negative influence of the plant on the biology of the insect attempting to use that plant as host; and tolerance indicates the ability of the plant to grow and reproduce in spite of harbour a pest population. Tolerance, however, only involves plant characteristics and is not part of the insect-plant interaction.
Quantify antixenosis may be accomplished by counting how many eggs, larvae, pupae and adult the plant is harbouring, by measuring leaf eating and/or larvae food intake, and by scoring how much damage is suffered by the plant (Stenberg and Muola 2017). Antibiosis is usually expressed as fertility rate, development time and body weight. However, antixenosis and antibiosis are often correlated and difficult to disentangle. Insect herbivores choose to lay eggs on plants that are more palatable to their offspring, but the offspring survival and development may also depend on plant chemical defences, which is a matter of antibiosis (Stenberg and Muola 2017).
The Misión Biológica de Galicia (Spanish Council for Scientific Research) stores a collection of B. rapa subsp. rapa accessions as part of the Brassica genus germplasm bank. The objective of this study was to identify sources of resistance via tolerance, antibiosis or antixenosis mechanisms to D. radicum in 56 local varieties of turnips, turnip tops and turnip greens. To this end, no-choice tests, searching for tolerance and antibiosis/antixenosis evidence, were performed under controlled conditions. The best accessions selected from this first screening were transplanted in the field and exposed to natural infestation of D. radicum.
Materials and methods
Plant material
Fifty-one local varieties of Brassica rapa subsp. rapa from Galicia (NW Spain) and five from Asturias (N Spain) including 22 turnips (the root is the edible part), 31 turnip greens (the leaves harvested in the vegetative period) and 3 turnip tops (the fructiferous stems with the flower buds and the surrounding leaves) and kept at the Brassica germplasm bank at the Misión Biológica de Galicia (MBG-CSIC), were used in this study. Plants were initially grown in glasshouse at 20 °C, under natural photoperiod and 80% RH for 40 days, in 8 × 8 × 8 cm plastic pots filled with Sphagnum peat (GRAMOFLOR GmbH & Co., Drepholzer, Germany). Regular manual watering (without fertilization), was adopted.
Insects
A laboratory culture of D. radicum was established in 2010 from a population collected in turnip crops at the Misión Biológica de Galicia (Salcedo, Pontevedra province, NW Spain, 42°24′N, 8°38′W) and used to provide eggs for artificial infestation. The cabbage fly larvae were reared on small pieces of fresh turnips, whereas the adults were fed with a mixture of brewer’s yeast:honey:water (1:1:2). The insectaries (mesh cages 65 × 65 × 65 cm) were maintained at 19 ± 1 °C, 60 ± 5% of RH and a photoperiod of L16:D8. Males and females were kept together for mating. Female flies laid eggs around pieces of turnip placed in plastic Petri-dishes with damp filter paper on the bottom. Eggs were collected with a fine camel hair paint brush.
Laboratory experiment
From October 2010 to November 2011, 56 B. rapa local varieties were tested for resistance to D. radicum larvae attack by using a no-choice test. Ten plants per variety were randomly arranged in a climatic chamber at 19 ± 1 °C, 60 ± 5% of RH and a photoperiod of L16:D8. The plants were inoculated with five D. radicum eggs (2–3 day old), when they had reached the 5–6 true leaf stage. The eggs were gently laid close the plant stem with a fine paint brush. Upon hatching, larvae were allowed to feed on the taproots. The plants were scored daily for symptoms of attack, such as leaf wilting and plant collapse (death). For each plant, the number of days without collapse (tolerance) was recorded as well as the survival rate per accession after 20 and 30 days from the infestation. During the experiment, the plants were watered as they needed for their maintenance in turgid conditions. The experimental unit was the plant.
