Abstract
The bracts of the globe artichoke inflorescence vary in their pigmentation and the extent of fleshy thorn development. Here, a genetic analysis of these two traits is presented, based on a pre-existing and well-characterized mapping population derived from a cross between a globe artichoke variety and a cultivated cardoon. While both traits appeared to be simply inherited when the number of trait classes was limited to three (pigmentation) or two (thorniness), extending the classification to a larger number of states generated continuous distributions appropriate for a quantitative trait locus (QTL) analysis. The seven QTL identified by this means, mapping to five different linkage groups, were all expressed across two growing seasons. The largest effect QTL mapped onto homologues linkage group in each parental maps, and explained up to 69 and 73 % of the phenotypic variance respectively for bracts pigmentation and thorniness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Cynara cardunculus complex includes three cross-compatible taxa: the progenitor var. sylvestris (wild cardoon) and the two cultivated forms var. scolymus (globe artichoke) and var. altilis (cultivated cardoon). Globe artichoke production has risen from about 1.5 Mt in 1993 to 1.8 Mt in 2013 (FAOSTAT 2013), with some 60 % of this amount generated in Europe, predominantly Italy. The primary product of the globe artichoke is its inflorescence (more formally referred to as the capitulum), the inner bracts and the fleshy receptacle. Each plant produces various sizes of capitulum, with the largest formed on its central stem and the smaller ones on its lateral branches (Lanteri and Portis 2008). Compared to most vegetables, the globe artichoke capitulum harbours a high level of polyphenols (Moglia et al. 2008) some of which are of medicinal value (Wang et al. 2003; Pinelli et al. 2007; Lombardo et al. 2012; Pandino et al. 2012; 2013). As a result, and according to the definition of the European Commission on Functional Food Science in Europe (FuFoSE), the globe artichoke is considered as a functional food (Roberfroid 2000). Its non-food uses include the pharmaceutical exploitation of its polyphenols (Comino et al. 2007, 2009) and sesquiterpene lactones (Eljounaidi et al. 2014) extracted from in the foliage, while its roots and capitula contain inulin, a potent prebiotic and probiotic substance (Rijnierse et al. 2011). Its biomass has been suggested as a source of green forage, and the oil extracted from its seed is suitable for both edible and bioenergy purposes (Ierna and Mauromicale 2010; Portis et al. 2010; Ierna et al. 2012; Acquadro et al. 2013; Mauromicale et al. 2014).
Most commercial globe artichoke production relies on vegetatively reproduced autochthonous landrace materials, although in recent years seed propagated cultivars have begun to make an impact. Landraces are conventionally distinguished from one another by their capitulum shape, the presence/absence of spines and the colour of the bracts, although genotypic assays have shown a deal of within landrace heterogeneity (Lanteri et al. 2001; Portis et al. 2005). The four major morphological groups recognized are the “spinosi”, which develop long, sharp spines on their bracts and leaves, the “violetti”, which produce violet coloured, moderately spiny capitula, the “romaneschi”, which develop spherical or near-spherical, non-spiny capitula and the “catanesi”, which produce small, elongated, non-spiny capitula (Porceddu et al. 1976). Pigmentation type (green or violet) is less robust as a descriptor, since colour intensity can vary substantially within a landrace and is also modulated by the growing environment.
Varietal improvement of globe artichoke has lagged behind that achieved in most vegetable crops, nevertheless, its genome organization has been explored in some detail (Scaglione et al. 2012) and its genome sequence is currently being acquired (http://compgenomics.ucdavis.edu/). As the species does not tolerate self-fertilization, populations developed for mapping purposes have been based on a double pseudo-test-cross approach (Lanteri et al. 2006; Acquadro et al. 2009), which has led elaboration of genetic maps covering all 17 major linkage groups (LGs). The most recently derived genetic maps have been generated from the progeny of a cross between the globe artichoke ‘Romanesco C3’ and the cultivated cardoon genotype ‘Altilis 41’ (Portis et al. 2009). The maps have been exploited to expose the mode of inheritance of precocity with respect to capitulum production (Portis et al. 2012), as well as to derive marker/trait associations involving a number of yield components (Portis et al. 2014). Here, the identification of genomic regions influencing bract colour and the presence of fleshy thorns on the bracts is described.
