Abstract
In this study, the morphology, cytology and crossability of amphidiploid hybrids (2n = 8x = 80; WWW′W′TTT′T′) between Nicotiana wuttkei × Nicotiana tabacum were studied. An R1 progeny of the hybrids were produced through in vitro chromosome doubling of the N. wuttkei × N. tabacum cv. Wiślica amphihaploids. The resulting amphidiploids showed enough self-fertility to be perpetuated by self-pollination. The plants preserved their phenotypes and the number of chromosomes over subsequent R2–R4 selfed generations. During metaphase I the bivalent number increased progressively over the generations R1 to R4 and averaged 36.9, 37.4, 38.6 and 38.7, respectively. In addition, a progressive increase in pollen viability was observed: 50.8, 55.5, 62.3 and 65.7 % in generations from R1 to R4, respectively. Genomic in situ hybridization of R4 plants revealed recombination between the parental species on two chromosomes. Reciprocal crosses of R2 generation amphidiploids to N. tabacum cv. Wiślica yielded sesquidiploid hybrids (2n = 6x = 64; WW′TTT′T′). When N. tabacum cv. Wiślica is the pollinator, the amphidiploid gave rise to a total of 25 sesquidiploid plants with the cytoplasmic genome of N. wuttkei, while the reverse mating resulted in a mixed population consisting of a single sesquidiploid plant with the cytoplasm of N. tabacum and 203 plants of supposedly maternal origin. Our amphidiploid N. wuttkei × N. tabacum cv. Wiślica can be concluded as sufficiently stable and is regarded as a new artificial hybrid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first plant hybridizations ever undertaken were between two Nicotiana species (N. paniculata × N. rustica) by Koelreuter in 1760 (Kostoff 1943). Hybridization barriers in Nicotiana developed relatively slower and were less hampering interspecific DNA transfer compared to many other genera (Goodspeed 1954). Up to now, more than 300 interspecific Nicotiana hybrids have been synthesized (Goodspeed 1954; Moav and Cameron 1960; Gopinath et al. 1965; Smith 1968; Williams and Pandey 1974; Gerstel et al. 1979; Gangadevi et al. 1988; Laskowska and Berbeć 2003, 2012).The majority of the interspecific amphihaploid Nicotiana hybrids are sterile. In order to circumvent this obstacle, chromosome doubling of the sterile amphihaploid hybrids was performed to converted its to amphidiploids. If the conversion results in at least partial restoration of fertility, amphidiploids provide one of two, besides sesquidiploids, conventional points of issue for interspecific gene transfer in Nicotiana (Patel and Gerstel 1961; Reed and Collins 1978).
In the tobacco breeding, successful transfer of the disease resistance genes from wild Nicotiana species is of greatest importance. However, the application of wide hybridization and the creation of allopolyploids in Nicotiana did rarely result in cultivars with an added practical value and only a few Nicotiana species have been successfully used for the introgression of valuable genes into cultivated tobacco (Lewis 2011). Up to now resistances towards tobacco mosaic virus (TMV) from N. glutinosa (Holmes 1938; Henderson 1949; Heggestad 1966), blue mold and black root rot from N. debneyi (Clayton 1969; Miller 1987; Rufty 1989; Brandle et al. 1997; Lea 1999), black shank from N. plumbaginifolia (Apple 1962), wildfire from N. longiflora (Clayton 1947; Clayton et al. 1951), and tomato spotted wilt virus (TSWV) from N. alata (Gajos 1981, 1988) were introgressed in the cultivated N. tabacum.
Nicotiana wuttkei is one of recently reported new species in the section Suaveolentes (Knapp et al. 2004). Although N. wuttkei is an interesting source for resistance to Peronospora hyoscyami de Bary (Peronospora tabacina Adam.) (Laskowska and Berbeć 2003), causing blue mold in tobacco, it has never been explored for its breeding potential.
We successfully performed interspecific hybridization between N. wuttkei Clarkson and Symon (2n = 4x = 32) and N. tabacum L. (2n = 4x = 48). However, the initial amphihaploid seedlings were unviable. Therefore, in order to obtain viable N. wuttkei × N. tabacum hybrids, in vitro cotyledon culture was applied and yielded both amphihaploids (2n = 4x = 40), near-amphidiploids (2n = 78) and amphidiploids (2n = 8x = 80) (Laskowska and Berbeć 2012).
In the present study, the morphology, meiotic behavior, fertility and crossability of the amphidiploids N. wuttkei × N. tabacum are described.
Materials and methods
Nomenclature
Following the common nomenclature for Nicotiana allopolyploids (Webber 1930; Goodspeed 1954; Chaplin and Mann 1961; Burk 1975; Pittarelli and Sisson 1989; Zhou et al. 1997), our allopolyloid hybrids are referred to as amphidiploids and sesquidiploids, depending on their derivation and cytological status. The application of the terms “amphidiploid” (allotetraploid) and “sesquidiploid” (allotriploid) assumes that Nicotiana species can be regarded, at least cytologically, as functional diploids even if they are phylogenetically seen as allotetraploids, as is the case of N. wuttkei and N. tabacum (Chase et al. 2003). The validity of this approach was also confirmed by the recent studies on phylogeny and genome rearrangements in Nicotiana allotetraploids (Renny-Byfield et al. 2013).
