Abstract
Late leaf spot (LLS), caused by Phaeoisariopsis personata, is an important foliar fungal disease of groundnut (Arachis hypogaea L.), which causes significant economic losses globally to the crop. Inheritance of resistance to LLS disease was studied in three crosses and their reciprocals involving two resistant interspecific derivatives and a susceptible cultivar to refine strategy for LLS resistance breeding. The traits associated with LLS resistance, measured both in the field and under controlled conditions were studied following generation mean analysis. Results suggested that resistance to LLS is controlled by a combination of both, nuclear and maternal gene effects. Among nuclear gene effects, additive effect controlled majority of the variation. In JL 24 × ICG 11337 cross and its reciprocal only additive effects were important, while in JL 24 × ICG 13919 cross and its reciprocal, both additive and dominance effects contributed to the variation. Among digenic epistatic effects, additive × dominance interactions were significant. Additive–maternal effects were significant in both the crosses, while dominance–maternal effects also contributed to the variation in the crosses between the parents, JL 24 and ICG 13919. Due to significant contribution of additive effects of both nuclear and maternal inheritance to resistance to LLS, the parent, ICG 11337 would be a good donor in breeding programs. It would be worthwhile to use the resistance donor as female parent to tap maternal effects of resistance to LLS. Disease score is the best selection criterion in the field for use in breeding programs because of its high heritability and ease in measurement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundnut (Arachis hypogaea L.), an annual leguminous oilseed crop, is valued as a rich source of high quality edible oil, protein, minerals and vitamins. In 2009, it was grown in 23.95 million ha with a production of 36.45 million tons globally (FAOSTAT 2010). It is cultivated primarily in the semi-arid tropical regions of Asia and Africa, which together account for over 96 % of world groundnut area and 92 % of total global groundnut production. Groundnut belongs to the family Fabaceae (Leguminosae). It is an allotetraploid (2n = 2x = 40) with ‘A’ and ‘B’ genomes, contributed by diploid progenitors, A. duranensis and A. ipaensis, respectively (Kochert et al. 1996). Southern Bolivia and Northern Argentina are thought to be the center of origin of this crop (Gregory et al. 1980; Kochert et al. 1996). The genetic diversity of the genus is classified into four gene pools (Singh and Simpson 1994). Groundnut is a self-pollinated crop with cleistogamous flowers and the breeding methods used for self-pollinated crops are applied in its breeding.
Foliar fungal diseases are the major production constraints of groundnut crop globally. Of these, late leaf spot (LLS), caused by Phaeoisariopsis personata (Berk. & M.A. Curtis) van Arx, is a major and widely distributed disease. It can cause total defoliation and reduce pod and fodder yields to an extent of over 50 % and affect adversely quality of its produce (Subrahmanyam et al. 1984; Waliyar 1991). Chemical control measures are available but they increase production costs by 10 % (Coffelt and Porter 1986) and are beyond the reach of small and marginal farmers, who are the major producers of this crop. Therefore, development and adoption of resistant cultivars is the best option as it minimizes losses at farm level and maintains good product quality (Dwivedi et al. 1993).
Several sources of resistance to LLSs have been identified in groundnut (Subrahmanyam et al. 1982; Walls et al. 1985; Anderson et al. 1993; Waliyar et al. 1993; Singh et al. 1997). These genotypes include both wild and cultivated Arachis species and their interspecific derivatives. Resistance in cultivated types is associated with low yield, poor pod and kernel characteristics and late maturity thus making breeding for LLS resistance long drawn and complex (Subrahmanyam et al. 1995; Mehan et al. 1996; Singh et al. 1997). Sporulation, lesion size, lesion number and latent period are important components of resistance to LLS (Chiteka et al. 1988; Waliyar et al. 1993; Aquino et al. 1995). Disease score, which is primarily based on percentage defoliation, integrates all components of resistance and their optimum combination brings out the lower score (Dwivedi et al. 2002).