The health status of the plants was checked twice, at 20 and 30 days after the infestation. Leaf wilting was evaluated by using a subjective rating scale from 1 to 5 points (1 = all the leaves are green and turgid; 2 = one leaf is wilted; 3 = the 50% of the leaves are wilted; 4 = more than 50% of the leaves are wilted; 5 = 100% of the leaves are wilted).The roots were carefully examined to determine the existence of feeding injury by using a rating scale from 1 to 5 points (1 = all the roots are healthy; 2 = the surface of the main root shows some feeding marks; 3 = the main root is injured and the secondary roots are scarce; 4 = the main root is seriously injured and the secondary roots are missing; 5 = the main root is dead, completely withered). At the end of experiment, 30 days after the infestation, plants were removed from the pots and the pupae per plant were scored and weighed to the nearest 0.1 mg with an electronic high precision balance (COBOS PrecisionAX 120). The plants that received the highest point at the rating scale for both leaves and roots were considered dead. Each variety was considered putatively resistant (via tolerance evaluation) when they satisfied all the following criteria: (1) a mean of 28–30 days without collapse, (2) more than 80% of survival plants at 30 days after infestation; (3) the average rating scale for leaves and roots ranged between 1 and 2 at 20 and 30 days after infestation. Those varieties which exhibited tolerance below the standards required were considered putatively susceptible. The existence of significant differences among the varieties was statistically assessed. Successively, the most representative, non-ambiguous, ten putatively resistant accessions along with ten susceptible ones (control group) were selected to confirm their resistance under field conditions and to investigate the existence of antixenosis/antibiosis elements.
Field experiment
Plants were initially grown in multi-pot trays in a greenhouse at 20 °C for 40 days under the above mentioned conditions. On September 2012, then they were transplanted into the field (Salcedo, Pontevedra province, NW Spain, 42°24′N, 8°38′W), under natural conditions, at the 5–6 true leaf stage. Soil at the experimental plots is acid (pH 5.3), with very high content of organic matter (6.6%), and mostly sandy-loam textures. During the experiment, from September 2012 to June 2013, the mean temperature was 11.5 ± 1 °C, with 83.4% of mean RH and 190.26 ± 33.3 mm of rain. Environmental parameters were obtained from an official climate station of the regional Government (Consellerıa de Medio Ambiente, Xunta de Galicia). The varieties were evaluated in a randomized complete block design with two replications. Each experimental block consisted of twenty rows of 10 plants each (one variety per row, randomly assigned). Rows were spaced 0.8 m apart and plants within rows were spaced 0.5 m apart. No insecticide of fungicide was applied to the plants. On April 2013, root position in the soil was scored by using a rating scale from 1 to 5 points (1 = completely buried, 2 = mostly buried, 3 = half buried, 4 = largely above soil line, 5 = above soil line), root diameter was measured at widest point, root colour was evaluated (1 = white, 2 = purple), and plant stem diameter was measured at the base (IBPGR 1990). Measurements were done with a digital calliper. On June 2013, all the plants were collected and transported to the laboratory to assess the root damage as the number of galleries per root. It was assumed that each gallery referred to one larva, according to our previous observations during the D. radicum rearing. To assess the existence of antixenosis or antibiosis, all the larvae and pupae found in the roots and/or in the soil around the plant were counted and individually weighed.
Statistical analysis
The count data, such as the number of root galleries and pupae, were square root transformed prior the analysis of variance. Rating scales were transformed as log10(x + 1) prior to be subjected to the tests to stabilize the variance.
For the experiment developed in the climatic chamber, the existence of significant differences for the number of days without collapse (tolerance), pupae number (antixenosis) and pupal weight (antibiosis) among the 56 plant accessions (fixed factor) was obtained with a one-way ANOVA. The survival rate at 20 and 30 days after infestation was subjected to a Generalized Linear Model (GLM) with binomial distribution and logit link function, with main effect from the variety. Significant differences for leaves and roots damaged after 20 and 30 days among the varieties (fixed factor) by using a visual scale were evaluated with a Kruskal–Wallis one-way analysis of variance. When the putatively resistant (R) and susceptible (S) groups were constituted, the same statistical tests were repeated to assess the existence of significant differences within each group and between them.