Plant materials
Among the 38 viable plants regenerated from cotyledon culture of the amphihaploid seedlings N. wuttkei × N. tabacum cv. Wiślica WW′TT′ (2n = 4x = 40) (Laskowska and Berbeć 2012), seven were found to have 2n = 8x = 80 chromosomes. This amphidiploids WWW′W′TTT′T′ (R1 generation) and their selfed progeny (R2 to R4 generations) provided the material for our investigation. The hybrid plants were grown in a greenhouse in pots filled with standard soil medium at 24 °C and with 16/8 h light–dark photoperiod.
Self-pollination and hybridization experiments
Self-pollination and interspecific hybridization was done under greenhouse conditions. Prior to hybridization stamens were removed from the juvenile flowers of the female parent. The pollen collected previously from the donor flowers was transferred to the stigmas of the emasculated female parent. For each self and cross-pollination treatment, 7 plants of the amphidiploid R1 generation and 10 plants of R2, R3 and R4 were included, and approximately 20 flowers were pollinated on each plant. After pollination, all the other buds and flowers were removed from the plants and the pollinated inflorescences were covered with paper bags until capsule formation.
Seed germination rate was verified using the seed germination apparatus (Jacobsen) for 100 seeds per plant. The seed germination rate was calculated as the percentage of seeds that germinated over a period of 14 days per number of sown seeds. Seedling survival rate was expressed as the percentage of seedlings capable of growing into flowering plants per number of the germinated seeds.
Amphidiploid plants were briefly observed for their leaf and flower morphology.
Cytogenetic studies: chromosome counts and GISH
Mitotic chromosome counts were performed on juvenile corollas of greenhouse-grown plants using the method of Burns (1964). Corolla were pretreated for 5 h in a 0.44 % solution of 8-hydroxyquinoline with saturated maltose solution, added just before using (0.05 ml maltose per 3 ml 8-hydroxyquinoline). The pretreated material was fixed in the Carnoy solution (ethanol, chloroform and acetic acid—6:3:1) and stained with acetocarmine (1.5 %) after squashing the corolla fragments. Chromosome numbers were determined for each plant of the R1 generation and 10 plants of R2, R3 and R4, ten metaphase of each plant was analyzed.
Genomic in Situ Hybridization (GISH) were carried out on five plants of the amphidiploid R4 generation. Young root tips were incubated for 5 h in a 0.44 % 8-hydroxyquinoline solution at room temperature. Then the roots were fixed for 2 h at room temperature in Farmer solution (3:1 ethanol: acetic acid). Enzyme incubation was done using an enzyme mixture containing 0.6 % Cellulase, 0.6 % Pectolyase, 0.6 % Cytohellicase for 1.5 h at 37 °C. Cell suspensions and chromosome slides were prepared according to the Steam Drop Method of Kirov et al. (2014). Total genomic DNA was extracted from young leaves using the CTAB protocol of Doyle and Doyle (1990) and purified by adding 1 µl RNAse (10 mg/ml). N. wuttkei genomic DNA was sonicated (ultrasonic Homogenizer Biologics) to obtain 1–3 kb fragments and labeled with digoxygenin-11-dUTP (Dig-Nick Translation Mix, Roche) according to the manufacturer’s indications. N. tabacum genomic DNA was sheared by autoclaving to obtain fragments of 200–400 bp and used as blocking DNA in 50 times excess than probe DNA. Denaturation, hybridization and detection was performed as described in Van Laere et al. (2010). A 82 % stringency wash was obtained by washing the slides twice in 0.1 % SSC at 37 °C for 15 min., twice in 0.1 % SSC at 52 °C for 7 min and in 2× SSC at room temperature (RT) for 5 min. Digoxigenin labeled signals were detected by anti-Dig-FITC (sheep) and anti-sheep-FITC (rabbit). The chromosomes were counterstained with 1 mg/ml DAPI and mounted in Vectashield. Chromosome analysis was done with an AxioImager M2 (Zeiss) fluorescence microscope equipped with an AxiocamMRm camera (Zeiss). Images were captured by ZEN software (Zeiss). Analysis of hybridization signals was carried out on at least 30 well-spread metaphases of each plant.
