Abstract
In order to explore the molecular mechanism of salt tolerance and heterosis in cotton, a methylation-sensitive amplification polymorphism method based on capillary electrophoresis was used to analyze DNA methylation level in a cotton hybrid CCRI 29 and its two parents. The major results and conclusions are as follows: Firstly, salt tolerance test showed that CCRI 29 had higher salt-tolerance level than its both parents. The global DNA methylation level in CCRI 29 under salt treatment significantly increased, whereas the two parents did not change significantly between salt treatment and control. All kinds of variation of DNA methylation happened in cotton under salt treatment, and hypermethylation happened at a significantly higher rate than that of hypomethylation in CCRI 29 but not in its two parents. The results suggested that the increase of global DNA methylation level in cotton genome and also different methylation types played an important role in tolerance to salt treatment in cotton. Secondly, both hypomethylation and hypermethylation happened to different genes and some genes maintained the same methylation level after salt stress, which meant that complex gene expression alterations occurred when responding to salt stress in cotton, indicating the complicated characteristics of roles that specific genes played in salt tolerance. Thirdly, although most cytosine methylation sites in hybrid CCRI 29 shared the same status as that of at least one of the parents, the site number of hypomethylation is significantly higher than that of hypermethylation in CCRI 29 compared to parents under both control and salt stress, indicating that demethylation could be the mechanism to explain heterosis in cotton hybrid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinity is a major abiotic stress in plant agriculture worldwide, which has led to research into salt tolerance with the aim of improving crop plants (Zhu 2001). High salt stress causes ion imbalance and hyperosmotic stress in plants and finally decease of plants. Besides natural causes such as salty raining waters near around the coasts, contamination from the parental rocks and oceanic salts, cultivation practices have also exacerbated growing concentration of salts in the rhizosphere (Mahajan and Tuteja 2005), and thus salinization of arable land is getting more widespread which decreased the yield from formerly productive soil throughout the world. The efforts to increase salt tolerance of crop plants bear remarkable importance to supply sustainable agriculture on marginal lands and could potentially improve crop yield overall. As the most important textile crop of the world and an important source of edible oil, cotton has a comparatively high level of salt tolerance with good economic returns, which makes it a pioneer crop suitable to be planted in saline-alkali soil if its salt tolerance can be further improved. It is reported that the soil salinity content below 0.2 % will benefit growth of cotton, whereas cotton growth will be hindered when soil salinity content increases up to 0.3 % (Jiang et al. 2006). Meanwhile, hybrid cotton has been widely used commercially and studied for many years in India and China (Khan 2002). However, the molecular mechanism of salt tolerance in cotton and also molecular mechanism of cotton heterosis have not been elucidated clearly yet.
DNA methylation, one of the most important epigenetic phenomena, plays a vital role in tuning gene expression during plant development as well as in response to environmental stimuli (Lukens and Zhan 2007), allowing relatively rapid adaptation to new conditions without changing the DNA sequence in plants (Tan 2010). DNA methylation in the regulation of plant gene expression in response to environmental stresses were widely studied, including cold (Shan et al. 2013), drought (Tan 2010; Wang et al. 2011a), salt (Dyachenko et al. 2006; Tan 2010; Wang et al. 2011b; Zhao et al. 2010; Mastan et al. 2012; Bilichak et al. 2012), and metals (Aina et al. 2004; Kimatu et al. 2011). In most cases, a global demethylation of genomic DNA occurs in response to abiotic stress. There might be a close correlation between demethylation and gene expression, although few studies provide powerful evidence. Contrastingly, sometimes hypermethylation occurs in response to stress, such as the response of tobacco to NaCl (Kovarik et al. 1997).
Heterosis is a well-known phenomenon showing superiority in hybrid progeny compared with their parents (Shull 1908). Two most important hypotheses explaining the genetic basis of heterosis include the dominance hypothesis (Bruce 1910; Jones 1917) and the over-dominance hypothesis (Shull 1908; East 1936), and later on it was found that epistasis also played an important role in the inheritance of heterosis (Yu et al. 1997). Although much research had been done in the past years, the mechanism of heterosis remains unconfirmed. Differential gene expression between cotton hybrids and their parents was proved to be closely connected with heterosis. Significant differences in gene expression were detected between the cotton hybrid and its parents during the growing stages, which might be responsible for the observed cotton heterosis (Xing et al. 2006; Zhao et al. 2009; Zhu et al. 2011). This implied that heterozygotes are not only capable of active genes expression but also inactive expression. Unfortunately, nothing is yet known about the regulatory mechanisms underlying these differential expression patterns. It is speculated that epigenetic control such as DNA methylation might be involved in the regulation (He et al. 2013). The association between DNA methylation and heterosis was also investigated in cotton. Zhao et al. (2008) found that the number of demethylation loci in highly heterotic hybrids was greater than that in lowly heterotic hybrids, and the level of DNA cytosine methylation in cotton at the seedling stage is higher than that of the flowering stage. Zhao et al. (2010) further pointed out that methylation, including hyper- and demethylation as well as the potential conversion among methylation types, had different changes in different growing stages of cotton. Thus, DNA methylation might play important roles in hybrid development.
Many kinds of techniques have been adopted to analyze DNA methylation, which generally can be assorted into two groups: detection of DNA methylation in whole genome and in specific loci. The method of methylation-sensitive amplified polymorphism (MSAP, Xiong et al. 1999; Xu et al. 2000) has been proved to be effective and reliable in detection of whole-genome DNA methylation. Here, capillary electrophoresis (CE), an electrophoresis performed in a capillary tube, was introduced into MSAP. CE is a very efficient separation technique available for the analysis of both large and small molecules, with the characteristics of high sensitivity, high resolution, high speed comparing to conventional electrophoresis, which will improve the resolution and efficiency of MSAP analysis. In the present investigation, DNA methylation of a cotton hybrid and its two parents under salt treatment and control was detected with a method of MSAP based on CE method, so as to elucidate the molecular mechanism of salt tolerance and heterosis in cotton from the viewpoint of epigenetics.