Both, simple and complex inheritance of resistance to LLS is reported in literature. Tiwari et al. (1984) and Motagi et al. (2000) reported a duplicate complementary recessive genes model and Nevill (1982) speculated a 5-gene model with significant non-additive gene action for inheritance of resistance to LLS in cultivated types. Sharief et al. (1978) reported high heritability estimates (81.7–92.9 %) for LLS disease index in three wild Arachis species. The variation in host reaction to LLS in their F2 populations was ascribed to multifactorial genetic differences. Coffelt and Porter (1986) suggested involvement of cytoplasmic factor and additive gene effects as they observed differences in reciprocal cross populations of a cross between two cultivated types. From generation mean analysis of three crosses, Jogloy et al. (1999) reported that additive and dominance gene actions were important in two crosses and in the third cross in addition to additive and dominance gene actions, epistasis (additive × additive) was also important. From their diallel study, Jogloy et al. (1987) observed highly significant general combining ability for most of the components of LLS resistance. However, they reported only low to moderate (13–68 %) broad sense heritability and low narrow sense heritability (0–12.8 %) for them. They found selection for LLS resistance in F2 population ineffective. From their field studies, Anderson et al. (1986a) also found general combining ability, attributed largely to additive genetic variance, responsible for most parameters of LLS resistance. They also found reciprocal effects significant. From their detached leaf study, Anderson et al. (1986a) reported moderate to high (40–80 %) broad sense heritability for the resistance components of LLS. Kornegay et al. (1980) also reported additive genetic effects responsible for resistance to LLS fungus and minimal leaf defoliation in F1 and F2 generations of two cultivated Virginia types. Transcripts involved in resistance responses to LLS were identified and these genes were found to be more greatly expressed in resistant genotypes as a result of response to the challenge by the pathogen (Luo et al. 2005). Quantitative trait loci (QTL) analysis based on phenotyping for the disease score detected minor QTLs for resistance to LLS accounting for <10 % phenotypic variability (Khedikar et al. 2010).
Breeding efforts to develop LLS resistant varieties of groundnut have led to the development of high yielding varieties with moderate levels of resistance to the disease. There is a need to further improve the levels of resistance to LLS so that the new varieties could withstand disease pressure, particularly in the case of disease epidemics or in disease endemic areas. A good knowledge on genetics of resistance will enable breeders to design an efficient breeding strategy. The currently available interspecific derivatives of groundnut carry high level of resistance to LLS with acceptable pod and seed traits, and good agronomic potential but they are late maturing. They offer a great opportunity to enhance the levels of resistance in breeding populations. Thus the objective of this study was to discern the genetic basis of LLS resistance in interspecific derivatives of groundnut under both field and controlled conditions.
Materials and methods
Two LLS resistant interspecific germplasm lines obtained from ICRISAT gene bank, ICG 11337 and ICG 13919, and a susceptible cultivar, JL 24, were used as parents. The study was conducted on three crosses, JL 24 × ICG 11337, JL 24 × ICG 13919 and ICG 11337 × ICG 13919 and their reciprocals, at ICRISAT, Patancheru, India. The genotypes, ICG 11337 and ICG 13919, are interspecific derivatives of A. hypogaea × A. cardenasii cross and were bred at ICRISAT after incorporating genes that confer resistance to LLS from A. cardenasii (Abdou et al. 1974; Singh et al. 1997). Both the genotypes have high agronomic potential besides offering high levels of resistance; ICG 11337 recorded a pod yield of 5,300 kg ha−1 with a LLS disease score of 3, while ICG 13919 recorded a pod yield of 2,300 kg ha−1 with a LLS score of 2.0 on a 1–9 scale (Singh et al. 1997). JL 24 was selected from EC 94943, an introduction from Taiwan, at the Oilseeds Research Station, Jalgaon, Maharashtra and was released for cultivation in India during 1979 (Patil et al. 1980). The F1’s were crossed to their parents P1 (female parent) and P2 (male parent) to derive BC1F1 and BC2F1 generations, respectively. On the same F1 plants, the F2 seed was generated by allowing some flowers to self. Similarly, with reciprocal of F1 (F1R), its backcross (BC1F1R and BC2F1R) and selfed generations (F2R) were derived. Thus, in each cross the following progenies were obtained: F1, F1R, F2, F2R, BC1F1, BC1F1R, BC2F1, and BC2F1R. During the 2008 rainy season, these eight different generation progenies of a cross along with their two parents were screened in a replicated trial for resistance to LLS under both field and controlled environment conditions.