Analysis of meiotic configurations
Flower buds at the appropriate stage (determined by a preliminary microscopic examination of one of the five anthers in a bud) were fixed in the Carnoy’s solution (ethanol, chloroform and acetic acid—6:3:1) for 24 h and stored in 70 % ethanol. Fixed anthers were squashed in a drop of 1.5 % acetocarmine. The meiotic analysis included observations of chromosome pairing at metaphase I, meiotic irregularities at further stages (chromatin bridges, laggards) and number of microspores and micronuclei produced at the tetrad phase. On the basis of chromosome pairing data, the means and ranges for the numbers of bivalents, univalent, trivalents and quadrivalents were estimated. Observations of meiotic configurations were performed in approximately 100 pollen mother cells (PMCs) prepared from anthers taken from four plants of each genotype.
Pollen viability tests
Pollen viability was determined as the percentage of mature pollen grains stainable in acetocarmine (1.5 %). Seven plants of amphidiploid R1 generation and 30 plants of R2–R4 generations were analyzed, 1000 pollen grains of each plant.
Results
Self-fertility and hybridization experiments
The efficiency to produce a selfed offspring of the amphidiploid N. wuttkei × N.tabacum cv. Wiślica was low in the R2 generation (Table 1). After pollination, 36.8 % of pollinated flowers yielded capsules with an average of 26.4 seeds per capsule. Seed germination capacity was 23.1 %, survival rate of seedlings—92.3 % and 416 plants were received (Table 1). The subsequent generations (R3 and R4) were substantially more self-fertile. Both the number of seeds per capsule and the germination rate increased by a factor more than 2 with practically the same survival rate of seedlings (91.9 and 92.2 % in R3 and R4 generations, respectively) (Table 1). As a consequence, the number of surviving progeny increased from 416 plants in generation R2, to 2565 and 3666 in R3 and R4, respectively.
The ability of the R2 amphidiploid N. wuttkei × N. tabacum cv. Wiślica to produce offspring by cross-pollination was considerably lower than by selfing, especially in terms of number of seeds per capsule and the survival rate of seedlings (Table 2). When used as female in crosses with ‘Wiślica’, the R2 plants yielded on average 18.3 seeds per capsule and the seeds showed a germination capacity of 23.9 %. In total, 25 progeny plants were obtained, which is several times less when comparing with the obtained plants after selfing. This was caused by a massive dieback of the juvenile plants (9.3 % of surviving seedlings). All the plants that survived to maturity, were confirmed as true sesquidiploid hybrids (2n = 64; Fig. 1).
Data on the male fertility of the amphidiploid N. wuttkei × N. tabacum cv. Wiślica are ambiguous. Backcrossed to ‘Wiślica’, a population of 204 surviving plants was obtained (Table 2). However, only one progeny plants was confirmed as a sesquidiploid and the remaining were the maternal ‘Wiślica’.
Pollen fertility of the amphidiploid hybrids
Flowers of the R1 plants of the amphidiploids N. wuttkei × N. tabacum cv. Wiślica produced abundant pollen. The average pollen viability in R1 generation was 50.8 % and varied among plants from 36.3 to 66.7 % (Table 3). The R1 plants were able to produce seeds after self-pollination. In the subsequent selfed generations (R2, R3, R4) the percentage of stainable pollen increased up to 65.7 % in R4, varying from 49.1 to 75.1 % (Table 3).
Morphology of amphidiploid hybrids
Nicotiana wuttkei × N. tabacum cv. Wiślica amphidiploids showed good vigor and resembled amphihaploids (Laskowska and Berbeć 2012) for most morphological attributes. The leaves and flowers of the amphidiploids were a little smaller in size and thicker compared to those of the amphihaploids as well as much smaller than those of the cultivated N. tabacum parent (Fig. 2). The R1–R4 plants did not exhibit any phenotypic variations both within and between individual generations.
Chromosome counts and GISH
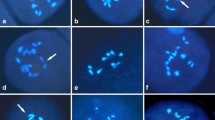
All the analysed plants from the R1–R4 amphidiploid generations had 80 chromosomes. For the R4 generation the number of 80 somatic chromosomes was furthermore confirmed in the GISH analysis (Fig. 3).
GISH analysis of the N. wuttkei (green fluorescence) × N. tabacum amphidiploid hybrid of the R4 generation. Pseudo colored merged image and grayscale version of the respective DAPI and FITC channels for complete metaphases (a, b, c) and for the pair of recombinant chromosomes on enlarged photos (d, e) are shown; f schematic drawing of the recombinant chromosomes. Arrows on a, b, c indicate the recombinant chromosomes; bar 10 µm
By GISH, the parental genomes in the hybrid could be clearly discriminated (Fig. 3). The amphidiploid hybrid contained 31 chromosomes from the wild species N. wuttkei, 47 chromosomes from the cultivated N. tabacum and 2 recombinant chromosomes (Fig. 3a, b, c). The recombination occurred on the short arm of the chromosomes involved (Fig. 3d, e, f).
Meiotic analysis
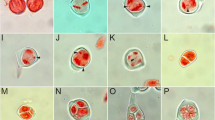
In the majority of metaphase I configurations, chromosomes predominantly formed bivalents (Table 4) and normal metaphase plates with bivalents only (Fig. 4i, k) or with occasional unpaired chromosomes (Fig. 4a, b, g, h). Among the bivalents some weakly paired associations were observed (Fig. 4b, i).
