Abstract
To enrich the source of germplasm of cultivated olive (Olea europaea subsp. europaea L.), inter-subspecific hybrid plants have been produced by experimental crosses between several varieties of cultivated olive and Asian and African accessions of the wild related subspecies cuspidata. Germination of putative hybrid seeds was enhanced by using in vitro embryo culture. The genetic make-up of germinated seedlings was assayed with the aid of both AFLP and SSR molecular markers and their hybrid nature was proved by the presence of male-specific alleles in their molecular patterns. Most of the parent specific alleles showed segregation among F1 progenies indicating high heterozygosity content of the parental lines. The majority of the hybrids derived from crosses in which an African accession of cuspidata was used as female parent. The overall morphological aspect of hybrids resembled that of the female parent. The production of inter-subspecific hybrid plants in Olea is discussed in relation to the genetic improvement of cultivated olive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cultivated olive (Olea europaea subsp. europaea L.) is one of the oldest, most widespread and economically important crops of the Mediterranean basin. Taxonomically, it belongs to the O. europaea complex, which includes five additional non cultivated subspecies widely distributed in Asia and Africa (Green and Wickens 1989; Green 2002). It is generally assumed that olive domestication began in the Near East around 6,000 years ago and spread to the western Mediterranean region following human migrations. The modern cultivars originated from recurrent hybridisation events between wild and cultivated Mediterranean genotypes (Besnard et al. 2013 and references therein). Although the autoctonous cultivars are well adapted to their environment and suited for long term established cultivation practices, in many cases these fail to respond adequately to the requirements of modern oliviculture (Lavee 2013). Furthermore, most of them are not resistant to insect and fungal diseases (Rugini 1986). For these reasons there is a need for new varieties characterized by resistance to major pests and abiotic stresses, early production without alternation, suitability for intensive cultivation and mechanized harvesting, and production of high quality oil and fruits (Bellini et al. 2008).
Implementation of olive breeding programs via inter-subspecific crosses might represent a useful strategy to exploit the enormous gene pool represented by the wild O. europaea subspecies (Rugini and Gutiérrez 2006; Rugini et al. 2011). As an example, the geographical isolation and adaptation to particular environmental conditions of some wild O. europaea subspecies led them to evolve an extent of inbreeding that might be a useful trait to be introgressed into cultivated olive trees in the perspective to develop self compatible new cultivars (Lavee 2013). Finally, the use of partially inbred plants belonging to other subspecies as parents in olive cross-breeding programs might exploit heterosis, leading to vigorous hybrids, as already reported in cultivated olive (Biton et al. 2012). O. europaea subsp. cuspidata, named Brown, or African or Indian olive, widespread in China, India as well as in North, East and South Africa, appears a promising candidate as source of useful traits for olive cultivars. It is adaptable to different climates, ranging from semi-arid to meso-humid ones, and shows resistance to fungal diseases (Hannachi et al. 2009). The high adaptability of O. cuspidata is witnessed in Australia where, from its introduction (in the mid of the 19th century) for horticultural purposes, is now considered an invasive and potentially dangerous weed (Spennemann and Allen 2000; Cuneo and Leishman 2006; Besnard et al. 2014). On the other hand, its wood is much appreciated in Africa for multiple purposes (Negash 2003).
Genetic affinity between cultivated O. europaea and cuspidata subspecies has been witnessed by the occurrence of spontaneous hybridisation events arisen in sympatrically grown natural populations or in the nearness of olive groves (Costa 1998; Belaj et al. 2001, 2004; Besnard et al. 2007; Omrani-Sabbaghi et al. 2007; Hannachi et al. 2009; Besnard et al. 2014). However, experimental evidences of sexual compatibility between the two subspecies are still lacking. The only documented experimental hybrids between olive and a wild form of the same genus were reported by Besnard et al. (2001). These authors characterised by means of low efficient RAPD markers, 14 putative hybrids between O. europaea subsp. europaea and O. africana (actually O. europaea subsp. cuspidata, Green and Wickens 1989) obtained in 1984 by Villemur from unspecified parents (cf. Besnard et al. 2001). On the other hand, hybrids between cultivated olive and any of the other subspecies of the same genus have never been found (Hannachi et al. 2009 and references therein).