Materials and methods
Plant materials and treatment conditions
The cotton accessions were collected from Cotton Research Institute, Chinese Academy of Agricultural Sciences (CCRI). A cotton hybrid CCRI 29 and its two parents, the maternal parent P1 and the paternal parent RP4 were used in this experiment, and a salt-tolerant cotton accession Zhong9807 was used as a check in the test of salt tolerance.
For MSAP analysis, twenty seeds of CCRI 29 and its two parents were selected, and soaked in water for 24 h. The seeds were transferred to a layer of filter paper in a petri dish, and then covered with another wet filter paper, and pure water was sprayed on the filter paper once a day to accelerate germination. The germinated seeds were then planted in plastic pots containing washed sand under conditions of 14 h-light/10 h-dark with the air temperature of 30 °C-light/25 °C-dark in a plant growth chamber. At the stage of 4th true leaves, 10 healthy and uniformly growing plants were selected from each cotton accession and were divided into 2 groups. These groups individually were watered either with 100 mL of NaCl solution containing a NaCl content of 0.4 % by weight of the sand in the pot as the salinization treatment or with the 100 mL of pure water as the control. The 3rd and 4th leaves from the salt-treated and control plants were collected on the 10th day after the beginning of salinization treatment and stored at −80 °C for later use.
To test the salt tolerance level of CCRI 29 and its two parents, the cotton materials were planted in single row with 3 replicates, with a salt-tolerant cotton accession Zhong9807 as check. The 0.4 % NaCl identification method was used to test the relative seedling survival rate, which is referred to as the salt-tolerance index to measure the salt tolerance at seedling stage, where salt-tolerance index (%) = seedling survival rate of each material × 0.5/seedling survival rate of CK × 100 (Zhang et al. 2010). The tolerance levels were divided as follows: Highly resistant, salt-tolerance level ≥90.00 %; Resistant, salt-tolerance level 75.00–89.99 %; Tolerant, salt-tolerance level 50.00–74.99 %; Sensitive, salt-tolerance level 0.00–49.99 %.
DNA isolation and MSAP assay
DNA extraction was performed according to Paterson et al. (1993). RNA in the DNA was digested with the final concentration of 20 ug/mL RNase for 30 min at room temperature. MSAP detection was adapted from Xu et al. (2000) using CE instead of conventional electrophoresis. First, we used several primers to identify the robustness of three biological replicates for each sample; the results indicated identical MSAP bands among three replicates. These replicates were then mixed to provide a pool sample for the following MSAP analysis. The sequence information for the adapters and primers of pre-amplification and selective amplification are provided in Supplemental Table 1.
Two sets of digestion reactions were performed simultaneously. Approximately 0.25 μg of cotton genomic DNA were digested with 10 units each of EcoRI and HpaII (Promega, USA) in a final volume of 25 μL of the appropriate buffer for 8 h at 37 °C, and then the enzymes were deactivated at 70 °C for 15 min. In the 2nd reaction, the same amount of cotton genome DNA was digested with EcoRI and MspI under the same reaction conditions. The digested fragments were ligated to the adapters in the ligation reaction mixture containing ligase buffer and T4 DNA ligase (Promega, USA), and incubated at 18 °C overnight (about 13 h). The ligation mixture was diluted at 1:5 (v/v) with Tris–EDTA (TE), and used as the template for the pre-selective amplification with EcoRI + A and HpaII/MspI + T primers.
The mechanism in which polymerase chain reactions (PCRs) were done for MSAP was addressed by Xu et al. (2000) and Xiong et al. (1999). Here PCRs were performed as follows: pre-denaturation at 94 °C for 5 min; 20 cycles of 30 s denaturation at 94 °C, 1 min annealing at 56 °C, and 1 min extension at 72 °C; 7 min extension at 72 °C; and finally a 10 °C hold. The pre-amplification products were diluted 20-fold (v/v) with TE buffer and used as the template for the selective amplification reaction. In this step, EcoRI and HpaII/MspI primers with two additional selective nucleotides were used. The selective PCR was performed in a final volume of 10 μL according to the protocol of Xu et al. (2000). The PCR reaction was performed for 36 cycles with the following cycle profile (Vos et al. 1995): pre-denaturation at 94 °C for 5 min; 13 touchdown cycles (30 s denaturation at 94 °C, 30 s annealing at 65 °C, which was subsequently reduced each cycle by 0.7 °C for the next 12 cycles, and 1 min extension at 72 °C); 23 cycles of 30 s denaturation at 94 °C, 30 s annealing at 56 °C, and 1 min extension at 72 °C; 7 min extension at 72 °C; and finally a 10 °C hold. The products of PCR were separated with CE on GenomeLab CEQ/GeXP genetic analysis system according to the manual (BECKMAN, USA). The scored MSAP bands were transformed into a binary character matrix, using “0” and “1” to indicate the absence and presence, respectively, of particular sites.