Meiosis of the amphidiploid hybrids N. wuttkei × N. tabacum ‘Wiślica’ (2n = 80); R1 generation: a the bivalents in equatorial plate and univalents scattered in and around the plate, b 4 I, 38 II and c 6 I, 37 II in metaphase I, d lagging chromosomes in anaphase I, e chromosomes not included in daughter nuclei in telophase II, f tetrad stage—pentad; R2 generation: g 2 I, 39 II and h 2 I, 37 II, 1 IV in metaphase I; R3 generation: i 40 II and j 7 I, 35 II, 1 III in metaphase I; R4 generation, PMC’s with regular meiosis: k 40 II in metaphase I, l anaphase I, m telophase II, n tetrad. Bar 5 µm; univalents, trivalents and tetravalents marked by arrows
Chromosome pairing observed at metaphase I varied among the four amphidiploid generations, increasing from 36.95 in R1 to 38.71 in R4 (Table 4; Fig. 4b—38 bivalents for R1; Fig. 4g—39 bivalents in R2; Fig. 4i—40 bivalents in R3; Fig. 4k—40 bivalents in R4). Highly regular meiosis in the amphidiploids was reflected by a relatively low number of unpaired chromosomes (univalents) per PMC, decreasing from R1 to R4 generation (5.75, 4.86, 2.69 and 2.52 in R1, R2, R3 and R4, respectively; Table 4). Also the number of trivalents decreased from 0.07 per PMC in R1 generation to 0.01 in R4 and quadrivalents from 0.05 to 0.004, respectively (Table 4).
Disjunctional abnormalities in later meiotic stages such as chromatin bridges and laggards at anaphase I and chromosomes not included in daughter nuclei at telophase II were rarely observed (Fig. 4d, e). Tetrads were formed in 97.4, 96.8, 98.0 and 98.8 % of the PMC’s examined at late telophase II in R1 to R4 amphidiploid generations, respectively (Table 5). In the remaining PMC’s abnormal microspores as tryads and pentads (Fig. 4f) were observed accounting for 0.4–2.0 % of the total number examined (Table 5). The micronuclei were found in 2.0 % of PMC’s in R1, 2.2 % in R2 and 1.0 % in R3 and R4 (Table 5).
Some of the amphidiploid plants, especially from R4 generation, were characterized by a completely regular meiosis (Fig. 4k, l).
Discussion
The closest relatives of N. wuttkei include N. velutina (2n = 32), N. maritima (2n = 32) and N. amplexicaulis (2n = 36) (Barbato 2009), all in the section Suaveolentes. Amphidiploids of these latter three species with N. tabacum have been reported (Wark 1970; Dorossiev et al. 1978; Berbeć and Doroszewska 1981; Palakarcheva and Dorossiev 1983; DeVerna et al. 1987; Nikova et al. 1991; Nikova and Vladova 2002). Our study here is the first to describe allopolyploids involving N. tabacum and N. wuttkei. In Laskowska and Berbeć (2012), the creation of the amphidiploids of N. wuttkei × N. tabacum (R1 generation) was described. Now, self-pollination and hybridisation experiments were performed with the amphidiploids and the subsequent generations (R1–R4) were analyzed in detail.
Amphidiploids in Nicotiana are known to show many variation concerning their cytogenetic stability. At one end of the spectrum amphidiploids showing full cytological stability are found, the so-called “synthetic species” such as N. edwardsonii, the hybrid of N. glutinosa with N. clevelandii (Christie 1969; Christie and Hall 1979), N. rustica × N. exigua (Bolsunov 1971), N. debneyi × N. tabacum (Smith 1941; Clayton et al. 1967), N. × digluta from the hybrid N. glutinosa × N. tabacum (Clausen and Goodspeed 1925), N. tabacum × N. glauca—N. × ditagla (Ternovski 1934; Modilevski 1936). At the other end of the spectrum, there are the unstable amphidiploids which rapidly disintegrate due to chromosome loss: N. tabacum × N. plumbaginifolia (Davies 1974), N. raimondii × N. tabacum (Berbec 1988), N. tabacum × N. africana (Doroszewska and Berbec 2000) and N. longiflora × N. tabacum (Nikova et al. 2001). Some of the amphidiploids, notably N. tabacum × N. glauca, were reported as either very stable by Ternovski (1934) and Modilevski (1936) (N. ditagla) or very unstable (Shilagyi 1975). According to Sybenga (1992) species with little chromosome homology will tend to form more stable allopolyploids than closely related species.