Screening under field conditions
The experimental material was evaluated in a disease screening nursery in split-plot design with two replications in an Alfisols (Alfisol-Patancheru Soil Series; Udic Rhodustolf) precision field under an infector row-system at ICRISAT Center, Patancheru. The crosses were assigned to main plots and their generations to subplots within each main plot. The plot size was a 4-m long row(s) in a ridge-furrow system; single row for parents, two rows for F1 hybrids, eight rows for backcross populations and 10 rows for F2 populations. Row to row distance was 60 cm and plant to plant distance within a row was 10 cm. After every 10 rows of test material, an infector row of susceptible variety JL 24 was planted to ensure uniform spread of disease inoculum. Standard package of practices were adopted to raise a healthy crop that included, 60 kg P2O5 as basal application, seed treatment with Mancozeb @ 2 g kg−1 of seed and Imidacloprid @ 2 ml kg−1 of seed, pre-emergence application of Pendimethalin @ 1 kg active ingredient per ha, irrigation soon after planting and subsequently as and when needed in the rainy season, gypsum @ 400 kg ha−1 at the peak flowering stage, and protection against insect pests. Rust was controlled by spraying Calyxin @ 1.5 ml l−1 of water regularly to avoid its interference with reaction to LLS. At 30 days after sowing (DAS), LLS infected potted plants from glasshouse were placed randomly throughout the infector rows of experimental plot. Artificial inoculation was done at 50 DAS by spraying the test plants and infector rows with conidial suspension of LLS pathogen to ensure uniform and heavy disease pressure in the experimental plot. After inoculation, perfo-irrigation was provided daily for 15 min in the evening hours for 30 days to promote disease development. Observations on disease score, defoliation percentage and leaf area damage (LAD) on 78, 89 and 104 DAS were recorded on each plant in each generation. A 9-point scale, as described by Subrahmanyam et al. (1995), was followed to record disease scores in the field. Area under the disease progress curve (AUDPC) was calculated based on the defoliation percentage and LAD on 78, 89 and 104 DAS.
Screening under controlled environment conditions
Detached leaf method is a rapid technique for screening resistance to leaf spots in groundnut (Foster et al. 1980). The fully expanded quadrifoliate leaves (third or fourth from top) from 45 days old plants were excised through pulvinus and planted in sterile sand culture (1.5 cm thick sand) in plastic trays. The leaves were collected from the plants of each generation for the study. The leaves in the tray were sprayed with LLS inoculum (30,000 conidia ml−1). The trays were covered with plastic bags and incubated in the growth chamber at a temperature of 24 °C and 85 % relative humidity. Water was sprayed on to the leaves once daily up to 5 days after inoculation. Disease development was determined every alternate day from 5 to 37 days after inoculation (DAI). Data on the following parameters were recorded:
-
(a)
Incubation period (IP): IP is defined as days from inoculation to appearance of first lesion. It is recorded on each leaf every alternative day from 5 days DAI.
-
(b)
Latent period (LP): LP is defined as days from inoculation to the appearance of first sporulating lesion. It is recorded on each leaf every alternative day from 5 to 37 DAI.
-
(c)
Lesion number (LN): LN was recorded on each leaflet every alternative day from 5 to 21 DAI and on 30 DAI.
-
(d)
Leaf area damage (LAD): The percent LAD was assessed by comparing the leaves with diagrams depicting leaves with known percentage of their areas affected (Hassan and Beute 1977). It was measured every alternative day from 5 to 21 DAI and on 30 DAI.
-
(e)
Lesion diameter (LD): LD is the average diameter of four lesions, randomly selected on each leaflet. It was measured at 25 DAI using vernier caliper under a magnifying glass.
Data analysis
The statistical analysis was performed using SAS 9.2. Reciprocal differences between the crosses were tested by t test. The means and variances from individual plant data were estimated for every generation separately, and generation mean analysis was performed. Six generations, the parents, F1, F2, BC1F1 and BC2F1 were used to fit in simple additive-dominance model in the generation means approach. Joint scaling test (Cavalli 1952) was conducted. Six parameter model proposed by Hayman (1958) was used, which estimates the mean (m), additive (d) and dominance (h) effects, and those caused by their interactions, i, j and l. The genetic model for estimation of additive- and dominance-genetic effects in the presence of maternal effects as given by Mather and Jinks (1971) was followed. This model includes the reciprocals of the generations, F1, BC1F1 and BC2F1 in addition to six generations mentioned above. This gives the effects of additive [d] and dominance [h] as well as additive—[dm] and dominance—[hm] maternal effects. Effective number of genes was computed using Castle-Wright’s formula (Castle 1921), a widely used tool for estimating the minimum number of genes affecting complex traits. Broad sense heritability for various traits was estimated as per Mahmud and Kramer (1951). The relative importance of the gene effects was studied following modified LMG method (Kruskal 1987a, b). This modified method is used extensively to determine relative importance of regressors (Gromping 2007).