HpaII and MspI both recognize the tetranucleotide sequence 5′-CCGG. However, HpaII is inactive if one or both cytosines are fully methylated (both strands methylated) but cleaves the hemimethylated sequence (only one DNA strand methylated), whereas MspI cleaves C5mCGG but not 5mCCGG (McClelland et al. 1994). For each single DNA sample, the MSAP patterns resulted from the digestions with the isoschizomers were divided into the following four types: Type I bands, present only for EcoRI/MspI (0,1), which represent the case of full methylation of internal cytosine of 5′-CCGG on the two strands; Type II bands, present only for EcoRI/HpaII (1,0), which represent the hemimethylated state of 5′-CCGG sites due to methylation in one DNA strand (external cytosine or both external and internal cytosines of 5′-CCGG) but not in its complementary strand; Type III bands, absent from both enzyme combinations (0,0), which represent full methylation of the two strands where at least one external cytosine of 5′-CCGG was methylated; Type IV bands, present for both enzyme combinations (1,1), which represent the case of no methylation, or the hemimethylated state of 5′-CCGG sites due to methylation of internal cytosine of 5′-CCGG on one DNA strand but not on its complementary strand, and Type IV bands were usually treated as no methylation in analysis although it will underestimate actual methylation level. Type I, II, IV can be identified according to any single DNA sample; however, Type III (0, 0) can only be detected when comparing to other samples. The percentage of polymorphic MSAP bands can be calculated using the following formula:
Cloning and BLAST search of differentially methylated fragments
The surplus samples used for CE were saved and then they were separated on 10 % conventional polyacrylamide gels so as to clone the interesting bands that were excised from polyacrylamide gels. DNA recovery, vector ligation, and transformation of DH5a Escherichia coli competent cells were conducted as described by Guo et al. (2003). At least three positive clones from each amplified fragment were simultaneously sequenced by Shanghai Generay Biotech Co., Ltd, China. Homology search was performed on the National Center for Biotechnology Information BLAST server (USA) using both the nucleotide sequence and its deduced amino acid sequence for each cloned sequence.
RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted with the EASY spin RNA plant kit (Aidlab, Beijing, China) according to the manufacturer’s instructions. RNA was extracted from 100 mg of leaf tissue sampled at the same time-points that DNA methylation was analyzed and stored at −80 °C. RNAs were then quantitated by UV spectrophotometry and cDNA was constructed using RNA samples and the M-MLV reverse transcriptase kit (Invitrogen). The RT-qPCR procedure was based on the iCycler iQTM real-time PCR detection system (Bio-Rad), following the manufacturer’s instructions. Primers were designed using the Primer 3 software (http://www.simgene.com/Primer3) and then synthesized and purchased from Takara Biotechnology (Dalian) Limited. The sequences of primers are described in Supplemental Table 2. For normalization, the G. hirsutum glyceraldehyde-3-phosphate dehydrogenase (GhGAPDH, GenBank accession numbers: FJ415206) was used as an endogenous control. All PCR reactions were performed in triplicate, and the specificity of the reaction was detected by melting-curve analysis at the dissociation stage. Comparative quantification of each target gene was performed based on cycle threshold (CT) normalized to GhGAPDH using the ΔΔCT method.
Results
Salt tolerance level of CCRI 29 and its two parents
The salt-tolerance level of CCRI29 and its two parents was shown in Table 1. The results showed that the paternal parent RP4 was sensitive to salt stress, with the salt-tolerance index of 42.39 %; the maternal parent P1 was salt tolerant, with the salt-tolerance index of 54.50 %; whereas the hybrid CCRI 29 had higher salt tolerance level, with the salt-tolerance index of 60.84 %, and the mid-parent heterosis of salt-tolerance index reached 25.59 % (Table 1).
DNA methylation level of CCRI 29 and its two parents
The detailed DNA methylation level of CCRI 29 and its two parents were listed in Table 2. Totally 32 primer combinations composed of 4 EcoRI selective primers and 8 HpaII/MspI selective primers were adopted to detect DNA methylation levels of CCRI 29 and its two parents under salt treatment and control. MSAP based on CE detected 2597 CCGG sites in CCRI 29 under salt treatment and the control (Fig. 1; Table 2), of which 2090 (80.5 %) and 1871 (72 %) sites were methylated respectively. The average sites detected per primer combination were 65.3 and 58.5 respectively, and the methylated site number under salt treatment is significantly higher than that of control (P < 0.05). For the type III methylation, 985 and 491 methylated sites were detected under salt treatment and control respectively; the methylation level under salt treatment is significantly higher than that of control (P < 0.05). For the type I methylation, 441 and 771 methylated sites were detected under salt treatment and control respectively; the methylation level under salt treatment is significantly lower than that of control (P < 0.05). MSAP based on CE detected 2848 CCGG sites in P1, the maternal parent of CCRI 29 under salt treatment and control (Table 2), of which 2094 (73.5 %) and 2193 (77 %) sites were methylated respectively. The average sites detected per primer combination were 65.4 and 68.5 respectively, and no significant difference existed between salt treatment and control. Totally 2572 CCGG sites were detected in RP4, the paternal parent of CCRI 29 under salt treatment and control (Table 2), of which 1914 (74.4 %) and 1968 (76.5 %) sites were methylated respectively. The average sites detected per primer combination were 59.8 and 61.5 respectively, and no significant difference existed between salt treatment and control.
DNA methylation patterns of CCRI 29 detected in CE with the primer combination of E1H6. a Digestion of control DNA with EcoR I + Hpa II. b Digestion of control DNA with EcoR I + Msp I. c Digestion of DNA under salt treatment with EcoR I + Hpa II. d Digestion of DNA under salt treatment with EcoR I + Msp I. Note The ordinate means the relative fluorescence intensity; the abscissa means the base number of PCR product and the internal lane standard; the red peaks refer to the internal lane standard; the blue peaks refer to PCR products
Variation of DNA methylation status of CCRI 29 and its two parents under salt stress
The methylation level compared between salt treatment and control for each cotton accession can be divided into four patterns including A, B, C and D, and these 4 patterns can be divided into 15 sub-patterns (Table 3). Pattern A indicated that the methylation status was the same between salt treatment and control without changes. Pattern B indicated that DNA methylation level decreased under salt treatment, or hypomethylation happened under stress compared to control. Pattern C indicated that DNA methylation level increased under salt treatment, or hypermethylation happened. Pattern D indicated that DNA methylation status changed between salt treatment and control, whereas we cannot tell the difference of methylation level.