On account of the preceding, we carried out experimental crosses by using cultivars of O. europaea and subspecies cuspidata plants as parents, with the dual purpose of i) verifying the genetic affinity between the two entities and ii) exploiting new sources of genetic variability for the genetic improvement of olive cultivars. Furthermore, it has been recently shown that some multigene characters are dominantly inherited from female parents in inter-varietal crosses (Lavee and Avidan 2011). Therefore, the direction of the crosses is crucial if a particular multigene trait should be transferred from one donor to a specific receiver parent. On this basis, several parental combinations in different cross directions were undertaken. The hybrid nature of the seeds obtained was corroborated with the aid of robust molecular markers. To overcome the known difficulties of olive seed germination (Cañas et al. 1987; Garcia et al. 2002), putative hybrid seeds were subjected to embryo culture that allows to obtain an higher percentage of germination in shorter time, thereby avoiding loss of potentially valuable hybrid genotypes (Istambouli and Neville 1977; Rugini 1986).
Materials and methods
Plant material and cross procedure
The plants of the cultivars and subspecies of O. europaea used in the crossing program were bred in pots according to the routine practices in greenhouse conditions at ISAFOM-CNR (Perugia, Italy). Genotypes of the subspecies cuspidata came from the areas of Himachal Pradesh (India), Sichuan (China), and from South Africa (accession A from Cape Town, and B from Morgenster). Olive varieties commonly cultivated in Umbria (Italy) chosen as parents, were Dolce Agogia, Frantoio, Leccino and Fs 17 (Fontanazza et al. 1998). Parent combinations and cross directions are reported in Table 1. Flowers of maternal parents were emasculated with the aid of fine scissors and their pistils were dusted with pollen collected at maturity from the paternal parent. Pollinated flowers were then bagged with plastic membranes (DuPont™Tyvek®; Smith and Mehlenbacher 1994) and allowed to set seeds in isolation. Open and self pollination seeds from both African and Asian cuspidata accessions, and from Fs 17 and Dolce Agogia cultivars, were collected as control (cf. Table 1).
Embryo culture and growth conditions
Immediately after collection, the stones were separated from flesh of mature fruits and kept in 2 % sodium hydroxide for 4 h, rinsed in H2O and left in tap water for about 2 h, then allowed to dry.
For embryo isolation, seeds isolated from sclerified endocarp were sterilized by dipping in 70 % ethanol for 3 min and then in 20 % sodium hypochlorite for 20 min. Finally, they were rinsed several times in sterile distilled water and stored in sterile Petri dishes containing filter paper imbibed with 5 ml of sterile distilled water. The number of stones containing more than one seed was recorded. After 2 days of imbibition at room temperature, embryos were dissected aseptically under stereomicroscope by cutting off two lateral sections of the endosperm and freeing the embryo from the remaining seed tissues (Acebedo et al. 1997; Germanà et al. 2009).
The embryos were then cultured individually in sterile test tubes containing 10 ml of culture medium according to Rugini (1988), closed with plastic caps and sealed with Parafilm®. To avoid germplasm loss, even the embryos that had lost hypocotyls during handling were subjected to in vitro culture. The cultured embryos were incubated in a controlled environment growth chamber at 23 ± 1 °C under a 16/8-h (day/night) photoperiod with fluorescent light at the intensity of 40 µE m−2 s−1, and weekly monitored. The opening of cotyledons and emergence of epicotyl were taken as evidence of germination, whereas the development of the hypocotyl and primary roots were not deemed essential to consider the embryo geminated. The shoots were cultured in OM medium according to Rugini (1984) and then transferred on a rooting medium (Mencuccini 2003). The hardening phase was carried out as described by Rugini (1984). Surviving plantlets were transplanted into pots and bred in the greenhouse according to the routine culture practices. The left stones derived from self and open pollination fruits not used for embryo culture (see Table 2) were mechanically scarified, sowed on trays containing potting soil and bred in the greenhouse.