Results and discussion
The susceptible parent, JL 24, consistently showed poor tolerance to LLS both in field and controlled conditions, while both the resistant parents showed tolerance to LLS under both the conditions. Susceptible parent had an average disease score of 5.9 at 78 days, while both the resistant parents had a disease score of ≤2.3 (Supplementary data). The susceptible and resistant parents differed similarly for disease score at 89 and 104 days. In a span of 26 days, the disease score of JL 24 increased from 5.9 to 8.5 indicating a quick progression of the disease in the susceptible parent, while that of the resistant parents rose from ≤2.3 to ≤3.6 only. Consequently, the resistant parents exhibited lower AUDPC than the susceptible parent. In controlled conditions, JL 24 had higher number of lesions and greater leaf area damage and lesion diameter than the resistant parents. After one month of inoculation, almost 70 % of the leaf area in JL 24 was damaged compared to less than 22 % in the resistant parents (supplementary data). The resistant parents, ICG 11337 and ICG 13919 differed significantly from the susceptible parent, JL 24 for all the traits studied for resistance (Electronic supplementary data). Interestingly, significant differences were also noticed between the two resistant parents although both are interspecific derivatives of, A. cardenasii, a wild species, resistant to disease (Abdou et al. 1974; Sharief et al. 1978). It appears that these two resistant parents may have some genes differing for resistance to LLS. The differences in the two derivatives may be either due to different alleles being fixed in them or due to expulsion of some genomic segments. After hybridization, while advancing the interspecific derivatives through selfing to a stable tetraploid level, the parts/segments of genome of wild species are expelled, which might result in differences in resistance genes in two interspecific derivatives originating from the same resistant source (Spielman et al. 1979; Garcia et al. 2006). The results also indicated that the resistance levels were higher in the cross where both the parents were resistant to LLS, than in the crosses, where only one of the parents was resistant to LLS. This again showed that the two resistant parents had some differing genes for resistance to LLS.
Significant reciprocal differences were observed for all the traits associated with LLS resistance except incubation period in susceptible × resistant (JL 24 × ICG 11337 and JL 24 × ICG 13919) crosses (Table 1). In the resistant × resistant cross (ICG 11337 × ICG 13919), the reciprocal differences were significant only for incubation period and leaf area damage on 29th day. Kornegay et al. (1980) and Coffelt and Porter (1986) also reported reciprocal effects influencing inheritance of resistance to LLS in groundnut. Reciprocal effects in plants are known to contribute to the variation of both, quantitative and qualitative traits (Roach and Wulff 1987). Both, cytoplasmic inheritance and maternal effects contribute to reciprocal effects. They may be distinguished by comparing F2 seed borne on reciprocal F1 plants (Knowles and Mutwakil 1963). This is based on the assumption that the F2 plants from reciprocal crosses have the same genotype average, i.e., they will have same mean value for the trait under study. If the differences exist between F2 and its reciprocal F2 populations, they would be expected to be due to cytoplasmic effects (Mosjidis and Yermanos 1984). In JL 24 × ICG 11337 cross, reciprocal differences in F2 generation were significant for all the traits except for disease scores, incubation period and latent period and in F2 generation of JL 24 × ICG 13919 cross, the reciprocal differences were significant for all the traits except for area under disease progress curve and lesion numbers. In F2 generation of ICG 11337 × ICG 13919 cross, the reciprocal differences were significant for leaf area damage on 29th day and disease score on 89th day (Table 1). These reciprocal differences for various traits can be attributed to cytoplasmic effects. Estimates of minimum number of genes affecting traits differed among crosses were given in Table 2. For some traits no nuclear gene(s) were detected which indicated that either the assumptions of Castle-Wright equation were not met or the cytoplasm effect overrode the genotype effect. The Castle-Wright equation rests on several simplifying assumptions. It assumes that all alleles behave additively with equal effect and two parental strains are homozygous for alternative alleles at all loci affecting the trait and all chromosomes are diploid (Jones 2001). Since in one of the resistant parents, both additive and dominance effects were significant, one of the assumptions of the equation was not met. The heritability estimates for various traits associated with resistance to LLS were highly variable from very low to high (Table 2). The area under disease progress curve registered the lowest estimates for the heritability. For disease scores, these estimates were on a higher side. Anderson et al. (1991) also reported moderate to high heritability for components of resistance to LLS.