The detailed composition of different patterns and their percentages are listed in Table 3. Especially, Pattern B is composed of 5 sub-patterns, and 24.5, 31.3 and 38.9 % of total sites belonged to this pattern in CCRI 29, P1 and RP4 respectively. Pattern C is also composed of 5 sub-patterns, and 49.6, 45.9 and 37.6 % of total sites belonged to this pattern in CCRI 29, P1 and RP4 respectively. In addition, the results showed that in CCRI 29, the site number of Pattern C or hypermethylation happened under salt treatment compared to control is significantly higher (P < 0.05) than that of Pattern B or hypomethylation. Meanwhile, in its two parents, the site number of Pattern C and Pattern B had no significant differences.
The difference of DNA methylation between CCRI 29 and its two parents
The DNA methylation status of CCRI 29 can be inherited from patent(s), or it will be different from the parents due to the hybridization. Here the DNA methylation pattern can be divided into 5 models regarding the inheritance of CCRI 29 from its two parents (Fig. 2; Supplemental Table 3). Model A indicated that CCRI 29 had the same DNA methylation status with its two parents. Model B indicated that CCRI 29 had the same DNA methylation status with one of the parents, whereas the two parents had different DNA methylation status. In these two models, the sites in parents followed a Mendelian inheritance, meaning the sites in CCRI 29 can be detected in one or both parents. Model C indicated that the sites detected by single EcoRI + HpaII or EcoRI + MspI enzyme combination in parents can be detected by both enzyme combinations in CCRI 29, which meant that the DNA methylation level decreased in CCRI 29, or hypomethylation happened. Model D indicated that the sites detected in parents cannot be detected in CCRI 29, which meant that the DNA methylation level increased in CCRI 29 (hypermethylation). Pattern E indicated that the DNA methylation status is different from two parents.
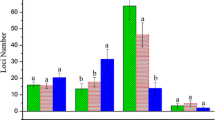
Variation of methylation banding model of CCRI29 (F1) and its two parents. a Same methylation level between F1 and two parents. b Same methylation level between F1 and one parent. c Methylation level decreased in F1 (hypomethylation). d Methylation level increased in F1 (hypermethylation). e F1 different methylation status with two parents
Model A is composed of 4 sub-models. The results showed that 2286 (44.6 %) and 2815 (55.0 %) sites had the same DNA methylation status between CCRI 29 and its two parents under control and salt treatment respectively. Model B is composed of 24 sub-models. The results showed that 1822 (35.6 %) and 1636 (31.9 %) sites had the same DNA methylation status between CCRI 29 and one of its two parents under control and salt treatment respectively. Model C is composed of 11 sub-models, where 524(10.2 %) and 335(6.5 %) sites had decreased level of DNA methylation level in CCRI 29 compared to parents under control and salt treatment respectively, where hypomethylation happened. Model D is also composed of 11 sub-models, where CCRI 29 had increased level of DNA methylation level compared to parents, and 197(3.8 %) and 163(3.2 %) sites followed this model under control and salt treatment respectively. Model E is composed of 14 sub-models, and 294(5.7 %) and 174(3.4 %) sites followed this model under control and salt treatment respectively.
As shown in Table S3 and Fig. 2, in most cases CCRI 29 had the same DNA methylation status as one or both parents both under control and salt treatment. It is noted that the site number of hypomethylation is significantly higher than that of hypermethylation (Fig. 2; P < 0.01 under control and P < 0.05 under salt treatment).
Differentially methylated DNA sequences and gene homology
Cloning and BLAST search analysis of methylated DNA sequences found 12 sequences that have high similarity to known genes/ESTs or non-coding regions (Table 4). Seven sequences had the same DNA methylation status between salt treatment and control, which are JP7, JP13, JP14, JP16, JP20, JP31 and JP37, with the DNA methylation pattern of A3, A3, A3, A3, A1, A3 and A2 as defined in Table 3 respectively. The sequences were homologous to genes related to Cc-nbs-lrr resistance, ATP synthase, peroxidase, glycosyltransferase and cytochrome respectively. Two sequences, namely JP25 and JP32 experienced hypomethylation under salt treatment, with the methylation pattern of B5 as shown in Table 4. JP25 is related to a cotton alcohol dehydrogenase gene, whereas JP32 is related to a ribosomal protein gene. Three sequences, namely JP6, JP19, and JP35 experienced hypermethylation under salt treatment, with the methylation pattern of C3, C3, and C5 respectively. JP19 is related to Glycosyltransferase gene, whereas JP35 is related to an ATP synthase gene.
Gene expression analysis of selected MSAP fragments
Two MSAP fragments that were selected on the basis of DNA methylation status in salt treatment versuss the control were analyzed for their transcript levels by means of RT-qPCR. Transcript abundance was assessed in all treated samples and normalized against controls. The sequence JP6 homologous to a Gossypium hirsutum clone NBRI_GE8639 microsatellite sequence (Accession No.: JX576916.1) experienced hypermethylation under salt treatment in the maternal parent of CCRI 29 (Table 4) and was down-regulated under salt treatment in P1 (Fig. 3). However, it was strongly up-regulated under salt treatment in CCRI 29, and it was also up-regulated under salt treatment in the RP4 (Fig. 3). The sequence JP31 related to Glycosyltransferase (Accession No.: XM_007045897.1) maintained the same methylation level between salt treatment and control in CCRI 29 (Table 4); its expression level did not vary significantly after salt treatment in CCRI 29. However, it was significantly up-regulated under salt treatment in P1 and RP4 (Fig. 3).