DNA isolation, AFLP and SSR procedures
Genomic DNA was purified from leaf tissue (100 mg) using the DNeasy Plant Mini Kit (Qiagen), following the manufacturer’s instructions. DNA concentration, purity and integrity were assessed by both spectrophotometric (Cary 100 Scan, Varian) and electrophoretic analyses. DNA concentrations were adjusted to 100 ng/µl in all samples.
AFLP procedure was as described by Labombarda et al. (2002) with minor modifications. Four hundreds (instead of 500) ng from each of 20 DNA samples (15 putative hybrids and 5 related parental lines) were restriction-ligated using 50 pmol (instead of 30) of MseI adapters. Pre- and selective amplifications were carried out as reported (Labombarda et al. 2002) using the combinations FEcoCAA/MseCCA and FEcoCAC/MseCGC as selective primers. AFLP amplicons were separated on a 6 % denaturing polyacrylamide gel and visualized in the Genomyx SC Scanner (Beckman). The pictures were collected as a TIFF image and the grey levels were adjusted manually by using the software Adobe Photoshop CS5 version 12.0 to enhance the sharpness of the images. Only clear and reproducible bands were considered and scored as 1 or 0 (for band presence or absence, respectively).
Three simple sequence repeat (SSR) markers, DCA5, DCA9 and GAPU103A developed in O. europaea subsp. europaea (Sefc et al. 2000; Carriero et al. 2002) and tested for their effectiveness (Baldoni et al. 2009) were used. PCR amplifications were performed in a volume of 25 µl containing 25 ng of template DNA, 5 µl of 5 × Colorless Go Taq® Flexi Buffer, 2 mM MgCl2, 0.3 µM of 5′ fluoresceine-labelled primer, 0.3 µM of 3′ primer, 0.5 mM of each dNTPs and 1.25 U of Go Taq® Hot Start Polymerase (Promega). Amplifications were performed on a 2720 Thermal Cycler (Applied Biosystems) using the following profile: an initial denaturation step of 95 °C for 5 min; 35 cycles of 20 s at 95 °C, 20 s at annealing temperature specific of each locus, 30 s at 72 °C; a final elongation step of 1 h at 72 °C. Amplicon lengths were detected loading 0.5 µl of each fluorescent sample mixed with 0.25 µl of LIZ 500 size standard (Applied Biosystems) up to a final volume of 10 µl with formamide. The DNA fragments were denatured and size fractioned using capillary electrophoresis on an ABI 310 Genetic Analyzer (Applied Biosystems). The software GeneMapper 4.0 (Applied Biosystems) was used to estimate allele size.
All the molecular analyses were repeated twice.
Results and discussion
Crossings
The results of 20 experimental crosses each involving an olive cultivar and a cuspidata accession as parents (cf. Table 1) are reported in Table 2. The limited pollen availability from poorly flowering cuspidata plants hampered to carry out some reciprocal crosses, thereby forcing us to use some genotypes instead of others as seed parent. From a total of 2,713 cross pollinated flowers, 65 fruits were obtained corresponding to an average fruit set of 2.47 %. Eight cross combinations did not yield any fruit. In most of the unsuccessful crosses, the female parent was a plant either from India or China cuspidata accessions. This could be due to the high rate of ovule abortion observed in the same accessions (data not shown). Conversely, the highest percentages of fruit set were obtained when the female parents were the African cuspidata accession B (C5 and C6) and cv. Dolce Agogia (C16 and C17). However, the seeds produced in C5, C6 and C7 cross combinations contained not viable embryos probably due to post-zygotic incompatibility. Moreover, a strict pre-zygotic genome incompatibility between African cuspidata accession A and cv. Fs 17 (C2 and C19) was recorded, as in neither parent directions fruit set was obtained. The cross combination cuspidata India and cv. Fs 17 was carried out in both directions too (C14 and C20). A nuclear-cytoplasmic incompatibility does exist between the two genomes because fruits were obtained only when cv. Fs 17 was used as maternal parent. Similar explanation could be given for the cross combinations involving African cuspidata accession A and cv. Dolce Agogia (C1 and C16). As a matter of fact, when cuspidata accession A was used as maternal plant, a hybrid was obtained from one of the two viable embryos (the other did not survive beyond the seedling stage), while a single individual arisen from self fertilization was obtained in the reciprocal cross (as showed by the molecular analyses, see below).