Among the traits studied, incubation period did not correlate with field measured traits—disease scores and area under disease progress curve. However, it was associated with latent period positively and all other laboratory-studied traits (lesion number, leaf area damage and lesion diameter) negatively with very low to low magnitude of relationship (Table 3). On the other hand, the latent period negatively correlated with field observations with low magnitude of relationship but with all other laboratory measured traits it correlated negatively with low to moderate magnitude of relationship. All disease scores and area under disease progress curve correlated positively with higher magnitude of relationship. The same was true for laboratory measured traits. Lesion numbers, leaf area damage and lesion diameter correlated moderately with field observations (disease scores and area under disease progress curve). Disease scores and area under disease progress curve are derived traits from leaf area damage. Incubation period and latent period seem to have little influence on disease scores and area under disease progress curve. However, earlier studies indicated that longer latent periods contributed to enhance resistance to LLS (Dwivedi et al. 2002; Cantonwine et al. 2008). From the results of the present study, it appears that observations on disease scores in the field should suffice to evaluate the breeding materials for resistance to LLS. Dwivedi et al. (2002) also identified a field measured trait, the remaining green leaf area on the plants, which is also a component in the disease score, as major selection criteria for resistance to LLS.
Invariably additive genetic effects were significant for majority of the traits studied in three crosses with a few exceptions where they were not. These included area under disease progress curve and lesion number on 22nd day in straight and reciprocal crosses of cross 3 (ICG 11337 × ICG 13919), which involved both LLS resistant parents (Table 4). Frequency of significance of dominance effects in three crosses was much lower. They were significant for disease scores, leaf area damage and lesion number at one or the other stage of observation recordings and incubation period, latent period and lesion diameter in some cases. There was preponderance of additive × dominance genetic interaction effects in cross 1 and cross 2 (both resistant × susceptible crosses and their reciprocals). However, in cross 3 (resistant × resistant cross), only 2 out of 13 traits showed significant additive × dominance genetic interaction effects. On overall basis, additive genetic effects were largely responsible for traits associated with LLS resistance in three crosses. In some cases additive × additive genetic interaction effects were also important. Being a self-pollinated crop, only additive genetic and additive × additive genetic interaction effects can be exploited in groundnut. Selection for LLS resistance can be practiced in early generations exploiting additive genetic effects. However, for exploitation of additive × additive genetic interaction effects, the selection should be delayed to later generations. Earlier studies have also indicated predominance of additive genetic effects (Sharief et al. 1978; Kornegay et al. 1980; Nevill 1982; Anderson et al. 1986b; Coffelt and Porter 1986). In almost all cases, h and l were in opposite direction indicating duplicate type of epistasis.
The estimates of additive—(dm) and dominance–maternal effects (hm) are given in Table 5. The m, d and h model was inadequate to explain the variability observed in P1, P2, F1, F1R, F2, BC1, BC1R, BC2 and BC2R. Therefore, the contributions of maternal effects (dm and hm) were estimated. In JL 24 × ICG 11337 cross and its reciprocal, significant Additive–maternal effects were detected, while in JL 24 × ICG 13919 cross and its reciprocal, both additive- and dominance–maternal effects were significant. Zhu and Weir (1994) suggested selection based on maternal plants when additive effects of maternal genes were the major contributors of genetic variation. Dominance–maternal effects were observed in ICG 11337 × ICG 13919 cross and its reciprocal, indicating that probably ICG 13919 contributed to dominance maternal effects. When ICG 11337 is used as resistant parent, additive effects of both nuclear genes and maternal effects were significant, while in the parent, ICG 13919, both additive and dominance effects were significant. Thus, when ICG 11337 is used as resistance donor, both additive-nuclear and—maternal effects can be tapped. On the other hand, the resistant parent ICG 13919, due to its dominance effects significantly contributing to resistance, cannot be fully exploited in breeding programs despite its higher levels of resistance.