Discussion
DNA methylation and salt stress
DNA methylation is a modification that occurs at the fifth carbon position of a cytosine ring. In both plants and animals, cytosine is primarily methylated in the CG dinucleotide context. However, DNA methylation in plants has been found in all three cytosine contexts, methylation at CG sites, CHG sites (H representing A, C or T) and CHH sites (Chen et al. 2010). Methylation in the 5′ portion of the gene and the 3′ portion may inhibit gene expression (Grativol et al. 2012). The least expressed genes are most likely to be methylated, and the most expressed genes are least likely to be methylated (Zemach et al. 2010).
In many cases, a global demethylation of genomic DNA occurs in response to abiotic stress, indicating that there might be a close correlation between demethylation and gene expression. Contrastingly, sometimes hypermethylation occurs in response to stress. It was reported that wild-type tobacco plants exposed to aluminum, salt, paraquat and cold stresses showed a selective decrease of CG methylation in the coding region of the glycerophosphodiesterase-like protein gene (Choi and Sano 2007); whereas tobacco cell cultures exposed to osmotic stress show CHG hypermethylation without changes in CG methylation in two heterochromatic sites (Kovarik et al. 1997). Chromium stress can promote methylation in radish (Yang et al. 2007) and oilseed rape (Labra et al. 2004) but induces a decrease in methylation in clover and hemp (Aina et al. 2004). These results imply that the DNA methylation pattern and alteration are related to the abiotic stress response in plants but that the association is obviously different between plant species, and there seems to be a relationship between DNA-methylation types and stress responses.
Under salt stress conditions, the level of CHG methylation of the halophyte Mesembryanthemum crystallinum increases in a satellite DNA on switching-over of C3-photosynthesis to the crassulacean acid metabolism pathway of carbon dioxide assimilation (Dyachenko et al. 2006). Wang et al. (2011b) found that demethylation of genes was an active epigenetic response to salt stress in roots at the rice seedling stage. In cotton research, Zhao et al. (2010) suggested that the demethylation positively contributed to salt tolerance and the hypermethylation had negative effect on salt tolerance; Li et al. (2009) found that the level of global DNA methylation in cotton roots decreased after salt stress, and there was a significantly negative correlation between salt concentration and DNA methylation level. In our current research, we found that the methylation level significantly increased under salt treatment comparing to control in salt-tolerant cotton hybrid CCRI 29 (Table 2). Meanwhile, 49.6 % of sites experienced hypermethylation under salt treatment, which is significantly higher than that of sites experienced hypomethylation (24.5 %) in CCRI 29 (Table 3). However, the DNA methylation level of the two parents, which had lower salt tolerance levels than CCRI 29, did not changed significantly under salt treatment comparing to control, and the number of sites experienced hypermethylation or hypomethylation had no significant difference between salt treatment and control (Table 3). The salt-tolerance level test showed that CCRI 29 had significantly higher salt tolerance than its two parents with mid-parent heterosis of 25.59 % (Table 1). The significant increase of global DNA methylation level under salt treatment in salt-tolerant hybrid CCRI 29 but not in parents with lower salt tolerance suggest that cotton accessions with high level of salt tolerance might have the potential mechanism of increasing methylation level when responding to salt treatment. It is also noted that different methylation type had different changes between salt treatment and control in CCRI 29, i.e., Type I methylation significant decreased under salt treatment, whereas Type III methylations significantly increased under salt treatment (Table 2). The increase of overall level of DNA methylation in cotton genome and also variation of different methylation types might play an important role in tolerance to salt treatment in cotton.
Variation of gene expression related to salt stress in cotton
Cloning and BLAST search analysis of methylated DNA sequences found 12 sequences that have high similarity to known genes/ESTs (Table 4), including important genes such as ATP synthase gene, alcohol dehydrogenase gene, and mitochondrion gene, and also genes controlling peroxidase and glycosyltransferase. In addition, some sequences were homologous to sequences with unknown function including non-coding regions (Table 4), which might play roles in regulating gene expressions. Bonasio et al. (2010) pointed out that noncoding regions of the genome are heavily transcribed, giving rise to a constellation of noncoding RNAs that often have regulatory functions; consequently, although their functions are unknown currently, the non-coding regions detected here are of great importance to be further investigated on their role in controlling genes related to salt tolerance. The sequence JP6 homologous to a Gossypium hirsutum clone NBRI_GE8639 microsatellite sequence (Accession No.: JX576916.1) experienced hypermethylation under salt treatment in P1 (Table 4) and RT-qPCR results did show that it was down-regulated under salt treatment in P1 (Fig. 3). However, it was strongly up-regulated under salt treatment in CCRI 29, and it was also up-regulated under salt treatment in the RP4 (Fig. 3). Its function needs to be further investigated. Previous research found that ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in Tobacco (Sun et al. 2013). Here we found that the sequence JP31 related to Theobroma cacao Glycosyltransferase family 61 protein (Accession No.: XM_007045897.1) maintained the same methylation level between salt treatment and control in CCRI 29 (Table 4), and its expression level detected in RT-qPCR did not significantly varied after salt treatment in CCRI 29; however, it was significantly up-regulated under salt treatment in both P1 and RP4 (Fig. 3). The results indicated that stable expression and over-expression of this glycosyltransferase gene might contribute to salt tolerance in cotton.