A poor rate of fruit set was obtained through self pollination in African accessions A and B (0.56 and 0.28 %, respectively), and in Asian accessions (China 0 %, India 0.3 %; Table 2) of cuspidata. In cultivars Dolce Agogia (S5) and Fs 17 (S6), both characterized by a variable extent of self compatibility (Fontanazza and Baldoni 1990; Fontanazza et al. 1998) this parameter raised up to over 5 % as expected.
Overall, higher percentage of fruit set was obtained through open pollination (Table 2). An average of 8.54 % was shown by both African cuspidata accessions (OP1 and OP2), 1.92 % by Asian accessions (OP3 and OP4), 10.95 % by cultivars Dolce Agogia (OP5) and Fs 17 (OP6). Either African or Asian accessions yielded very poor fruit set from self pollination, that could be due to a common high rate of self incompatibility expressed in the environment in which the crosses have been carried out. However, differently from the African accessions, the Asian ones showed also poor open pollination fruits, due to the high rate of observed ovule abortion (see above) likely depending on its scarce environmental adaptation.
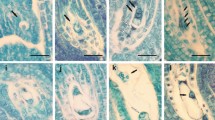
Stones containing more than one seed were frequently produced by crosses C3, C4, C6, and C7, involving both A and B African cuspidata accessions as female parent (Table 2; Fig. 1a, b). In olive cultivars, only one out of the four ovules, each containing an embryo sac, is usually fertilized whereas the others degenerate (Altamura Betti et al. 1982). As cyto-embryological studies in cuspidata are lacking, we assume that the multiple seeds observed in the stones analysed were likely arisen from fertilization of more than one embryo sac. Furthermore, about one third of the fruits produced by cross combination C5 (including African accession B as maternal parent) were parthenocarpic or characterised by an anomalous seed development (Table 2; Fig. 1c, d).
Transversal sections of stones containing two (a) or three (b) seeds, produced by crosses C3 and C4, respectively. Bar 5 mm. Longitudinal median sections of two fruits produced by cross C5 showing a parthenocarpic (c) or abnormally developed (d) seed. Bar 2.5 mm. e C3 well rooted seedling after 40 days of embryo culture. f C1 non rooted seedling after 40 days of embryo culture. g Eighteen month old plants in pots after the hardening phase. Dolce Agogia (1) and subsp. cuspidata A (2) plants from seeds self pollination-obtained; hybrid C16-1 obtained by crossing Dolce Agogia as maternal parent and subsp. cuspidata accession A as paternal parent (3)
To sum up, although variable rates of anomalies were observed on fruits derived from crosses involving African accessions, on the basis of the high rate of viable hybrid embryos obtained (see below) we are in the position to recommend these accessions as suitable female parents in crosses with cultivated olive.
Some stones obtained from open pollination were sowed in soil to analyse the germination in cuspidata, a subspecies for which this information is scarce. The seed sample OP1 showed a percentage of germination equal to 0.35 %, while seeds derived from OP2, OP3 and OP4 did not germinate at all. About 10 % of seeds derived from cvs. Dolce Agogia and Fs 17 (OP5 and OP6, respectively) showed active germination: a value similar to that reported for other olive cultivars (Acebedo et al. 1997).