Materials and methods
Plant materials and trait evaluation
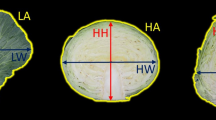
The C3 (Romanesco C3, female parent) × ALT (Altilis 41, male parent) mapping population (Portis et al. 2009) consists of 154 F1 progeny. The population, along with six clones of each of C3 and ALT, was raised at the University of Catania (Sicily, Italy) Experimental Station (37°25′N; 15°30′E; 10 m a.s.l) in both 2009–2010 (hereafter referred to as 2010) and 2010–2011 (2011) using standard agronomical practices. The area is representative of commercial globe artichoke cultivation, characterized by mild and wet winters and hot, dry summers. The two traits bc (bract colour) and bt (the extent of fleshy thorns developed on the bract) were assessed on capitula harvested prior to bract divergence, defined as “stage D” by Foury (1967). bc was initially evaluated by classifying the capitula as either ‘green’, ‘uneven purple’ or ‘even purple’, then refined by applying a 1–7 scale (1 green; 2 green with purple hue; 3 green-purple; 4 purple-green; 5 purple with green hue; 6 purple; 7 dark purple; Fig. 1a) was assigned to each individual. bt was initially classified using a simple presence/absence score, and subsequently refined to a 1–5 scale (1 absence; 2–5 from small and soft to fully developed; Fig. 1b).
Frequency distribution of bc and bt among the mapping population (2010 harvest). Parental perfomance (‘C3’ ‘Romanesco C3’, ALT ‘Altilis 41’) indicated by arrows. a Representative bracts illustrating the scale used to score bract pigmentation (bc). 1 green; 2 green with purple hue; 3 green-purple; 4 purple-green; 5 purple with green hue; 6 purple; 7 dark purple. b Representative bracts illustrating the scale used to score fleshy thorns (bt). 1 absence; 2 to 5: from small and soft to fully developed
Statistical analysis and quantitative trait locus (QTL) detection
Standard population metrics and trait correlations were obtained via algorithms implemented in R software (R Development Core Team 2006). Analyses of variance were based on treating each growing season as an independent replicate, following Zhang et al. (2010). Broad sense heritability was given by the expression \(h_{B}^{2} = {{y\sigma_{g}^{2} } \mathord{\left/ {\vphantom {{y\sigma_{g}^{2} } {(\sigma_{g}^{2} + \sigma_{e}^{2} )}}} \right. \kern-0pt} {(\sigma_{g}^{2} + \sigma_{e}^{2} )}}\), where \(\sigma_{g}^{2}\) refers to the genetic variance and \(\sigma_{e}^{2}\) to the error variance. The inter-trait correlation was calculated using the Spearman coefficient, and normality, kurtosis and skewness assessed using the Shapiro–Wilks test (α = 0.05). Segregation was considered as transgressive where at least one mapping population individual recorded a trait value differing from the relevant parental one by at least two standard deviations. QTL locations were, at first, based on the consensus genetic linkage map (Portis et al. 2012), which includes 694 markers and covers 1687.6 cM by applying the Kruskal–Wallis (KW) non-parametric test in conjunction with the simple interval mapping procedure (SIM) (Lander and Botstein 1989) implemented in MapQTL v4.0 software (van Ooijen et al. 2002); subsequently the two separate parental maps were used for a re-analysis based on the BC1 algorithm, using both SIM and multiple QTL mapping (MQM) (Jansen and Stam 1994). Among the markers lying within a region harbouring a QTL, the one associated with the highest LOD score was used as a co-factor. For the MQM, a backwards elimination procedure was applied to select the appropriate co-factors. LOD thresholds for QTL significance were confirmed using a permutation test comprising 1000 replications, which implies a genome-wide significance level of 0.05 (Churchill and Doerge 1994). Only those QTL associated with a LOD greater than either the genome-wide threshold or the LG threshold were considered. 1-LOD support intervals were determined for each LOD peak following van Ooijen (1992). The additive effect and the proportion of the overall phenotypic variance (PVE) associated with each QTL were both estimated from the MQM model. Linkage maps and QTL positions were drawn using MapChart (Voorrips 2002). Each QTL was designated by an abbreviated version of the trait name as a prefix, followed by the relevant LG; so for example “bcC3_5” indicates a QTL underlying the bracts colour traits, mapping to the female linkage group C3_5.