Morgan et al. (2014) proposed that the high plasma membrane H+-ATPase activity will increase the rate of proton pumping, ultimately acidifying the apoplastic pH, alkalizing the cytoplasmic pH and reducing the cytosolic free Na+ concentration in addition to increasing the resting cytosolic free Ca2+ concentration and increasing the cytosolic free K+ concentration. All these alterations introduced when calcium is supplied under saline conditions can improve the re-distribution of the ions between the cytosol and the apoplast and derive ion homeostasis and reduce the damage of salt stress. In our current research, the ATP synthase gene (JP13) maintained the same methylation level after salt treatment in CCRI 29 (Table 4), so their expression should not be inhibited by DNA methylation, which might partly explain that CCRI 29 are salt tolerant and can maintain normal growth after salt stress in a certain period. It is noted that hypermethylation happened in the ATP synthase gene (JP35) in RP4, the salt-sensitive paternal parent of CCRI 29, and its expression would be inhibited, suggesting that down-regulated expression of the ATP synthase gene will lead to less salt tolerance, which further confirmed the above mentioned points.
We found that methylation level decreased under salt treatment (hypomethylation) for an alcohol dehydrogenase gene (JP25), indicating that salt stress had the tendency of increasing expression level of the ethanol alcohol dehydrogenase gene in cotton. It is known that alcohol dehydrogenases (ADH) catalyze the interconversion between alcohols and aldehydes with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH, which is usually thought to be related to improve stress tolerance. In plants, expression of ADH is known to increase under various stresses, including oxygen-deficient conditions, waterlogging, low temperature, drought, abscisic acid, and salinity (Yamauchi et al. 2014; Davik et al. 2013; Sairam et al. 2009; Lindlöf et al. 2007). In particular, ADH genes are among the most commonly found cold-induced genes in cereal crops and Arabidopsis (Lindlöf et al. 2007).
DNA methylation and cotton heterosis
Heterosis describes a phenomenon in which offspring of diverse varieties of a species or crosses between different species exhibit greater biomass, speed of development, and fertility than both parents (James et al. 2010), which plays an important role in increasing productivity of crops. Although heterosis has been utilized widely to improve yield potential in crops, the underlying biological mechanisms remain poorly understood. As proposed by Groszmann et al. (2011), significant heterosis can be obtained even when the parents are genetically very similar, indicating that parents with highly similar genome sequences may have epigenomes which are sufficiently different from each other to be of significance in the generation of the heterotic phenotypes. Given the widespread and important effects of DNA methylation on gene expression, some researchers have tried to relate heterosis to the alterations of DNA methylation in hybrids relative to their parental lines (Xiong et al. 2013; Shen et al. 2012; Zhao et al. 2007; Zhang et al. 2007). DNA methylation will generally be inherited from parents to offsprings, although the DNA methylation pattern may be changed accompanying to hybridization (Liu and Wendel 2003). Tsaftaris and Kafka (1997) found that DNA methylation level in maize hybrid was lower than that of parents, and they further proposed that decrease of DNA methylation might be related to up-regulated gene expression, which was closely related to heterosis.
Here in our research, the DNA methylation pattern can be divided into 5 models regarding the inheritance of hybrid CCRI 29 from its two parents (Fig. 2, Table S3). The five models of cytosine methylation of hybrid identified from the present study could be interpreted as the inherited and altered patterns. Model A and B indicated that hybrid CCRI 29 had the same DNA methylation status with at least one parent, or inherited model. Most cytosine methylation sites in hybrid CCRI 29 shared the same status as that of at least one parent (Fig. 2). This observation was in accordance with many other reports (Xiong et al. 2013; Zhao et al. 2007; Zhang et al. 2007), indicating that the methylation status at most cytosine methylation sites displays stable inheritance from inbred parents to hybrids. This model of DNA methylation is considered as highly conserved among parents and hybrid and just passed onto the progeny without any change. These conserved genomic regions may be essential for proper plant growth and development and to avoid development-associated irregularities as well as abnormal phenotypes (Sakthivel et al. 2010).
Model C, D and E indicated that hybrid CCRI 29 had different DNA methylation status from its two parents, or altered patterns. Here the banding patterns were not inherited in a Mendelian fashion and were novel in hybrid. This indicates that certain sites in the genome get reprogrammed for methylation when they are transmitted to the progeny. It was suggested that the sites which undergo methylation alteration are more likely consequential to hybrid-specific gene expression leading to novel phenotypes (Sakthivel et al. 2010; Zhang et al. 2007). Moreover, the transgenerational inheritance and remodeling of parental methylation patterns upon sexual hybridization may have bearings on genome evolution and on the enhanced overall performance of hybrid.
It is noted that the sites of hypomethylation is significantly higher than that of hypermethylation under both control and salt treatment (Fig. 2; Table S3). The results are consistent with Tsaftaris and Kafka (1997) and Zhao et al. (2008), which may be due to the fact that higher level of demethylation events might be required for enhanced growth rate during the vegetative stage to realize early seedling vigor in hybrids (Joel and Zhang 2001; Sakthivel et al. 2010). The results indicated that demethylation could be one plausible mechanism to explain novel, hybrid-specific gene expression patterns, as suggested by Sakthivel et al. (2010). However, our observation relied upon a single technique to detect DNA methylation status, and this idea is proposed based on the assumption that DNA methylation suppresses gene expression as regulatory factors. In order to gain a better understanding of the molecular mechanisms of heterosis in plants as related to epigenetic regulation, more direct and stronger evidence is needed. This may be achieved by generating hybrids with loss of functions of various genes involved in epigenetic regulation and assessing the potential contribution of each gene to the growth vigor in hybrids. Also, comparative epigenomic analyses in different tissues at different developmental stages from various hybrid crosses should be conducted to elucidate the conservation and divergence of epigenetic variations in hybrids. As suggested by He et al. (2013), a complete understanding of the molecular mechanisms of heterosis in plants depends on the comprehensive analysis of the genetic and epigenetic regulation of gene expression variation in hybrids.