Embryo culture
All the cross-derived seeds were subjected to embryo culture. Few seeds obtained from open- and self-pollination involving both African and Asian cuspidata accessions as well as cultivars Dolce Agogia and Fs 17 were also subjected to embryo culture as control (Table 2).
Thirty, 4 and 19 actively growing embryos were obtained after 15 days of culture from 56, 6 and 31 cultured embryos which were extracted from seeds derived from cross, self and open pollinations, respectively (Table 2). The majority (63.33 %) of the 30 cross-derived viable embryos arose from crosses in which African cuspidata accession A was used as female parent. After 40 days, six embryos out of 30 showed a well-developed root system together with a vigorous shoot (Fig. 1e), whereas the others underwent a delayed development (Fig. 1f). After 90 further days of culture, nineteen well-developed plantlets from cross-derived seeds (C3, C4, C9, C16, C17, C18, and C20) were obtained. These were transferred in pots and maintained in the greenhouse for further growth, along with some self and open pollination-derived individuals as control. The remaining putative hybrids were considered not viable because they never reached a full development stage. This could be due to post-zygotic barriers, or even to the use of a culture medium unsuitable for subspecies cuspidata. Nine (47.37 %) out of the 19 successfully obtained plantlets derived from crosses involving African cuspidata accession A as female parent (cf. Table 4). This percentage value allows us to suggest that, among the accessions analysed, subsp. cuspidata A is the best candidate to cross with subsp. europaea in order to obtain viable embryos and plantlets.
AFLP analysis
Table 3 shows the results of AFLP analysis carried out on 15, early germinated, putative hybrids by using DNA extracted from leaflets of six-week-old plants. Two primer combinations were tested on hybrids belonging to the C1 (1 hybrid), C3 (6 hybrids), C4 (5 hybrids) and C17 (3 hybrids) cross combinations together with their related parental lines. A total of 463 scorable bands, with an average of 115.75 bands per parent combination, were obtained. Of these, 161 (34.77 %) were considered not informative because monomorphic between parents. Out of 286 polymorphic bands, 185 female- and, in particular, 101 male-specific bands were highly informative to assess the hybrid nature of cross-derived plants. Of the male-specific bands, 34 were not transmitted to any of the progenies, 8 were inherited by the unique putative hybrid of the cross C1, 4 were inherited by the whole progeny (not segregating, Table 3) of each of the two crosses C3 and C4, and 55 bands segregated in all crosses for which more than one putative hybrid was considered. The majority of parent specific bands (62.59 %) showed segregation in crosses C3, C4 and C17 indicating high level of heterozygosity of parental lines. Part of the AFLP profile obtained with the primer combination FEcoCAA/MseCCA in all the tested individuals is reported in Fig. 2: bands with asterisks are male-specific alleles transmitted to the progenies. All the putative hybrids showed one or more male-specific bands with the exception of individuals C3-3, C3-6 and C17-3 for which some bands, although present, appeared very faint. Such uncertainty on the hybrid nature of these individuals was ruled out by the results showed in Fig. 3, in which the male inherited bands were clearly detectable (asterisks). Moreover, 16 non parental bands were observed in the AFLP patterns of hybrid plants (Table 3). Non parental, PCR-amplified bands were frequently observed in interspecific crosses in plants (Yin et al. 2002; Luo et al. 2002) and in segregating populations of Populus (Wu et al. 2000), Pinus (Cato et al. 1999) and Phaseolus (Muñoz et al. 2004). Most of these non parental bands are believed to originate from heteroduplex molecules formed between sequences showing partial matching coming from both parents (Ayliffe et al. 1994), thereby constituting a further evidence of hybridity.