We received amphidiploids of N. wuttkei × N. tabacum (2n = 80) showing enough self-fertility and cytological stability to be reproduced over successive generations. Increased self-fertility in the R2 to R4 generations resulted in a more regular chromosome pairing reaching the expected number of 40 bivalents and elimination of higher chromosome associations (trivalents and tetravalents).This result is in contrast with the results obtained for the allopolyploids involving N. velutina and N. maritima, the closest relatives of N. wuttkei (Nikova and Zagorska 1987). In this study, the near-amphidiploids (82–84 chromosomes) regenerated from the amphihaploid hybrid N. velutina × N. tabacum, showed high cytological instability, segregating into male fertile and male sterile types. It was explained by massive disintegration of the allopolyploid genome through selective chromosome elimination in the first selfed generation, resulting in the appearance of N. tabacum-like male sterile phenotypes.
The strong tendency for chromosomes to associate as bivalents in the amphidiploid N. wuttkei × N. tabacum, can be explained by the existing structural differences between genomes of the N. wuttkei and N. tabacum and the consequently preferential pairing between duplicated chromosomes of each species. The GISH technique provide a valuable tool to verify the relationship and homology between species. In the present study, parental chromosomes in the amphidiploid R4 generation could be clearly distinguished by standard GISH. According to Schwarzacher et al. (1989) genomes sharing 80 % or less sequence homology can be discriminated by standard GISH conditions.
One pair of recombinant chromosomes in the amphidiploid N. wuttkei × N. tabacum detected by GISH was most probably the result of a single crossover event. It demonstrates that homoeologous pairing between these two species is possible, albeit very low and that it may occur within the first few generations in an amphidiploid lineage. Intergenomic translocations were also reported in the synthetic allopolyploid N. sylvestris × N. tomentosiformis of S4 generation (Skalicka et al. 2005) as well as in the hybrid N. tabacum × N. rustica (Kitamura et al. 1997).
The decreasing self- and cross fertility of the amphidiploids N. wuttkei × N. tabacum can be, at least partly, related to cytoplasmic male sterility (CMS). All 16 combinations of Suaveolentes cytoplasms with the nuclear genome of N. tabacum are known to have resulted in CMS (Gerstel 1980; Kubo 1985; Kaul 1988; Berbeć 2001; Nikova and Vladova 2002), N. wuttkei being no exception (Laskowska and Berbeć 2007). CMS may manifestate in the first generation of the hybrid, as in N. excelsior × N. tabacum amphihaploids (Nikova et al. 1997). CMS was also observed in highly unstable populations of the allopolyploid N. maritima × N. tabacum (Nikova and Zagorska 1987) and in the near-amphidiploid regenerants of the hybrid N. velutina × N. tabacum where anthers contained no sporogenic tissue (Nikova and Vladova 2002). Since the efficiency to produce true hybrid offspring is low, the amphidiploid N. wuttkei × N. tabacum may be considered as having its male fertility drastically reduced (single sesquidiploid plant out of more than 200 pollination events). On the other hand, the cross between N. tabacum as female and the amphidiploid N. wuttkei × N. tabacum as pollen donor, resulted in a large population of fully fertile non-hybrid plants, completely resembling the maternal parent. This phenomenon is described earlier in interspecific hybridization in Nicotiana. At first, Goodspeed (1915) dismissed diploid maternals (apomictic or otherwise) as products of experimental errors in disagreement to East (1930) but later on he revoked his former view (Goodspeed 1954). Alleged apomictics have continued to be reported either as induced by external factors (Pandey and Phung 1982) or as spontaneous by-products or even sole products of interspecific mattings (Berbeć and Doroszewska 1981; Sarychev 1987; Murthy and Subbarao 2004). According to Naumenko (2012) diploid maternals resulting from the cross N. tabacum × N. alata, described as “pseudogamic”, can be generated on a regular basis, even with N. tabacum male sterile plants used as female parents. Similar phenomenon were maternal haploids obtained in several Nicotiana cross combinations (Clausen and Mann 1924; Kumashiro and Oinuma 1985). One of these crosses, N. tabacum × N. africana, produces maternal haploids very regularly and this approach became one of the methods to generate haploid plants in N. tabacum (Burk et al. 1979; Nielsen and Collins 1989). Investigation of this phenomenon should get more attention than it has hitherto received.
The amphidiploids N. wuttkei × N. tabacum of this report could be successfully backcrossed to N. tabacum but, with a single exception, only used as females. This resulted in the substitution of an alien cytoplasm in the backcross products (Laskowska and Berbeć 2007) which is a serious limitation in terms of introgression since further backcrosses in alloplasmic lineages usually irrevocably lead to cytoplasmic male sterility. However, based on the results from this study, in which also a single sesquidiploid plant with N. tabacum cytoplasm was obtained, there is a theoretical possibility to circumvent the barrier. Therefore, similar work should be done on a larger scale.
In conclusion, the stable amphidiploid of N. wuttkei × N. tabacum can be regarded as a new artificial hybrid species which can extend the genetic variation within Nicotiana. This hybrid can serve as a starting point for transfer of useful genes, such as Peronospora tabacina resistance, from N. wuttkei to N. tabacum.