References
Aina R, Sgorbati S, Santagostino A, Labra A, Ghiani A, Citterio S (2004) Specific hypomethylation of DNA is induced by heavy metals in white clover and industrial hemp. Physiol Plant 121:472–480
Bilichak A, Ilnystkyy Y, Hollunder J, Kovalchuk I (2012) The progeny of Arabidopsis thaliana plants exposed to salt exhibit changes in DNA methylation, histone modifications and gene expression. PLoS One 7(1):e30515
Bonasio B, Tu S, Reinberg D (2010) Molecular signals of epigenetic states. Science 330:612–616
Bruce AB (1910) The Mendelian theory of heredity and the augmentation of vigor. Science 32:627–662
Chen M, Lv S, Meng Y (2010) Epigenetic performers in plants. Dev Growth Differ 52:555–566
Choi CS, Sano H (2007) Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol Genet Genomics 277:589–600
Davik J, Koehler G, From B, Torp T, Rohloff J, Eidem P, Wilson RC, Sønsteby A, Randall SK, Alsheikh M (2013) Dehydrin, alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.). Planta 237:265–277
Dyachenko OV, Zakharchenko NS, Shevchuk TV, Bohnert HJ, Cushman JC, Buryanov YI (2006) Effect of hypermethylation of CCWGG sequences in DNA of Mesembryanthemum crystallinum plants on their adaptation to salt stress. Biochemistry (Moscow) 71(4):461–465
East EM (1936) Heterosis. Genetics 21:375–397
Grativol C, Hemerly AS, Ferreira PCG (2012) Genetic and epigenetic regulation of stress responses in natural plant populations. BBA-Gene Regul Mech 1819:176–185
Groszmann M, Greaves IK, Albert N, Fujimoto R, Helliwell CA, Dennis ES, Peacock WJ (2011) Epigenetics in plants-vernalisation and hybrid vigour. BBA-Gene Regul Mech 1809:427–437
Guo WZ, Zhang TZ, Sheng XL, John Y, Kohel RJ (2003) Development of SCAR marker linked to a major QTL for high fiber strength and its molecular marker assisted selection in Upland cotton. Crop Sci 6:2252–2256
He GM, He H, Deng XW (2013) Epigenetic variations in plant hybrids and their potential roles in heterosis. J Genet Genomics 40:205–210
James AB, Hong Y, Sivanandan C, Daniel V, Reiner A (2010) Heterosis. Plant Cell 22:2105–2112
Jiang YR, Lv YJ, Zhu SJ (2006) Advance in studies of the mechanism of salt tolerance and controlling of salt damage in Upland cotton. Cotton Sci 18(4):248–254
Joel AJ, Zhang Q (2001) Direction of methylation and its effect on heterosis in rice. In: Book of Abstracts of 8th National Rice Biotechnology Network Meeting, Aurangabad, pp 231–237
Jones DF (1917) Dominance of linked factors as a means of accounting for heterosis. Genetics 2:466–479
Khan UQ (2002) Study of heterosis in fiber quality traits of Upland cotton. Asian J Plant Sci 1:593–595
Kimatu JN, Diarso M, Song CD, Agboola RS, Pang JS, Qi X, Liu B (2011) DNA cytosine methylation alterations associated with aluminium toxicity and low pH in Sorghum bicolor. Afr J Agric Res 6:4579–4593
Kovarik A, Koukalova B, Bezdek M, Opatrny Z (1997) Hypermethylation of tobacco heterochromatic loci in response to osmotic stress. Theor Appl Genet 95:301–306
Labra M, Grassi F, Imazio S, Fabio TD, Citterio S, Sgorbati S, Agradi E (2004) Genetic and DNA-methylation changes induced by potassium dichromate in Brassica napus L. Chemosphere 54(8):1049–1058
Li XL, Lin ZX, Nie YC, Guo XP, Zhang XL (2009) MSAP analysis of epigenetic changes in cotton (Gossypium hirsutum L.) under salt stress. Acta Agro Sin 35(4):588–596
Lindlöf A, Bräutigam M, Chawade A, Olsson B, Olsson O (2007) Identification of cold-induced genes in cereal crops and Arabidopsis through comparative analysis of multiple EST sets. In: Hochreiter S, Wagner R (eds) Bioinformatics research and development—first international conference BIRD ‘07, LNBI, vol 4414. Springer, Berlin, pp 48–65
Liu B, Wendel JF (2003) Epigenetic phenomena and the evolution of plant allopolyploids. Mol Phylogenet Evol 29(3):365–379
Lukens LN, Zhan SH (2007) The plant genome’s methylation status and response to stress, implications for plant improvement. Curr Opin Plant Biol 10:317–322
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Mastan SG, Rathore MS, Bhatt VD, Yadav P, Chikara J (2012) Assessment of changes in DNA methylation by methylation-sensitive amplification polymorphism in Jatropha curcas L. subjected to salinity stress. Gene 508:125–129
McClelland M, Nelson M, Raschke E (1994) Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 22:3640–3659
Morgan SH, Maity PJ, Geilfus CM, Lindberg S, Mühling KH (2014) Leaf ion homeostasis and plasma membrane H+-ATPase activity in Vicia faba change after extra calcium and potassium supply under salinity. Plant Physiol Bioch 82:244–253
Paterson AH, Brubaker CL, Wendel JF (1993) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep 11(2):122–127
Sairam RK, Dharmar K, Chinnusamy V, Meena RC (2009) Waterlogging-induced increase in sugar mobilization, fermentation, and related gene expression in the roots of mungbean (Vigna radiata). J Plant Physiol 166:602–616
Sakthivel K, Girishkumar K, Ramkumar G, Shenoy VV, Kajjidoni ST, Salimath PM (2010) Alterations in inheritance pattern and level of cytosine DNA methylation, and their relationship with heterosis in rice. Euphytica 175:303–314