For the field experiment, firstly we tested the existence of differences among the accessions within the R and S groups for root position, root colour, root diameter, plant diameter, number of galleries, pupae number and pupal weight (response variates) by using the ANOVA analysis. Successively, comparisons between R and S groups were done by using an unbalanced ANOVA. The means were separated by Fisher’s least significant difference (LSD). Blocks were considered as random factor. Finally, to investigate the relationships between the agronomical characters and the indicators of D. radicum performance, such as the number of galleries (antixenosis), pupae number (antixenosis) and pupal weight (antibiosis) found within the R and S groups, a multiple regression analysis was performed.
Significance was declared at P < 0.05. Statistical tests were performed using the software GenStat12.1 (VSN International Ltd., Hemel Hempstead, UK).
Results
Laboratory experiment
The fifty-six tested varieties were significantly different for the number of days without collapse, the survival rate at 20 and 30 days, leaf damage at 20 and 30 days, root damage at 20 and 30 days, the number of retrieved pupae and pupal weight (Supplementary Table S1). Based on these data and on the criteria to evaluate the tolerance under controlled conditions, the plants were categorized into putatively resistant (R) and susceptible group (S). Ten, non-ambiguous, putatively resistant accessions were selected for the field experiment. Data showed significant differences between the R and S groups for all the variables, with the exception of the pupal weight (Table 1). In addition, within the R group, the accessions performed similarly, without differences for tolerance or survival rate, whereas within the S group, there were significant differences for all the tested variables (Table 1).
Field experiment
The comparisons among the accessions within the R and S groups showed the existence of significant differences for all the studied variables (Table 2). In particular, within the R group, the accessions MBGBR0178, MBGBR0570 and MBGBR0371, stand out for the low number of galleries (Table 2; Fig. 1).
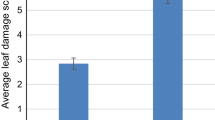
When the R and S groups were compared, it was found that the mean number of galleries per plant (2.12 ± 0.23) was significantly lower in the first group than in the second one (4.15 ± 0.37) (Table 2) and the mean number of pupae collected per plant was significantly higher in the S group (0.49 ± 0.10 and 1.38 ± 0.26, respectively) (Table 2; Fig. 2). The pupal weight did not vary between resistant (8.09 ± 0.67 mg) and susceptible plants (9.3 ± 0.34 mg) (Table 2; Fig. 2). Root diameter, root position, root colour and plant diameter were significantly different between R and S group. The putatively resistant plants had in general smaller size, smaller and mostly buried roots and the proportion of white roots was higher than in the susceptible group (94 and 64%, respectively) (Table 2; Fig. 2).
Mean values of root diameter at the widest point, plant diameter, root position in the soil, root colour, number of pupae and pupal weight of resistant (N = 20 plants/accession) and susceptible (N = 20 plants/accession) plants subjected to D. radicum attack in the field. Root position in the soil was described by using a 1–5 points rating scale (1 = completely buried, 2 = mostly buried, 3 = half buried, 4 = largely above soil line, 5 = above soil line). Root colour was described as 1 = white, 2 = purple. Error bars mean SD
The proportion of successfully developing larvae (i.e. the ratio between the pupae number found per plant and the number of galleries) should account for antibiosis to D. radicum, although these data should be taken with caution because in the field many other factors could lead to pupal mortality. However, for both R and S groups, the result was similar (31 and 31.6%, respectively). The existence of a relationship between pupae number and pupal weight could account for the presence of antibiosis mechanism through larval competition for the resources. By using a linear regression analysis, we found that a significant and negative relationship does exist only among the R accessions (F < 0.001, r = − 0.41).