Results
Phenotypic variation, heritability and frequency distributions of phenotypes
The population displayed a very high diversity of phenotype and some individuals displayed aspects of morphology not previously observed in cultivated types. A summary of trait performance and the associated heritability values is given in Table 1. C3 and ALT differed significantly from one another (p < 0.05) in both 2010 and 2011 (Table 1). The former produced purple-green capitula (bc score of 4 corresponding to prevalence of purple over the green, see Fig. 1a) and lacked fleshy thorns (bt score of 1, see Fig. 1b), while the latter recorded a bc score of 3 (green-purple capitula, with prevalence of green over the purple) and a bt score of 2 (presence of small and soft thorns). A high broad sense hereditability was observed for both traits ranging from a minimum of 0.91 (bt) to a maximum of 0.94 (bc) (Table 1). There was no significant correlation between the traits in either season.
As the population distributions for both traits were similar between years, only the 2010 data have been graphically presented (Fig. 1). On the basis of the initial three state classification of bc, the segregation in the mapping population was 39 green, 86 uneven purple and 29 even purple, fitting a 4:9:3 ratio (χ2 = 0.01, n.s.) predicted by the action of two independent genes interacting epistatically, as previously suggested by Cravero et al. (2005). The use of a 1–7 scale generated a continuous distribution in the mapping population, while each set of six clonally propagated individuals of both C3 and ALT was homogeneous. The mapping population mean value was close to the mid-parent value, but the distribution was skewed towards each parent, as F1 capitula ranged from wholly green to dark purple (Fig. 1a). With respect to bt, the segregation pattern based on presence vs absence fitted the monogenic 3:1 ratio (χ2 = 0.70, n.s.) indicating that this trait is mainly controlled by a single gene. When the trait was scored on the 1–5 scale, the distribution became continuous, with the population mean lying above the mid-parent value. About one-third of the population recorded a higher bt score than ALT (Fig. 1b).
QTL identification
An independent QTL analysis was performed for each season (Table 2). The initial identification procedure highlighted three genomic regions influencing bc and two influencing bt. Each of the five QTL regions mapped to a different LG. The same QTL regions were identified in both seasons. The separate analysis of the C3 and ALT maps further validated the QTL regions; each of which was thus taken forward into the MQM procedure. The relationship between the two parental maps and the location of the bt and bc QTL are shown in Fig. 2. Table 2 documents the properties of each of the QTL: maximum LOD value, location on the genetic maps, additive marker value effects and proportion of phenotypic variance (PV) explained.
Considering bract pigmentation as a simple presence/absence trait (ignoring the effect of the proposed epistatic gene), the 9:4:3 ratio resolved into a monogenic 3:1 one (115 uneven or even purple vs 39 green). This pattern of segregation is consistent with the existence of a locus, termed P, present in the heterozygous state in both C3 and ALT; it was used as an intercross marker (ab × ab) and mapped onto the homologues LGs C3_5 and Alt_1, in the neighbourhood of two AFLP loci (Fig. 2). When the phenotypic data were treated as varying from 1 to 7, three QTL regions, each stable across the two seasons, were identified. One was represented in both parents (homologues QTL), one in C3 but not ALT, and the third in ALT but not C3. The largest effect QTL (PVE of 66–69 %) mapped to C3_5/Alt_1, coincident with P. The C3 QTL (PVE of 16–17 %) was located on LG C3_13 and the ALT QTL (PVE of 21–23 %) mapped to Alt_11 (Fig. 2).