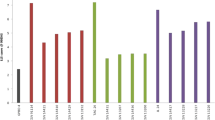
The contribution of genetic effects was studied to determine the relative importance of the gene effects. The contribution of genetic and maternal factors to the traits associated with LLS resistance is represented in Fig. 1. The contribution of additive, dominance and epistatic component to the traits of LLS resistance are shown in Fig. S1. In the crosses derived from the parents, JL 24 and ICG 11337, the contribution of additive effect range from 40 to 55 %, followed by contribution of Additive–maternal effects (about 40 %), thus additive nuclear and maternal effects explained over 80 % of contribution for the field measured traits. For lab measured traits, lesion number and diameter, the additive effects contributed of additive effects range from 55 to 80 %. On contrary, in the crosses derived from the parents, JL 24 and ICG 13919, additive effect contributed to 43–93 % for field measured traits, but for lab measured traits, the contribution of dominance effects was more. Interestingly, in these crosses, the Additive–maternal effects were large for all lab measured traits (latent period, lesion numbers on 20th, 22nd and 29th day, leaf area damage on 20th, 22nd and 29th day and lesion diameter on 25th day). In the resistant × resistant cross, dominance maternal effect contributed to 97 % of the variation for area under disease progress curve. dominance–maternal effect also contributed to latent period, lesion numbers on 20th and 29th day, leaf area damage on 22nd day and lesion diameter on 25th day, (17–57 %). The additive- maternal effects could be tapped in groundnut breeding programs to realize high level of resistance by selecting for maternal plant types.
Graphical representation of contribution of additive- and dominance- nuclear and maternal gene effects (d, h, dm and hm) to the traits of LLS resistance in the crosses involving three parents in groundnut. DS78, DS89, DS104, are disease score at 78, 89 and 104 days, respectively; AUDPC, area under disease progress curve; IP, incubation period; LP, latent period; LN20, LN22, LN29, are lesion number at 20, 22 and 29 days, respectively; LAD20, LAD22, LAD29, are leaf area damage at 20, 22 and 29 days, respectively; LD-25, lesion diameter at 25 days
Resistance to LLS in groundnut is controlled by both nuclear and maternal factors. The nature of resistance and its level are different in the two resistant parents, ICG 11337 and ICG 13919, despite their common origin (A. cardenasii is LLS resistance donor in both). Among genetic effects, both additive and dominance effects are important, but bulk of the variation seems to be controlled by additive gene effects. Among maternal effects, both additive and dominance–maternal effects were found to be governing resistance to LLS; although the additive effects were predominant. In LLS resistance breeding program, it is important to use the resistance donor as female parent to tap cytoplasmic inheritance of resistance to LLS. Given the polygenic nature of the LLS resistance, recombination breeding coupled with some amount of recurrent selection to accumulate minor genes in elite susceptible/tolerant backgrounds can be rewarding. Wild species of Arachis are known for resistance to LLS (Abdou et al. 1974) and therefore, it would be necessary to include other species as donors to broaden the genetic base of resistance to LLS. Such an attempt would enable the development of varieties with genes accumulated from different donors, thus enhancing the levels of resistance to the disease without compromising on yield or quality traits.
To hasten the fixation of desirable alleles for LLS resistance in the breeding populations, selection based on field based disease score can be advantageous as they not only have higher heritability but are also easy to record than the measurements taken under controlled conditions. In addition, disease score is also positively correlated to area under disease progress curve. Lesion number, leaf area damage and lesion diameter, which are the component traits for resistance to LLS would be useful in phenotyping for QTLs detection. Walls et al. (1985) also suggested that latent period, lesion area and amount of sporulation could be used as measurements of resistance to predict the disease reaction of a line in the field. They further suggested that lines with resistance to LLS can be selected from a population of lines which have been selected for resistance to ELS. In many places both ELS and LLS occur together, however, their resistance is inherited independently (Anderson et al. 1986b; Abdou et al. 1974). Anderson et al. (1991) reported moderate to high correlations between ELS and LLS disease components indicating possible genetic linkage or host-plant physiology that confers resistance to both diseases in one population. From their study, they further reported that selection based on family means rather than individual plant selection within families would be more successful. Iroume and Knauft (1987) also observed that selection among crosses would be more advantageous as compared to individual plant selection or within family selection. Whereas Jogloy et al. (1987) observed that selection for resistance to LLS in F2 generation would be ineffective due to low narrow sense heritability; the contrasting observation was made by Anderson et al. (1986b) who concluded from their study that individual plant selection for LLS resistance would be effective during early generations. The preponderance of additive genetic effects in the present study also supports selection for LLS resistance in early generations. It has been observed that high yield potential and a high degree of resistance do not generally go together (Jogloy et al. 1987; Nigam et al. 1991). Lower dry matter partitioning in rust- and LLS-resistant genotypes of groundnut was also observed (Williams et al. 1984). Nevertheless, other studies have suggested the possibility of combining high levels of resistance to leaf spots with superior yield and quality factors (Ouedraogo et al. 1994; Tallury et al. 2009), for which selection for yield under disease pressure may be advantageous (Iroume and Knauft 1987).