The accessions of the R group, showed significant and positive correlations between plant diameter and pupae number (P = 0.001, r = 0.45), root colour and pupae number (P = 0.020, r = 0.49) and root colour and pupal weight (P = 0.007, r = 0.48), which indicates that purple roots had a larger number of pupae with higher body weight. Among the S accessions, a similar trend was found for the correlation between plant diameter and pupae number (P = 0.001, r = 0.52), root colour and pupae number (P = 0.020, r = 0.45) and root colour and pupal weight (P = 0.007, r = 0.49). In addition, plant diameter (P = 0.048, r = 0.54) and root position (P = 0.025, r = 0.49) did account for the higher number of galleries among the susceptible plants.
Discussion
Knowledge of mechanisms of host plant resistance to insect pests is a crucial first step in developing insect resistant cultivars. Indeed, the combination of more elements of resistance is highly desirable because it ensure a good control of a resident pest population. The existence of both antixenosis and antibiosis increase the probability of a long-lasting resistance to insect herbivores, in comparison with antibiosis or antixenosis alone (Jyoti et al. 2001). However, the relative importance of antibiosis and antixenosis may depend also on the type of herbivore (generalist vs. specialist) and the lifetime of the plant. Generalist herbivore are not always able to make the optimal plant choice. Also, if the plant live longer than the generation time of the herbivore, antibiosis gains more importance than antixenosis because the initial plant choice become less significant (Stenberg and Muola 2017). It is well known that environmental conditions can affect the outcome of evaluations aimed at identifying insect resistant varieties, and especially when natural infestation is used one runs the risk of susceptible plants escaping infestation. To prevent this, we firstly used a no-choice test to eliminate the obviously susceptible accessions. Plant performance observed in the field, especially among the putatively resistant accessions, mostly agreed with the results obtained under controlled conditions by D. radicum egg infestation, which shows that the adopted indicators of plant resistance, such as plant tolerance, survival rate, leaf wilting and collapse, and root damages were effective.
Antibiotic effect on insect herbivores could be due to single or multiple traits including low nutritional value of the host plant, the presence of biologically active inhibitory plant chemicals or morphological traits. Antibiosis typically increases mortality or, in alternative, surviving individuals may experience small body size, low weight, prolonged developmental period, and low female fecundity (Mithöfer and Boland 2012). We did not find evidence of antibiosis effect on the pupal weight under controlled conditions and in the field. Nonetheless, the lower pupae number found among the putatively resistant accessions subjected to the no-choice test may indicate certain level of antibiosis/antixenosis, for example through reduced feeding efficiency or plant palatability (Mitchell et al. 2016). In the field, where the plants were exposed to natural infestation, the scarce number of pupae retrieved from resistant accessions could indicate the existence of both antibiosis and antixenosis elements, possibly through increased larval mortality and low attractiveness to egg laying. The existence of a negative relationship between pupae number and pupal weight among the resistant accessions, suggests the existence of a mechanism of antibiosis, possibly through larval competition for the resources. Anyway, precaution should be adopted in the interpretation of this result from the field, because the immature stages of the cabbage root fly may suffer certain rate of mortality due to soil conditions, drought or flooding, and predator or parasitoid attack. Indeed, for the future, the use of other insect traits primarily related to antibiosis, such as egg-to adult development time and the proportion of the enclosed flies should provide more reliable results (Shuhang et al. 2016). Actually, the scarce evidence of resistance based on antibiosis mechanisms among our accessions was expected because of this kind of resistance to D. radicum among Brassica crops is very low in comparison to wild Brassica species (Ellis et al. 1999; Jensen et al. 2002; Felkl et al. 2005; Shuhang et al. 2016).
D. radicum larvae feeding usually damage the tissue of the root epidermis and phloem and xylem parenchyma, provoking plant wilting and collapse. We did not check for such symptoms among the plants in the field as a measure of tolerance, as we did in the climatic chamber, because under natural conditions it should be caused by several other biotic and abiotic factors (Shuhang et al. 2016). However, at the end of the field experiment the 9% of the putatively resistant plants died, in contrast to the 18% for the susceptible ones. Resistant varieties had in the field a significantly small number of root injuries by D. radicum than the susceptible ones. Dosdall et al. (1994) showed that root damage is generally correlated with oviposition rate, which imply that the mechanism of resistance by brassicaeous plants to D. radicum is mainly by antixenosis or non-preference mechanisms.