On the basis of the 3:1 segregation ratio detected for presence:absence of soft thorns, conventional linkage analysis located a gene underlying bt (denoted Th) on the same LG in both maps (C3_14/Alt_7). Scoring bt on a 1–5 scale identified a QTL (PVE of 68–73 %) mapping in the same region as Th, along with a second locus (PVE of 11–12 %) exclusive to ALT mapping to Alt_4, in the neighbourhood of a CAPS and a SSR marker (Fig. 2).
Discussion
Genetic control of capitulum colour
Differences in capitulum colour in globe artichoke reflect the content and distribution of anthocyanin. High levels of anthocyanin are considered to be a positive attribute of plant-based foods (Lattanzio et al. 2009) and pigmented globe artichoke capitula are well regarded by consumers. The synthesis and accumulation of anthocyanin are strongly affected by genotype, are developmental stage dependent and are influenced by temperature (Prior et al. 1998; Cohen et al. 2012; Yamane et al. 2011); the intensity of pigmentation in the capitulum is particularly sensitive to temperature (Foury 1969; Pochard et al. 1969; Basnitzki and Zohary 1994). Nevertheless, the present experiments recorded a very high broad sense heritability for capitulum pigmentation. According to Pochard et al. (1969), pigmentation is determined by the presence of a dominant allele, interacting epistatically with an inhibitor. However, later evidence has suggested that a series of modifiers is additionally involved (Basnitzki and Zohary (1994). The genetic model proposed by Cravero et al. (2005) on the basis of segregation patterns observed in various populations is that bc is determined by the two independent genes P and U, where plants of genotype PP or Pp produce purple bracts and the pp genotype produces green ones; meanwhile the UU or Uu genotype confers an uneven distribution of pigment, while uu individuals form uniformly pigmented bracts. Superimposed on this simple genetic system is the action of various modifier genes, which result in a gradation of pigmentation intensity and the formation of colour streaks.
The pigmentation segregation pattern observed in the present mapping population was consistent with both C3 and ALT being of genotype PpUu as it was possible to identify the predicted three classes of capitulum colouration (uneven purple, green and even purple) segregating in the di-genic ratio of 4:9:3 [ppU_ + ppuu (4)]:[P_U_ (9)]:[P_uu (3)]. The pre-availability of the genome-wide genetic map allowed the placement of P onto LG C3_5 and Alt_1 (these two LGs are homologues) by analyzing its 3:1 monogenic segregation [(P_U_ + P_uu):(ppU_ + ppuu) = pigmented:green]. By treating the trait as a QTL showed that the most important locus (PVE of 66–69 %) mapped to the same region of this LG. Following the strategy proposed by Monforte et al. (2004), the possibility that the other QTL (PVE of 16–23 %) mapping to C3_13 and Alt_11 corresponded to U was tested by repeating the linkage analysis after excluding those individuals, which produced a green capitulum: the resulting segregation of uneven purple versus even purple fitted the segregation of a single dominant locus [P_U_ (3)]:[P_uu (1)]. However, since the restricted size of this portion of the population hindered the placement of U on the map, it was not possible to confirm whether the minor bc QTL revealed on both C3_13 and Alt_11 was identical to U. The segregation of pigmentation among a population bred from a cross between a green capitulum wild cardoon and a purple capitulum globe artichoke variety was consistent with the two parents having the allelic constitution ppUu and Ppuu, as reported by Martin et al. (2013). On the resulting map associated with the wild cardoon parent, the presumptive location for U mapped to an LG corresponding with C3_10, rather than with C3_13. This lack of correspondence may reflect a number of factors, most notably the imprecision associated with the detection of minor effect QTL (Beavis 1994). However, as other loci could be involved in the interaction, the number of modifier loci involved in the genetic control of anthocyanin distribution might be variable in different progenies.