References
Abdou YA-M, Gregory WC, Cooper WE (1974) Sources and nature of resistance to Cercospora arachidicola Hori and Cercosporidium personatum (Beck & Curtis) Deighton in Arachis species. Peanut Sci 1:6–11
Anderson WF, Wynne JC, Green CC, Beute MK (1986a) Combining ability and heritability of resistance to early and late leaf spot of peanut. Peanut Sci 13:13–14
Anderson WF, Wynne JC, Green CC (1986b) Potential for incorporation of early and late leafspot resistance in peanut. Plant Breeding 97:163–170
Anderson WF, Holbrook CC, Wynne JC (1991) Heritability and early-generation selection for resistance to early and late leafspot in peanut. Crop Sci 31:588–593
Anderson WF, Holbrook CC, Brenneman TB (1993) Resistance to Cercosporidium personatum within peanut germplasm. Peanut Sci 20:53–57
Aquino VM, Shokes FM, Gorbet DW, Nutter FW Jr (1995) Late leaf spot progression on peanut as affected by components of partial resistance. Plant Dis 79:74–78
Cantonwine EG, Culbreath AK, Holbrook CC, Gorbet DW (2008) Disease progress of early leaf spot and components of resistance to Cercospora arachidicola and Cercosporidium personatum in runner-type peanut cultivars. Peanut Sci 35:1–10
Castle WE (1921) An improved method of estimating the number of genetic factors concerned in cases of blending inheritance. Proc Natl Acad Sci USA 81:6904–6907
Cavalli LL (1952) An analysis of linkage in quantitative inheritance. In: Rieve ECR, Waddington CH (eds) Quantitative inheritance. HMSO, London, pp 135–144
Chiteka JA, Gorbet DW, Knauft DA, Shokes FM, Kucharek TA (1988) Components of resistance to late leaf spot in peanut. II. Correlation among components and their significance in breeding for resistance. Peanut Sci 15:76–81
Coffelt TA, Porter DM (1986) Field screening of reciprocal Chico × Florigiant peanut populations for resistance to leaf spot in virginia. Peanut Sci 13:57–60
Dwivedi SL, Nigam SN, Subrahmanyam P, Jambunathan R, Nagabhushanam GVS, Reddy PM, Raghunath K, McDonald D (1993) Effect of foliar diseases control by chlorothalonil on pod yield and quality characteristics of confectionery groundnuts (Arachis hypogaea L.). J Sci Food Agric 63:265–271
Dwivedi SL, Pande S, Rao JN, Nigam SN (2002) Components of resistance to late leaf spot and rust among interspecific derivatives and their significance in a foliar disease resistance breeding in groundnut (Arachis hypogaea L.). Euphytica 125:81–88
FAOSTAT (2010) http://faostat.fao.org. Accessed 17 Nov 2011
Foster DJ, Wynne JC, Beute MK (1980) Evaluation of detached leaf culture for screening peanuts for leaf spot resistance. Peanut Sci 7:98–100
Garcia GM, Tallury SP, Stalker HT, Kochert G (2006) Molecular analysis of Arachis interspecific hybrids. Theor Appl Genet 112:1342–1348
Gregory WC, Krapovickas A, Gregory MP (1980) Structure, variation, evolution and classification in Arachis. In: Summerfield RJ, Bunting AH (eds) Advances in legume science. Royal Botanic Gardens, Kew, pp 469–481
Gromping U (2007) Estimators of relative importance in linear regression based on variance decomposition. Am Stat 61:139
Hassan HN, Beute MK (1977) Evaluation of resistance to Cercospora leafspot in peanut germplasm potentially useful in breeding program. Peanut Sci 4:78–83
Hayman BI (1958) The separation of epistatic from additive and dominance variation in generation mean. Heredity 12:371–390
Iroume RN, Knauft DA (1987) Heritabilities and correlations for pod yield and leaf spot resistance in peanut (Arachis hypogaea L.): implications for early generation selection. Peanut Sci 14:46–50
Jogloy S, Wynne JC, Beute MK (1987) Inheritance of late leafspot resistance and agronomic traits in peanut. Peanut Sci 14:86–90