Antixenosis is characterized by the presence of morphological or chemical plant factors that significantly alter insect behaviour and the selection of the host plant. This may depend to morphological characters that create physical barriers to pest attachment, feeding and oviposition, such as glandular trichomes or leaf waxes, or to the presence of chemicals that repel insect from oviposition and larval feeding (Jyoti et al. 2001; Mitchell et al. 2016). The study of turnip agronomical characters revealed significant differences within and between R and S group. Because plants tested belong to the same plant species, we avoided differences due to different leaf and root morphology and/or plant phenology. The resistant accessions had a smaller, white, mostly buried root, and lower plant diameter in comparison to susceptible varieties, which implies that these agronomic traits may protect the plants to the cabbage root fly attack. Multiple regression analyses showed that larger plants and purple roots were mostly preferred for egg laying, because they harboured a significant higher number of pupae in both R and S groups. Host plant finding for oviposition by D. radicum female is mainly based on visual cues, such as leaf colour, plant shape and size, on the detection of host-finding cues such as isothiocyanates and on oviposition stimulant such as sinigrin (Nottingham 1988; Städler and Schöni 1990; Baur et al. 1996; Roessingh et al. 1997). Further investigation should be directed to assess whether biochemical differences between white and purple root does exist. Three turnip varieties from the putatively resistant group (MBGBR0178, MBGBR0570 and MBGBR0371), stand out for resistance to D. radicum through antixenosis mechanisms, because they had the lowest number of galleries per plant. Selection of the most resistant individuals within these accessions could provide the better candidates for a breeding programme against the cabbage root fly.
To conclude, the present study represents a first approach to find B. rapa subs. rapa local varieties that represent a potential source of desirable traits in order to develop a resistance breeding programme against the cabbage root fly. Several accessions tested in this work are promising genotypes and should be deeply investigated to unravel the genetics of the resistance and for identification of repellent or deterrent factors which interfere with oviposition stimulants. In addition, glucosinolate content and root sugar content, particularly sucrose, which is an important insect phagostimulants (Hopkins et al. 1999), should be also quantified in the selected varieties, unveiling whether biochemical contents of purple root may differ from the white roots.
References
Baur R, Birch ANE, Hopkins RJ, Griffiths DW, Simmonds MSJ, Städler E (1996) Oviposition and chemosensory stimulation of the root flies Delia radicum and D. floralis in response to plants and leaf surface extracts from resistant and susceptible Brassica genotypes. Entomol Exp Appl 78:61–75. https://doi.org/10.1111/j.1570-7458.1996.tb00765.x
Biron D, Coderre D, Boivin G, Brunel E, Nénon J (2002) Genetic variability and expression of phenological and morphological differences in populations of Delia radicum (Diptera: Anthomyiidae). Can Entomol 134:311–327. https://doi.org/10.4039/Ent134311-3
Bomford MK, Vernon RS, Päts P (2000) Importance of collection overhangs on the efficacy of exclusion fences for managing cabbage flies (Diptera: Anthomyiidae). Environ Entomol 29:795–799. https://doi.org/10.1603/0046-225X-29.4.795