Genetic control of the presence of fleshy thorns
The lack of both spines and fleshy thorns is a positive attribute for both the consumer and the processing industry. The genetic basis of spine formation relates to the allelic constitution at the Sp locus, with the spiny “thistle-like” phenotype determined by the recessive allele sp (Pochard et al. 1969; Basnitzki and Zohary 1994); the map location of Sp has been reliably established with a few mapping exercises (Lanteri et al. 2006; Sonnante et al. 2011; Martin et al. 2013). Both C3 and ALT are non-spiny types; the lack of any segregation for spininess in the mapping population, together with its segregation among progeny derived from other crosses involved the same female parent (Lanteri et al. 2012), implies that the allelic constitution of the globe artichoke must have been Spsp and that of the cultivated cardoon SpSp. With respect to fleshy thorns, which can develop in non-spiny types either solely on the leaves or in some cases on both the leaves and capitulum (Lanteri et al. 2012), the discrete nature of its segregation is fully consistent with its monogenic control involving a gene Th mapping to LG C3_14/Alt_7. The dominant allele confers the absence of fleshy thorns, with C3 and ALT sharing the allelic constitution Thth. Unlike those of ALT, the capitula produced by C3 lack fleshy thorns; the simplest genetic model consistent with this phenotype is that C3 is homozygous for i, a recessive inhibitor gene, while ALT is homozygous for the dominant allele I. The variation observed with respect to the length of the fleshy thorn types suggests the existence of modifier genes, possibly related to the minor QTL (PVE = 11–12 %) identified on Alt_4.
Conclusions
The evolving C. cardunculus genetic map has sufficient resolution to identify the genomic region(s) influencing any heritable agronomic trait, such as the oligogenic traits (capitulum pigmentation and fleshy thorniness) described here. For the purpose of marker-assisted selection—and definitely for the QTL isolation where this may be of interest—a greater level of resolution is still needed. This goal should be accomplished in the near future given the recent preparation of a draft genome assembly (Acquadro et al. 2014; Scaglione et al. 2014), the large-scale re-sequencing of C3 and ALT and ongoing efforts to acquire low coverage genotyping-by-shotgun-sequencing of the mapping population.
References
Acquadro A, Lanteri S, Scaglione D, Arens P, Vosman B, Portis E (2009) Genetic mapping and annotation of genomic microsatellites isolated from globe artichoke. Theor Appl Genet 118:1573–1587
Acquadro A, Portis E, Scaglione D, Mauro RP, Campion B, Falavigna A, Zaccardelli R, Ronga D, Perrone D, Mauromicale G, Lanteri S (2013) CYNERGIA Project: exploitability of Cynara cardunculus L. as energy crop. Acta Hortic (ISHS) 983:109–115
Acquadro A, Scaglione D, Portis E, Tirone M, Mauro R, Lo Monaco A, Mauromicale G, Froenicke L, Reyes Chin Wo S, Michelmore R, Lanteri S (2014) The globe artichoke genome sequence. In: Proceedings of the 58th Italian Society of Agricultural Genetics Annual Congress: http://www.geneticagraria.it/attachment/SIGA_2014/1_06.pdf
Basnitzki J, Zohary D (1994) Breeding of seed planted artichoke. Plant Breed Rev 12:253–269
Beavis WD (1994) The power and deceit of QTL experiments: lessons from comparative QTL studies. In: Proceeding of the annual corn and sorghum research conference, vol 49, pp 250–266
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Cohen SD, Tarara JM, Gambetta GA, Matthews MA, Kennedy JA (2012) Impact of diurnal temperature variation on grape berry development, proanthocyanidin accumulation, and the expression of flavonoid pathway genes. J Exp Bot 63:2655–2665
Comino C, Lanteri S, Portis E, Acquadro A, Romani A, Hehn A, Larbat R, Bourgaud F (2007) Isolation and functional characterization of a cDNA coding a hydroxycinnamoyltransferase involved in phenylpropanoid biosynthesis in Cynara cardunculus L. BMC Plant Biol 7:14