AFLP band patterns of female (1) and male (2) parents, and individuals forming their progenies (from 3 to 8) in the C3 and C17 crosses. The AFLP patterns were obtained by using the primer combination FEcoCAC/MseCGC. Asterisks indicate the male-specific bands inherited by C3-3, C3-6, and C17-3 plants showing their hybrid nature
SSR analysis
SSR analysis was undertaken to confirm the hybrid nature of the plants screened with AFLP and to analyse additional putative hybrids that developed with delay compared to the previous ones. Three SSR markers, DCA5, DCA9 and GAPU103A, were used and the microsatellite profiles of the hybrids were compared with those of their related parental lines. Eleven hybrids previously analysed by means of AFLP, together with 7 hybrids generated from 4 new cross combinations (C9, C16, C18, and C20) and an additional delayed hybrid from the C3 cross, were subjected to SSR analysis (Table 4). The allele sizes were compared to those reported in Oleadb (www.oleadb.it), a database collecting information about SSR markers in olive cultivars. Minor variations were recorded between our material and the database. The most remarkable difference concerned the locus GAPU103A, showing a biallelic pattern in cv. Dolce Agogia (172–176 bp) as analysed by us, instead of the reported monoallelic one (174 bp). This discrepancy, like others in allele length that we consider less significant, could be addressed to the different resolution power of the instrument and the standard size used, as already stated by Baldoni et al. (2009). Anyway, the same locus showed a null allele in all the cuspidata accessions, thereby rendering this marker highly informative for hybridity test when these accessions were used as maternal parent.
SSR analysis thereby confirmed the hybrid nature of the individuals previously analysed with AFLP. Furthermore, seven (C3-1, C9-1, C16-1, C18-1, C20-1, C20-2, C20-3) out of the additional cross-derived individuals tested were also considered hybrid on the basis of the presence of paternal specific SSR alleles, whereas the individual C20-4 was probably arisen from self pollination.
Morphological analysis
A preliminary evaluation of the morphological aspect of the hybrids was carried out after 18 months of their maintenance in greenhouse. The overall morphological habit, and in particular plant height and leaf morphology, did not differ from that of the maternal parent while branching was more similar to the paternal one (Fig. 1g). Other morphological traits, including those presumably related to heterotic effects, have not been noticed because the hybrid plants are still in juvenile phase.
Conclusions
The use of the molecular markers allowed us to assess the hybrid nature of 23 genotypes obtained by crossing O. europaea subsp. europaea with subsp. cuspidata. Four of them did not survive the hardening phase. Among all the cross derived seedlings analysed, only one resulted a self pollination product. The remaining eighteen plants are vigorously and actively growing. They represent the first example of intraspecific hybrids obtained from different subspecies of O. europaea and for which a robust evidence of hybridity, based on highly efficient molecular markers of different nature, was provided. The obtainment of these plants demonstrated the feasibility of inter-subspecific crosses in O. europaea. Moreover, the possibility to obtain hybrids in multiple cross directions opens interesting opportunities to exploit alternative nucleus-cytoplasm interactions aimed at the production of specific superior genotypes. Hybrids are under observation to assess the inheritance of traits from the progenitors.
The possibility to use these hybrids in olive breeding will depend on their genetic stability in terms of both male and female gametophyte development. The formation of balanced gametes (gametes with the appropriate haploid chromosome set) is a key factor influencing hybrid fertility and therefore the transfer of traits to the progenies. These parameters, together with morphological traits related to fruit production, will be analysed as soon as the hybrids will reach maturity.