Bruck DJ, Snelling JE, Dreves AJ, Jaronski ST (2005) Laboratory bioassays of entomopathogenic fungi for control of Delia radicum (L.) larvae. J Invertebr Pathol 89:179–183. https://doi.org/10.1016/j.jip.2005.02.007
Cartea ME, Velasco P, Vilar M, Francisco M, Lema M (2010a) Plagas y enfermedades de los cultivos de Brásicas. Deputación de Pontevedra Editorial, Pontevedra
Cartea ME, Francisco M, Lema M, Soengas P, Velasco P (2010b) Resistance of cabbage (Brassica oleracea capitata group) crops to Mamestra brassicae (L.). J Econ Entomol 103:1866–1874. https://doi.org/10.1603/EC09375
Cartea ME, de Haro A, Obregón S, Soengas P, Velasco P (2012) Glucosinolate variation in leaves of Brassica rapa crops. Plant Food Hum Nutr 67:283–288. https://doi.org/10.1007/s11130-012-0300-6
Dosdall LM, Herbut MJ, Cowle NT (1994) Susceptibilities of species and cultivars of canola and mustard to infestation by root maggots (Delia sp.) (Diptera: Anthomyiidae). Can Entomol 126:251–260. https://doi.org/10.4039/Ent126251-2
Dosdall LM, Florence LZ, Conway PM, Cowle NT (1998) Tillage regime, row spacing, and seeding date influence infestation of root maggots (Delia sp.) (Diptera: Anthomyiidae) in canola. Can J Plant Sci 78:671–681. https://doi.org/10.4141/P98-001
Dosdall LM, Good A, Keddie BA, Ekuere U, Stringam G (2000) Identification and evaluation of root maggot (Delia spp.) (Diptera: Anthomyiidae) resistance within Brassicaceae. Crop Prot 19:247–253. https://doi.org/10.1016/S0261-2194(00)00015-6
Dreves AJ, Dalthorp D, Stone AG, Fisher G (2006) Spring emergence and seasonal flight of Delia radicum L (Diptera: Anthomyiidae) in Western Oregon. Environ Entomol 35:465–477. https://doi.org/10.1603/0046-225X-35.2.465
Ellis PR, Pink DAC, Barber NE, Mead A (1999) Identification of high levels of resistance to cabbage root fly, Delia radicum, in wild Brassica species. Euphytica 110:207–214. https://doi.org/10.1023/A:1003752801143
Eyre MD, Labanowska-Bury D, Avayanos JG, White R, Leifert C (2009) Ground beetles (Coleoptera, Carabidae) in an intensively managed vegetable crop landscape in eastern England. Agric Ecosyst Environ 131:340–346. https://doi.org/doi.org/10.1016/j.agee.2009.02.006
Felkl G, Jensen EB, Kristiansen K, Andersen SB (2005) Tolerance and antibiosis resistance to cabage root fly in vegetable Brassica species. Entomol Exp Appl 116:65–71. https://doi.org/10.1007/s10681-016-1724-0
Fernandes F, Valentão P, Sousa C, Pereira JA, Seabra RM, Andrade PB (2007) Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem 105:1003–1010. https://doi.org/10.1016/j.foodchem.2007.04.063
Francisco M, Velasco P, Romero A, Cartea ME (2009a) Sensory quality of turnip greens and turnip tops grown in northwestern Spain. Eur Food Res Technol 230:281–290. https://doi.org/10.1007/s00217-009-1163-4
Francisco M, Moreno DA, Cartea ME, Velasco P (2009b) Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J Chromatogr A 1216:6611–6619. https://doi.org/10.1016/j.chroma.2009.07.055
Francisco M, Velasco P, Moreno DA, García-Viguera C, Cartea ME (2010) Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Res Int 43:1455–1463. https://doi.org/10.1016/j.foodres.2010.04.024
Francisco M, Velasco P, Lema M, Cartea ME (2011a) Genotypic and environmental effects on agronomic and nutritional value of Brassica rapa. Agron J 103:735–742. https://doi.org/10.2134/agronj2010.0439
Francisco M, Cartea ME, Soengas P, Velasco P (2011b) Effect of genotype and environmental conditions on health-promoting compounds in Brassica rapa. J Agric Food Chem 59:2421–2431. https://doi.org/10.1021/jf103492r