Comino C, Hehn A, Moglia A, Menin B, Bourgaud F, Lanteri S, Portis E (2009) The isolation and mapping of a novel hydroxycinnamoyltransferase in the globe artichoke chlorogenic acid pathway. BMC Plant Biol 9:30
Cravero V, Picardi L, Cointry E (2005) An approach for understanding the heredity of two quality traits (head color and tightness) in globe artichoke (Cynara scolymus L.). Genet Mol Biol 28:431–434
Eljounaidi K, Cankar K, Comino C, Moglia A, Hehn A, Bourgaud F, Bouwmeester H, Menin B, Lanteri S, Beekwilder J (2014) Cytochrome P450 s from Cynara cardunculus L. CYP71AV9 and CYP71BL5, catalyze distinct hydroxylations in the sesquiterpene lactone biosynthetic pathway. Plant Sci 223:59–68
FAOSTAT 2013. http://faostat.fao.org/
Foury C (1967) Étude de la biologie florale de l’artichaut (Cynara scolymus L.), application à la sélection, 1ère partie: données sur la biologie florale. Annales d’Amelioration des Plantes 17:357–373
Foury C (1969) Étude de la biologie florale de l’artichaut (Cynara scolymus L.), application a la sélection, 2ème partie: étude des descendances obtenues en fécondation contrôlée. Annales d’Amelioration des Plantes 19:23–52
Ierna A, Mauromicale G (2010) Cynara cardunculus L. genotypes as a crop for energy purposes in a Mediterranean environment. Biomass Bioenergy 34:754–760
Ierna A, Mauro RP, Mauromicale G (2012) Biomass, grain and energy yield in Cynara cardunculus L. as affected by fertilization, genotype and harvest time. Biomass Bioenergy 36:404–410
Jansen RC, Stam P (1994) High-resolution of quantitative traits into multiple loci via interval mapping. Genetics 136:1447–1455
Lander ES, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Lanteri S, Portis E (2008) Globe artichoke and cardoon. In: Springer (ed) Vegetables I, vol 1. Springer, New York, pp 49–74
Lanteri S, Di Leo I, Ledda L, Mameli M, Portis E (2001) RAPD variation within and among populations of globe artichoke cultivar ‘Spinoso sardo’. Plant Breed 120:243–246
Lanteri S, Acquadro A, Comino C, Mauro R, Mauromicale G, Portis E (2006) A first linkage map of globe artichoke (Cynara cardunculus var. scolymus L.) based on AFLP, S-SAP, M-AFLP and microsatellite markers. Theor Appl Genet 112:1532–1542
Lanteri S, Portis E, Acquadro A, Mauro RP, Mauromicale G (2012) Morphology and SSR fingerprinting of newly developed Cynara cardunculus genotypes exploitable as ornamentals. Euphytica 184:311–321
Lattanzio V, Kroon PA, Linsalata V, Cardinali A (2009) Globe artichoke: a functional food and source of nutraceutical ingredients. J Funct Foods 1:131–144
Lombardo S, Pandino G, Ierna A, Mauromicale G (2012) Variation of polyphenols in a germplasm collection of globe artichoke. Food Res Int 46:544–551
Martin E, Cravero V, Portis E, Scaglione D, Acquaviva E, Cointry E (2013) New genetic maps for globe artichoke and wild cardoon and their alignment with an SSR-based consensus map. Mol Breed 32:177–187
Mauromicale G, Sortino O, Pesce GR, Agnello M, Mauro RP (2014) Suitability of cultivated and wild cardoon as a sustainable bioenergy crop for low input cultivation in low quality Mediterranean soils. Ind Crops Prod 57:82–89
Moglia A, Lanteri S, Comino C, Acquadro A, de Vos R, Beekwilder J (2008) Stress-induced biosynthesis of dicaffeoylquinic acids in globe artichoke. J Agric Food Chem 56:8641–8647
Monforte AJ, Oliver M, Gonzalo MJ, Alvarez JM, Dolcet-Sanjuan R, Arús P (2004) Identification of quantitative trait loci involved in fruit quality traits in melon (Cucumis melo L.). Theor Appl Genet 108:750–758
Pandino G, Lombardo S, Mauro RP, Mauromicale G (2012) Variation in polyphenol profile and head morphology among clones of globe artichoke selected from a landrace. Sci Hortic 138:259–265
Pandino G, Lombardo S, Mauromicale G (2013) Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind Crops Prod 44:44–49