References
Apple JL (1962) Transfer of resistance to black shank Phytophthora parasitica var. nicotianae from Nicotiana plumbaginifolia to N. tabacum. (Abstr). Phytopathology 52:1
Barbato L (2009) Relazioni genetiche, basate sull’analisi del polimorfismo ISSR, tra la Nicotiana wuttkei Clarkson & Symon edaltre specie del genere Nicotiana appartenenti alla sezione Suaveolentes. PhD thesis, Universita degli Studii di Napoli “Federico II”
Berbeć A (1988) Morphology, cytogenetics and resistance of amphidiploid Nicotiana raimondii Macbride x N. tabacum L. (F1 cv. Zamojska 4 x cv. LB-838) to potato virus Y. Genet Pol 29:41–52
Berbeć A (2001) Floral morphology and some other characteristics of iso-genomic alloplasmics of Nicotiana tabacum L. Beitr Tab 19:309–314
Berbeć A, Doroszewska T (1981) Investigations of the interspecific hybrid Nicotiana amplexicaulis Burbidge x Nicotiana tabacum L. Genet Pol 22:197–207
Bolsunov I (1971) New Nicotiana amphidiploid as a valuable starting material for the control of virulent blue-mold lines. In: Proceedings of Fifth International Tobacco Science Congress, Hamburg, 1970, 160
Brandle JE, Rogers WD, Ankersmit JCD (1997) AC Gayed flue-cured tobacco. Can J Plant Sci 77(1):157–158
Burk LG (1975) Hybrid fertility and aphid resistance in the cross Nicotiana tabacum x N. gossei. Euphytica 24:59–63
Burk LG, Gerstel DU, Wernsman EA (1979) Maternal haploids of Nicotiana tabacum L. from seed. Science 206:585
Burns JA (1964) A technique for making preparations of mitotic chromosomes from Nicotiana flowers. Tob Sci 8:1–2
Chaplin JF, Mann TJ (1961) Interspecific hybridization, gene transfer and chromosome substitution in Nicotiana. N C State Coll Agric Exp Stn Tech Bull 145:1–31
Chase MW, Knapp S, Cox AV, Clarkson JJ, Butsko Y, Joseph J, Savolainen V, Parokonny AS (2003) Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann Bot 92:107–127
Christie SR (1969) Nicotiana hybrid developed as a host for plant viruses. Plant Dis Rep 53:939–941
Christie SR, Hall DW (1979) A new hybrid species of Nicotiana (Solanaceae). Baileya 20:133–136
Clausen RE, Goodspeed TH (1925) Interspecific hybridization in Nicotiana. II. A tetraploid glutinosa-tabacum hybrid, an experimental verification of Winge’s hypothesis. Genetics 10:278–284
Clausen RE, Mann MC (1924) Inheritance in Nicotiana tabacum. V. The occurrence of haploid plants in interspecific progenies. Proc Natl Acad Sci USA 10:121–124
Clayton EE (1947) A wildfire resistant tobacco. J Hered 38:35–40
Clayton EE (1969) The study of resistance to the black root rot disease of tobacco. Tob Sci 13:30–37
Clayton EE, Heggestad HE, Gross JG, Bowman DR, Schneider EO (1951) Breeding behaviour and growth responses resulting from the transfer of wildfire resistance from N. longiflora to N. tabacum. Phytopathology 41:7
Clayton EE, Heggestad HE, Grosso JJ, Burk LG (1967) The transfer of blue mold resistance to tobacco from Nicotiana debneyi. Part I. Breeding Progress 1937-1954. Tob Sci 11:91–99
Davies DR (1974) Chromosome elimination in interspecific hybrids. Heredity 2:267–271
DeVerna JW, Myers JR, Collins GB (1987) Bypassing prefertilization barriers to hybridization in Nicotiana using in vitro pollination and fertilization. Theor Appl Genet 3:665–671
Dorossiev L, Palakarcheva M, Stoyanova M (1978) Overcoming the sterility in F1 of interspecific hybrids of genus Nicotiana using the methods of tissue culture. CORESTA Inf Bull Sofia (Special) 1978:80–81
Doroszewska T, Berbeć A (2000) Cytogenetical investigations of polyploid interspecific hybrids of Nicotiana africana with different cultivars of N. tabacum. J Genet Breed 54:77–82
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus (Life Technologies Inc.) 12:13–15
East EM (1930) The origin of the plants of maternal type which occur in connection with interspecific hybridizations. Proc Natl Acad Sci USA 16(6):377–380
Gajos Z (1981) Transfer of resistance to Tomato spotted wilt virus from Nicotiana alata Link et Otto to tobacco by crossing the two species (in Polish). Biul CLPT 1–2:3–24
Gajos Z (1988) Polalta: a tobacco variety resistant to Tomato spotted wilt virus (TSWV) and black root rot (Thielaviopsis basicola Ferr.) (in Polish). Biul CLPT 1–4:7–25