Francisco M, Cartea ME, Butrón A, Velasco P (2012) Environmental and genetic effects on yield and secondary metabolite production in Brassica rapa crops. J Agric Food Chem 60:5507–5514. https://doi.org/10.1021/jf301070q
Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS, Samish M, Tan L, Torri P, van Tol RWHM (2006) Successes and failures in the use of parasitic nematodes for pest control. Biol Control 38:103–123. https://doi.org/10.1016/j.biocontrol.2005.11.005
Gómez-Campo C, Prakash S (1999) Origin and domestication. In: Gómez-Campo C (ed) Biology of Brassica coenospecies. Elsevier, Amsterdam, pp 33–58
Griffith GCD (1986) Phenology and dispersion of Delia radicum (L.) (Diptera: Anthomyiidae) in canola fields at Morinville, Alberta. Quaest Entomol 22:29–50
Hemachandra KS, Holliday NJ, Mason PG, Soroka JJ, Kuhlmann U (2007) Comparative assessment of the parasitoid community of Delia radicum in the Canadian prairies and Europe: a search for classical biological control agents. Biol Control 43:85–94. https://doi.org/10.1016/j.biocontrol.2007.07.005
Hopkins RJ, Griffiths DW, McKinlay RG, Birch NE (1999) The relationship between cabbage root fly (Delia radicum) larval feeding and the freeze-dried matter and sugar content of Brassica roots. Entomol Exp Appl 92:109–117. https://doi.org/10.1046/j.1570-7458.1999.00530.x
Hummel JD, Dosdall LM, Clayton GW, Harker KN, O’Donovan JT (2012) Ground beetle (Coleoptera: Carabidae) diversity, activity density, and community structure in a diversified agroecosystem. Environ Entomol 41:72–80. https://doi.org/doi.org/10.1603/EN11072
IBPGR (1990) Descriptors for Brassica and Raphanus. International Board for Plant genetic Resources, Rome
Jensen EB, Felkl G, Kristiansen K, Andersen SB (2002) Resistance to the cabbage root fly, Delia radicum, within Brassica fruticulosa. Euphytica 124:379–386. https://doi.org/10.1023/A:1015755306547
Joseph SV, Zarate J (2015) Comparing efficacy of insecticides against cabbage maggot (Diptera: Anthomyiidae) in the laboratory. Crop Prot 77:148–156. https://doi.org/10.1016/j.cropro.2015.07.022
Jyoti JL, Shelton AM, Earle ED (2001) Identifying sources and mechanisms of resistance in crucifers for control of cabbage maggot (Diptera: Anthomyidae). J Econ Entomol 94:942–949. https://doi.org/10.1603/0022-0493-94.4.942
Kergunteuil A, Dugravot S, Danner HJ, van Dam NM, Cortesero AM (2015) Characterizing volatiles and attractiveness of five brassicaceous plants with potential for a “push-pull” strategy toward the cabbage root fly, Delia radicum. Chem Ecol 41:330. https://doi.org/10.1007/s10886-015-0575-9
Krumbein A, Schonhof I, Schreiner M (2005) Composition and contents of phytochemicals (glucosinolates, carotenoids and chlorophylls) and ascorbic acid in selected Brassica species (B. juncea, B. rapa subsp. nipposinica var. chinoleifera, B. rapa subsp. chinensis and B. rapa subsp. rapa). J Appl Bot Food Qual 79:168–174
Lema M, Cartea ME, Francisco M, Soengas P (2015) Screening for resistance to black rot in a Spanish collection of Brassica rapa. Plant Breed 134:551–556. https://doi.org/10.1111/pbr.12293
Mitchell C, Brennan R, Graham J, Karley AJ (2016) 9. Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Front Plant Sci 7:1132. https://doi.org/10.3389/fpls.2016.01132
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450. https://doi.org/10.1146/annurev-arplant-042110-103854
Nottingham SF (1988) Host-plant finding for oviposition by adult cabbage root fly, Delia radicum. J Insect Physiol 3:227–234. https://doi.org/10.1016/0022-1910(88)90053-4