Pinelli P, Agostini F, Comino C, Lanteri S, Portis E, Romani A (2007) Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. Food Chem 105:1695–1701
Pochard E, Foury C, Chambonet D (1969) Il miglioramento genetico del carciofo. In: Proceedings of the first international congress on artichoke, Bari, Italy, pp 117–155
Porceddu E, Dellacecca V, Bianco V (1976) Classificazione numerica di cultivar di carciofo. In: Proceedings of the IInd international congress on artichoke, Ed Minerva Medica, Torino, pp 1105–1119
Portis E, Mauromicale G, Barchi L, Mauro R, Lanteri S (2005) Population structure and genetic variation in autochthonous globe artichoke germplasm from Sicily Island. Plant Sci 168:1591–1598
Portis E, Mauromicale G, Mauro R, Acquadro A, Scaglione D, Lanteri S (2009) Construction of a reference molecular linkage map of globe artichoke (Cynara cardunculus var. scolymus). Theor Appl Genet 120:59–70
Portis E, Acquadro A, Longo A, Mauro R, Mauromicale G, Lanteri S (2010) Potentiality of Cynara cardunculus L. as energy crop. J Biotechnol 150:165–166
Portis E, Scaglione D, Acquadro A, Mauromicale G, Mauro R, Knapp S, Lanteri S (2012) Genetic mapping and identification of QTL for earliness in the globe artichoke/cultivated cardoon complex. BMC Res Notes 5:252
Portis E, Mauro RP, Barchi L, Acquadro A, Mauromicale G, Lanteri S (2014) Mapping yield-associated trait QTL in globe artichoke. Mol Breed 34:615–630
Prior RL, Cao GH, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM (1998) Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem 46:2686–2693
R Development Core Team (2006) R: a language and environment for statistical computing. http://www.R-project.org
Rijnierse A, Jeurink PV, van Esch BCAM, Garssen J, Knippels LMJ (2011) Food-derived oligosaccharides exhibit pharmaceutical properties. Eur J Pharmacol 668:117–123
Roberfroid MB (2000) A European consensus of scientific concepts of functional foods. Nutrition 16:689–691
Scaglione D, Lanteri S, Acquadro A, Lai Z, Knapp SJ, Rieseberg L, Portis E (2012) Large-scale transcriptome characterization and mass discovery of SNPs in globe artichoke and its related taxa. Plant Biotechnol J 10:956–969
Scaglione D, Reyes Chin Wo S, Lanteri S, Acquadro A, Portis E, Tirone M, Froenicke L, Beitel C, Korf I, Rieseberg L, Michelmore R (2014) De novo genome assembly and ultra-dense mapping of artichoke, chicory and endive, and syntenic comparisons across the Compositae. Plant and Animal Genome XXIInd Conference: https://pag.confex.com/pag/xxii/webprogram/Paper12727.html
Sonnante G, Gatto A, Morgese A, Montemurro F, Sarli G, Blanco E, Pignone D (2011) Genetic map of artichoke x wild cardoon: toward a consensus map for Cynara cardunculus. Theor Appl Genet 123:1215–1229
Van Ooijen JW (1992) Accuracy of mapping quantitative trait loci in autogamous species. Theor Appl Genet 84:803–811
Van Ooijen JW, Boer MP, Jansen RC, Maliepaard C (2002) MapQTL 4.0: software for the calculation of QTL positions on genetic maps. Plant Research International, Wageningen
Voorrips R (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Wang MF, Simon JE, Aviles IF, He K, Zheng QY, Tadmor Y (2003) Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J Agric Food Chem 51:601–608
Yamane T, Jeong ST, Goto-Yamamoto N, Koshita Y, Kobayashi S (2011) Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am J Enol Vitic 57:54–59
Zhang GR, Sebolt AM, Sooriyapathirana SS, Wang DC, Bink M, Olmstead JW, Iezzoni AF (2010) Fruit size QTL analysis of an F-1 population derived from a cross between a domesticated sweet cherry cultivar and a wild forest sweet cherry. Tree Genet Genomes 6:25–36
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Portis, E., Mauro, R.P., Acquadro, A. et al. The inheritance of bract pigmentation and fleshy thorns on the globe artichoke capitulum. Euphytica 206, 523–531 (2015). https://doi.org/10.1007/s10681-015-1521-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1521-1