Shan XH, Wang XY, Yang G, Wu Y, Su SZ, Li SP, Liu HK, Yuan YP (2013) Analysis of the DNA methylation of Maize (Zea mays L.) in response to cold stress based on methylation-sensitive amplified polymorphisms. J Plant Biol 56:32–38
Shen H, He H, Li J, Chen W, Wang X, Guo L, Peng Z, He G, Zhong S, Qi Y, Terzaghi W, Deng XW (2012) Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell 24(3):875–892
Shull GH (1908) The composition of a field of maize. Ann Breed Assoc Rep 4:296–301
Sun YG, Wang B, Jin SH, Qu XX, Li YJ, Hou BK (2013) Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in Tobacco. PLoS One 8(3):e59924
Tan MP (2010) Analysis of DNA methylation of maize in response to osmotic and salt stress based on methylation-sensitive amplified polymorphism. Plant Physiol Bioch 48:21–26
Tsaftaris AS, Kafka M (1997) Mechanisms of heterosis in crop plants. J Crop Prod 1(1):95–111
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Fijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang WS, Pan YJ, Zhao XQ, Dwivedi D, Zhu LH, Ali J, Fu BY, Li ZK (2011a) Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.). J Exp Bot 62:1951–1960
Wang WS, Zhao XQ, Pan YJ, Zhu LH, Fu BY, Li ZK (2011b) DNA methylation changes detected by methylation-sensitive amplified polymorphism in two contrasting rice genotypes under salt stress. J Genet Genomics 38:419–424
Xing CZ, Zhao YL, Yu SX, Guo LP, Zhang XL, Wang HL (2006) Relationship between leaves gene differential expression in full opening flower stages of hybrids & their parents and heterosis in pest-resistant cotton. Acta Genet Sin 33:948–956
Xiong LZ, Xu CG, Saghai Maroof MA, Zhang QF (1999) Patterns of cytosine methylation pattern in an elite rice hybrid and its parental lines detected by a methylation-sensitive amplification polymorphism technique. Mol Gen Genet 261(3):439–446
Xiong WS, Li XR, Fu DH, Mei JQ, Li QF, Lu GY, Qian LW, Fu Y, Disi JO, Li JN, Qian W (2013) DNA Methylation alterations at 5′-CCGG Sites in the Interspecific and Intraspecific Hybridizations Derived from Brassica rapa and B. napus. PLoS One 8(6):e65946
Xu ML, Li XQ, Korban SS (2000) AFLP-based detection of DNA methylation. Plant Mol Biol Rep 18:361–368
Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65(1):261–273
Yang JL, Liu LW, Gong YQ, Huang DQ, Wang F, He LL (2007) Analysis of genomic DNA methylation level in radish under cadmium stress by methylationsensitive amplified polymorphism technique. J Plant Physiol Mol Biol 33(3):219–226
Yu SB, Li JX, Tan YF, GaoYJ Li XH, Zhang QF, Saghai Maroof MA (1997) Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 94:9226–9231
Zemach A, McDaniel IE, Silva P, Zilberman D (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328(5980):916–919
Zhang MS, Yan HY, Zhao N, Lin XY, Pang JS, Xu KZ, Liu LX, Liu B (2007) Endosperm-specific hypomethylation, and meiotic inheritance and variation of DNA methylation level and pattern in sorghum (Sorghum bicolor L.) interstrain hybrids. Theor Appl Genet 115:195–207
Zhang LN, Ye WW, Wang JJ, Fan BX, Wang DL (2010) Genetic diversity analysis of salinity related germplasm in cotton. Biodivers Sci 18(2):142–149
Zhao XX, Chai Y, Liu B (2007) Epigenetic inheritance and variation of DNA methylation level and pattern in maize intra-specific hybrids. Plant Sci 172:930–938
Zhao YL, Yu SX, Xing CZ, Fan SL, Song MZ (2008) Analysis of DNA methylation in cotton hybrids and their parents. Mol Biol 42:169–178
Zhao YL, Yu SX, Xing CZ, Fan SL, Song MZ, Ye WW (2009) Differential gene expression between hybrids and their parents during the four crucial stages of cotton growth and development. Agric Sci China 8(2):144–153
Zhao YL, Yu SX, Ye WW, Wang HM, Wang JJ, Fand BX (2010) Study on DNA cytosine methylation of cotton (Gossypium hirsutum L.) genome and its implication for salt tolerance. Agric Sci China 9(6):783–791
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6(2):66–71
Zhu XX, Ainijiang Zhang YM, Guo WZ, Zhang TZ (2011) Relationships between differential gene expression and heterosis in cotton hybrids developed from the foundation parent CRI-12 and its pedigree-derived lines. Plant Sci 180:221–227
Acknowledgments
Research supported by the Natural Science Foundation of Jiangsu Province of China (BK20131204), the National Natural Science Foundation of China (31000729), Key Research and Development Project of Jiangsu Province (Modern Agriculture, BE2015353), Autonomous Innovation Project of Jiangsu Agricultural Science & Technology [CX(15)1005], the State Key Laboratory of Cotton Biology Open Fund (CB2015A09), Qing Lan Project of Colleges and Universities in Jiangsu Province, Scientific Research and Innovation Projects for Graduate Students in Jiangsu Province (YKC15071).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Zhang, M., Fu, R. et al. Epigenetic mechanisms of salt tolerance and heterosis in Upland cotton (Gossypium hirsutum L.) revealed by methylation-sensitive amplified polymorphism analysis. Euphytica 208, 477–491 (2016). https://doi.org/10.1007/s10681-015-1586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1586-x