References
Acebedo MM, Lavee S, Liñán J, Troncoso A (1997) In vitro germination of embryos for speeding up seedling development in olive breeding programmes. Sci Hort 69:207–215
Altamura Betti MM, Pasqua G, Mazzolani G (1982) Embryogenesis in Olea europaea L. Ann Bot (Roma) 40:141–152
Ayliffe MA, Lawrence GL, Ellis JG, Pryor AJ (1994) Heteroduplex molecules between allelic sequences cause nonparental RAPD bands. Nucleic Acids Res 22:1632–1636
Baldoni L, Cultrera NG, Mariotti R, Ricciolini C, Arcioni S et al (2009) A consensus list of microsatellite markers for olive genotyping. Mol Breed 24:213–231
Belaj A, Trujillo I, De La Rosa R, Rallo L, Gimenez MJ (2001) Polymorphism and discrimination capacity of randomly amplified polymorphic markers in an olive germplasm bank. J Am Soc Hort Sci 126:64–71
Belaj A, Rallo L, Trujillo I, Baldoni L (2004) Using RAPD and AFLP markers to distinguish individuals obtained by clonal selection of ‘Arbequina’ and ‘’Manzanilla de Sevilla’ olive. Hort Sci 39:1566–1570
Bellini E, Giordani E, Rosati A (2008) Genetic improvement of olive from clonal selection to cross-breeding programs. Adv Hort Sci 22:73–86
Besnard G, Baradat P, Chevalier D, Tagmount A, Bervillé A (2001) Genetic differentiation in the olive complex (Olea europaea) revealed by RAPDs and RFLPs in the rRNA genes. Genet Resour Crop Evol 48:165–182
Besnard G, Henry P, Wille L, Cooke D, Chapuis E (2007) On the origin of the invasive olives (Olea europaea L., Oleaceae). Heredity 99:608–619
Besnard G, Khadari B, Navascués M, Fernández-Mazuecos M, El Bakkali A et al (2013) The complex history of the olive tree: from Late Quaternary diversification of Mediterranean lineages to primary domestication in the northern Levant. Proc R Soc B 280:20122833
Besnard G, Dupuy J, Larter M, Cuneo P, Cooke D, Chikhi L (2014) History of the invasive African olive tree in Australia and Hawaii: evidence for sequential bottlenecks and hybridization with the Mediterranean olive. Evol Appl 7:195–211
Biton I, Shevtsov S, Ostersetzer O, Mani Y, Lavee S, Avidan B, Ben-Ari G (2012) Genetic relationships and hybrid vigour in olive (Olea europaea L.) by microsatellites. Plant Breed 31:767–774
Cañas LA, Carramolino L, Vicente M (1987) Vegetative propagation of the olive tree from in vitro cultured embryos. Plant Sci 50:85–90
Carriero F, Fontanazza G, Cellini F, Giorio G (2002) Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.). Theor Appl Genet 104:301–307
Cato SA, Corbett GE, Richardson TE (1999) Evaluation of AFLP for genetic mapping in Pinus radiata D. Don. Mol Breed 5:275–281
Costa C (1998) Olive production in South Africa. Agricultural Research Council, Pretoria, A handbook for olive growers, p 124
Cuneo P, Leishman MR (2006) African Olive (Olea europaea subsp. cuspidata) as an environmental weed in eastern Australia: a review. Cunninghamia 9:545–557
Fontanazza G, Baldoni L (1990) Proposta per un programma di miglioramento genetico dell’olivo. Olivae 34:32–39
Fontanazza G, Bartolozzi F, Vergari G (1998) Fs 17. Riv Frutticol 5:61
Garcia JL, Troncoso J, Sarmiento R, Troncoso A (2002) Influence of carbon source and concentration on the in vitro development of olive zygotic embryos and explants raised from them. Plant Cell Tiss Organ Cult 69:95–100
Germanà MA, Patricolo G, Chiancone B (2009) In vitro germination of stoneless seeds and isolated embryos of 14 sicilian cultivars of Olea europaea L. In: Hanke M-V, Dunemann F, Flachowsky H (eds) I International Symposium on Biotechnology of Fruit Species: BIOTECHFRUIT 2008. Acta Hort 839:173–180
Green PS (2002) A revision of Olea L. (Oleaceae). Kew Bull 57:91–140
Green PS, Wickens GE (1989) The Olea europaea complex. In: Tan K (ed) The davis and hedge festschrift. Edinburg University Press, Edinburgh, pp 287–299