Gangadevi T, Rao PN, Satyanarayana KV (1988) Cytogenetic studies of some synthetic amphiploids. J Hered 79(2):119–122
Gerstel DU (1980) Cytoplasmic male sterility in Nicotiana (A review). N C Agric Res Serv Tech Bull 263:1
Gerstel DU, Burns JA, Burk LG (1979) Interspecific hybridization with an African tobacco Nicotiana africana Merxm. J Hered 70:342–344
Goodspeed TH (1915) Parthenogenesis, parthenocarpy and phenospermy in Nicotiana. Calif Univ Publ Bot 5(8):249–272
Goodspeed TH (1954) The genus Nicotiana. Chronica Botanica Co., Waltham
Gopinath DM, Krishnamurthy KV, Krishnamurthy AS (1965) Cytological studies on interspecific hybrids in Nicotiana involving a new Australian species, Nicotiana amplexicaulis. Can J Genet Cytol 7:328–340
Heggestad HE (1966) Registration of Burley 1, Burley 2, Burley 11 A, Burley 11B, Burley 21, Burley 37 and Burley 49 tobaccos. Crop Sci 6:612–613
Henderson RG (1949) Vamorr 48 and 50: two new flue-cured varieties of tobacco resistant to mosaic and root rot, vol 427. Virginia Agricultural Experiment Station B, Virginia Polytechnic Institute, Blacksburg
Holmes FO (1938) Inheritance of resistance to tobacco-mosaic disease in tobacco. Phytopathology 28:553–561
Kaul MLH (1988) Male sterility in higher plants, vol 10., Monographs on theoretical and applied geneticsSpringer, Berlin
Kirov I, Divashuk M, Van Laere K, Soloviev A, Khrustaleva L (2014) An easy “SreamDrop” method for high quality plant chromosome preparation. Mol Cytogenet 7:21. doi:10.1186/1755-8166-7-21
Kitamura S, Inoue M, Ohmido N, Fukui K (1997) Identification of parental chromosomes in the interspecific hybrids of Nicotiana rustica L. × N. tabacum L. and N. gossei Domin × N. tabacum L., using genomic in situ hybridization. Breed Sci 47:67–76
Knapp S, Chase MW, Clarkson JJ (2004) Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 53(1):73–82
Kostoff D (1943) Cytogenetics of the genus Nicotiana. State Printing House, Sofia
Kubo T (1985) Studies on hybrid breeding by means of cytoplasmic male sterility in tobacco. Bull Iwata Tob Exp Stn 17:69–138
Kumashiro T, Oinuma T (1985) Comparison of genetic variability among anther derived and ovule derived doubled haploid lines of tobacco. Jpn J Breed 35:301–310
Laskowska D, Berbeć A (2003) Preliminary study of the newly discovered tobacco species Nicotiana wuttkei Clarkson et Symon. Genet Resour Crop Evol 50(8):835–839
Laskowska D, Berbeć A (2007) The new alloplasmic Nicotiana tabacum L. line with Nicotiana wuttkei Clarkson et Symon cytoplasm (in Polish). Biul Inst Hod Rośl 244:289–296
Laskowska D, Berbeć A (2012) Production and characterization of amphihaploid hybrids between Nicotiana wuttkei Clarkson et Symon and N. tabacum L. Euphytica 183:75–82
Lea HW (1999) Resistance of tobacco to pandemic blue mould Peronospora hyoscyami de Bary (syn. P. tabacina Adam): a historical overview. Aust J Exp Agric 39(1):115–118
Lewis RL (2011) Nicotiana. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Plantation and ornamental crops. Springer, New York, pp 185–208
Miller RD (1987) Registration of TN 86 burley tobacco. Crop Sci 27:365–366
Moav R, Cameron DR (1960) Genetic instability in Nicotiana hybrids. I. The expression of instability in N. tabacum x N. plumbaginifolia. Am J Bot 47(2):87–93
Modilevski J (1936) Cytogenetical investigation of the genus Nicotiana I. Cytology and embryology of the amphidiploid Nicotiana ditagla. J Inst Bot Acad Sci Ukr 7(15):21–39
Murthy TGK, Subbarao IV (2004) Some new interspecific hybrids in the genus Nicotiana: characterization and utilization. Tob Res 30:33–41
Naumenko SA (2012) Particular qualities of the development of flue-cured and burley tobacco varieties in Russia (in Russian). DSc Thesis, Russian Timiryazev State Agrarian University, Moscow
Nielsen MT, Collins GB (1989) Variation among androgenic and gynogenic doubled haploids of tobacco (Nicotiana tabacum). Euphytica 43:263–267
Nikova V, Vladova R (2002) Wild Nicotiana species as a source of cytoplasmic male sterility in Nicotiana tabacum. Beitr Tab 20:301–311