Padilla G, Cartea ME, Rodríguez VM, Ordás A (2005) Genetic diversity in a germplasm of Brassica rapa subsp rapa L. from northwestern Spain. Euphytica 145:171–180. https://doi.org/10.1007/s10681-005-0895-x
Padilla G, Cartea ME, Velasco P, de Haro A, Ordás A (2007) Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 68:536–545. https://doi.org/10.1016/j.phytochem.2006.11.017
Roessingh P, Städler H, Baur R, Hurter J, Ramp T (1997) Tarsal chemoreceptors and oviposition behaviour of the cabbage root fly (Delia radicum) sensitive to fractions and new compounds of host-leaf surface extracts. Physiol Ent 80:140–148. https://doi.org/10.1111/j.1365-3032.1997.tb01151.x
Rousse P, Fournet S, Porteneuve C, Brunel E (2003) Trap cropping to control Delia radicum populations in crouciferous crops: first results and future applications. Entomol Exp Appl 109:133–138. https://doi.org/10.1046/j.1570-7458.2003.00098.x
Schreiner M, Krumbein A, Knorr D, Smetanska I (2011) Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J Agric Food Chem 59:1400–1405. https://doi.org/10.1021/jf103585s
Shuhang W, Voorrips RE, Steenhuis-Broers G, Vosman B, van Loon JJA (2016) Antibiosis resistance against larval cabbage root fly, Delia radicum, in wild Brassica-species. Euphytica 211:139–155. https://doi.org/10.1007/s10681-016-1724-0
Smith CM (1989) Plant resistance to insects. A fundamental approach. John, Chichester
Smith CM (2005) Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecth
Soengas P, Me Cartea, Francisco M, Velasco P (2011) Genetic structure and diversity of a collection of Brassica rapa subsp. rapa L. revealed by simple sequence repeat markers. J Agric Sci 149:1–8. https://doi.org/10.1017/S002185961100013X
Städler E, Schöni R (1990) Oviposition behavior of the cabbage root fly, Delia radicum, influenced by host plant extracts. J Insect Behav 3:195–209
Stenberg JA, Muola A (2017) How should plant resistance to herbivore be measured? Front Plant Sci 8:663
Takuno S, Kawahara T, Ohnishi O (2007) Phylogenetic relationships among cultivated types of Brassica rapa L. em. Metzg. as revealed by AFLP analysis. Genet Resour Crop Evol 54:279–285. https://doi.org/10.1007/s10722-005-4260-7
Thiruvengadam M, Chung I-M (2015) Selenium, putrescine, and cadmium influence health-promoting phytochemicals and molecular-level effects on turnip (Brassica rapa ssp. rapa). Food Chem 173:185–193. https://doi.org/10.1016/j.foodchem.2014.10.012
Vogl-Lukasser B, Vogl CR, Reiner H (2007) The Turnip (Brassica rapa L. subsp. rapa) in Eastern Tyrol (Lienz district; Austria). Ethnobot Res Appl 5:305–317. https://doi.org/10.17348/era.5.0.305-317
Zhao J, Wang X, Deng B, Lou P, Wu J, Sun R, Xu Z, Vromans J, Koornneef M, Bonnema G (2005) Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor Appl Genet 110:1301–1314. https://doi.org/10.1007/s00122-005-1967-y
Acknowledgements
Research was supported by the National Plan for Research and Development AGL-2012-35539, AGL2015-66256-C2-1-R and financed by the European Regional Development Funds (FEDER). Authors thank Fátima Nogueira Míguez, Rosaura Abilleira, and César González, for their valuable help during laboratory and field works. Serena Santolamazza-Carbone acknowledges a post-doctoral research contract (JAE-Doc) from the Consejo Superior de Investigaciones Científicas (CSIC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santolamazza-Carbone, S., Velasco, P. & Cartea, M.E. Resistance to the cabbage root fly, Delia radicum (Diptera, Anthomyiidae), of turnip varieties (Brassica rapa subsp. rapa). Euphytica 213, 274 (2017). https://doi.org/10.1007/s10681-017-2069-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-2069-z