Hannachi H, Sommerlatte H, Breton C, Msallem M, El Gazzah M, Ben El Hadj S, Bervillé A (2009) Oleaster (var. sylvestris) and subsp. cuspidata are suitable genetic resources for improvement of the olive (Olea europaea subsp. europaea var. europaea). Genet Resour Crop Evol 56:393–403
Istambouli A, Neville P (1977) Etudé de la “dormance” des semences d’olivier (Olea europaea L.). Mise en evidence d’une dormance embryonaire. C R Acad Paris Set D 284:2503–2506
Labombarda P, Busti A, Cáceres ME, Pupilli F, Arcioni S (2002) An AFLP marker tightly linked to apomixis reveals hemizygosity in a portion of the apomixis controlling locus in Paspalum simplex. Genome 45:1–7
Lavee S (2013) Evaluation of the need and present potential of olive breeding indicating the nature of the available genetic resources involved. Sci Hortic 161:333–339
Lavee S, Avidan B (2011) Heredity diversity in populations of free-, self-, and specific cross-pollinated progenies of some olive (Olea europaea L.) cultivars. Israel J Plant Sci 59:29–37
Luo S, He P, Zheng X, Zhou P (2002) Inheritance of RAPD markers in an interspecific F1 hybrid of grape between Vitis quinquangularis and V. vinifera. Sci Hortic 93:19–28
Mencuccini M (2003) Effect of medium darkening on in vitro rooting capability and rooting seasonality of olive (Olea europaea L.) cultivars. Sci Hortic 97:129–139
Muñoz LC, Blair W, Duquea MC, Tohmea J, Rocab W (2004) Introgression in common bean × tepary bean interspecific congruity-backcross lines as measured by AFLP markers. Crop Sci 44:643–645
Negash L (2003) Vegetative propagation of the threatened African wild olive [Olea europaea L. subsp. cuspidata (Wall. ex DC.) Ciferri]. New Forest 26:137–146
Omrani-Sabbaghi A, Shahriari M, Falahati-Anbaran M, Nankali A, Gharehyazei B (2007) Microsatellite markers based assessment of genetic diversity in Iranian olives (Olea europaea L.) collections. Sci Hortic 112:439–447
Rugini E (1984) In vitro propagation of some olive (Olea europaea sativa L.) cultivars with different root-ability, and medium development using analytical data from developing shoots and embryos. Sci Hortic 24:123–134
Rugini E (1986) Olive (Olea europaea L.). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry. Vol 1. Trees. Springer-Verlag, New York, pp 253–267
Rugini E (1988) Somatic embryogenesis and plant regeneration in olive (Olea europaea L.). Plant Cell Tiss Organ Cult 14:207–214
Rugini E, Gutiérrez P (2006) Genetic improvement of olive. Pomol Croat 12:43–74
Rugini E, De Pace C, Gutiérrez-Pesce P, Muleo R (2011) Olea. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Temperate fruits. Springer-Verlag, Berlin, Heidelberg, pp 79–118
Sefc KM, Lopes MS, Mendonca D, Dos Santos MR, Machado MLD, Machado AD (2000) Identification of microsatellite loci in olive (Olea europaea) and their characterization in Italian and Iberian olive trees. Mol Ecol 9:1171–1193
Smith DC, Mehlenbacher SA (1994) Use of Tyvek housewarp for pollination bags in breeding hazelnut (Corylus avellana L.). Hort Sci 29:918
Spennemann DHR, Allen LR (2000) Feral olives (Olea europaea) as future woody weeds in Australia: a review. Austral J Exp Agr 40:889–901
Wu RL, Han YF, Hu JJ, Fang JJ, Li L et al (2000) An integrated genetic map of Populus deltoides based on amplified fragment length polymorphisms. Theor Appl Genet 100:1249–1256
Yin T, Zhang X, Huang M, Wang M, Zhuge Q et al (2002) Molecular linkage maps of the Populus genome. Genome 45:541–555
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cáceres, M.E., Ceccarelli, M., Pupilli, F. et al. Obtainment of inter-subspecific hybrids in olive (Olea europaea L.). Euphytica 201, 307–319 (2015). https://doi.org/10.1007/s10681-014-1224-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1224-z