Nikova V, Zagorska N. (1987) Nicotiana maritima as a source of tobacco male sterility. Genet Sel 20:224–231 [CORESTA Inf Bul1 9873-4:224–231]
Nikova VM, Zagorska NA, Pundeva RS (1991) Development of four tobacco cytoplasmic male sterile sources using in vitro techniques. Plant Cell Tissue Organ Cult 27:289–295
Nikova V, Vladova R, Pundeva R, Shabanov D (1997) Cytoplasmic male sterility in Nicotiana tabacum L. obtained through interspecific hybridization. Euphytica 94(3):375–378
Nikova V, Pundeva R, Vladova R, Petkova A (2001) A new tobacco cytoplasmic male sterile source from the hybrid combination Nicotiana longiflora Gav. and N. tabacum L. using in vitro techniques. Isr J Plant Sci 49:9–13
Palakarcheva M, Dorossiev L (1983) Amphidiploids N. maritima x N. tabacum (4n = 80) and N. noctiflora x N. tabacum (4n = 72), a useful source of disease resistance. Rast Nauk 20(3):68–72
Pandey KK, Phung M (1982) “Hertwig effect” in plants: induced parthenogenesis through the use of irradiated pollen. Theor Appl Genet 62:295–300
Patel KA, Gerstel DU (1961) Additional information on the mechanism of chromosome substitution in Nicotiana. Tob Sci 5:19–20
Pittarelli GW, Sisson VA (1989) Registration of cytoplasmic male sterile tobacco germplasm Bel MS-2. Crop Sci 29(3):836–837
Reed SM, Collins GB (1978) Interspecific hybrid of Nicotiana through in vitro culture of fertilized ovules: N. stoctonii x N. tabacum, N. nesofila x N. tabacum, N. repanda x N. tabacum. J Hered 69:311–315
Renny-Byfield S, Kovarik A, Kelly LJ, Macas J, Novak P, Chase MW, Nichols RA, Pancholi MR, Grandbasien MA, Leitch AR (2013) Diploidization and genome size change in allopolyploids is associated with differential dynamics of low- and high-copy sequences. Plant J 74:829–839
Rufty RC (1989) Genetics of host resistance to tobacco blue mold. In: McKeen WE (ed) Blue mold of tobacco. American Phytopathological Society Press, St Paul, pp 141–164
Sarychev YF (1987) A new method of inducing diploid apomixis in Nicotiana tabacum L. Sov Genet 22:871–875
Schwarzacher T, Leitch AR, Bennett MD, Heslop-Harrison JS (1989) In situ localization of parental genomes in a wide hybrid. Ann Bot 64:315–324
Shilagyi L (1975) Elimination of chromosomes in an alloploid hybrid of Nicotiana tabacum × Nicotiana glauca. Acta Bot (Budapest) 21:433–441
Skalicka K, Lim KY, Matyasek R, Matzke M, Leitch AR, Kovarik A (2005) Preferential elimination of repeated DNA sequences from the paternal, Nicotiana tomentosiformis genome donor of a synthetic, allotetraploid tobacco. New Phytol 166:291–303
Smith HH (1941) Polyploidy in Nicotiana: discussion. Am Nat 75(759):307–309
Smith HH (1968) Recent cytogenetic studies in the genus Nicotiana. Adv Genet 14:1–43
Sybenga J (1992) Cytogenetics in plant breeding, vol 17., Monographs on theoretical and applied geneticsSpringer, New York
Ternovski MF (1934) Die Fragen der Immunitat bei Vertretern der Gattung Nicotiana. Der Zuchter 6:140–144
Van Laere K, Khrustaleva L, Van Huylenbroeck J, Van Bockstaele E (2010) Application of GISH to characterize woody ornamental hybrids with small genomes and chromosomes. Plant Breed 129(4):442–447
Wark DC (1970) Development of flue cured tobacco cultivars resistant to common strain of blue mold. Tobacco 171(16):19–22
Webber JM (1930) Interspecific hybridization in Nicotiana. XI. The cytology of a sesquidiploid hybrid between tabacum and sylvestris. Univ Calif Publ Bot 11:319–354
Williams E, Pandey KK (1974) Meiotic chromosome pairing in interspecific hybrids of Nicotiana II. South American species hybrids: the influence of genotype on pairing. N Z J Bot 13:611–622
Zhou WM, Yoshida KT, Takeda G (1997) Studies on hybrid inviability of interspecific hybridization in Nicotiana. III. Production of sesquidiploid and amphidiploid in Nicotiana tabacum and N. repanda crosses and the view on hybrid inviability. Chin Agron J 7:181–191
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laskowska, D., Berbeć, A., Van Laere, K. et al. Cytology and fertility of amphidiploid hybrids between Nicotiana wuttkei Clarkson et Symon and N. tabacum L.. Euphytica 206, 597–608 (2015). https://doi.org/10.1007/s10681-015-1459-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1459-3