Jogloy S, Pensuk V, Patanothai A, Wongkaew S (1999) Generation mean analyses of late leaf spot and rust resistance in peanut (Arachis hypogaea L.). Thai J Agric Sci 32:423–433
Jones CD (2001) Extension of the Castle-Wright effective factor estimator to sex linkage and haplodiploidy. J Hered 92:274–276
Khedikar YP, Gowda MVC, Sarvamangala C, Patgar KV, Upadhyaya HD, Varshney RK (2010) A QTL study on late leaf spot and rust revealed one major QTL for molecular breeding for rust resistance in groundnut (Arachis hypogaea L.). TAG 121:971–984
Knowles PF, Mutwakil A (1963) Inheritance of low iodine value of safflower selection from India. Econ Bot 17:139–145
Kochert G, Stalker TH, Gimenes M, Galgaro L, Romero Lopes C, Moore K (1996) RFLP and cytogenetic evidence on the origin and evaluation of allotetraploid domesticated peanut, Arachis hypogaea (Leguminosae). Am J Bot 83:1282–1291
Kornegay JL, Beute MK, Wynne JC (1980) Inheritance of resistance to Cercospora arachidicola and Cercosporidium personatum in six virginia-type peanut lines. Peanut Sci 7:4–9
Kruskal W (1987a) Relative importance by averaging over orderings. Am Stat 41:6–10
Kruskal W (1987b) Correction to relative importance by averaging over orderings. Am Stat 41:341
Luo M, Dang P, Bausher MG, Holbrook CC, Lee RD, Lynch RE, Guo BZ (2005) Identification of transcripts involved in resistance responses to leaf spot disease caused by Cercosporidium personatum in peanut (Arachis hypogaea). Phytopathology 95:381–387
Mahmud I, Kramer HH (1951) Segregation for yield, height, and maturity following a soybean cross. Agron J 43:605–609
Mather K, Jinks JL (1971) Biometrical genetics: the study of continuous variation. Chapman & Hall, London
Mehan VK, Reddy PM, Rao KV, McDonald D (1996) Identification of new sources of resistance to rust and late leaf spot in peanut. Int J Pest Manag 42:267–271
Mosjidis JA, Yermanos DM (1984) Maternal effects and cytoplasmic inheritance of oleic and linoleic acid contents in sesame. Euphytica 33:427–432
Motagi BN, Gowda MVC, Naidu GK (2000) Inheritance of late leafspot resistance in groundnut mutants. Indian J Genet 60:347–352
Nevill DJ (1982) Inheritance of resistance to Cercosporidium personatum in groundnuts: a genetic model and its implications for selection. Oleagineux 37:355–362
Nigam SN, Dwivedi SL, Gibbons RW (1991) Groundnut breeding: constraints, achievements and future possibilities. Plant Breed Abstr 61:1127–1136
Ouedraogo M, Smith OD, Simpson CE, Smith DH (1994) Early and late leaf spot resistance and agronomic performance of nineteen interspecific derived peanut lines. Peanut Sci 21:99–104
Patil GD, Desale SC, Patil PS, Patil SS (1980) ‘Phule-Pragati’ a high yielding early bunch groundnut for Maharashtra. J MAU 5:47–52
Roach DA, Wulff RD (1987) Maternal effects in plants. Ann Rev Ecol Syst 18:209–235
Sharief Y, Rawlings JO, Gregory WC (1978) Estimates of leaf spot resistance in three interspecific hybrids of Arachis. Euphytica 27:741–751
Singh AK, Simpson CE (1994) Biosystemic and genetic resources. In: Smartt J (ed) The groundnut crop: a scientific basis for improvement. Chapman and Hall, London, pp 96–137
Singh AK, Mehan VK, Nigam SN (1997) Sources of resistance to groundnut fungal and bacterial diseases: an update and appraisal. Information Bulletin no. 50. International Crops Research Institute for the Semi-Arid Tropics, Hyderabad, p 48
Spielman IV, Burge AP, Moss JP (1979) Chromosome loss and meiotic behavior in interspecific hybrids in the genus Arachis L. and their implications in breeding for disease resistance. Z Pflanzenzüchtg 83:236–250
Subrahmanyam P, McDonald D, Gibbons RW, Nigam SN, Nevill DJ (1982) Resistance to rust and late leaf spot diseases in some genotypes of Arachis hypogaea. Peanut Sci 9:6–10
Subrahmanyam P, Williams JH, McDonald D, Gibbons RW (1984) The influence of foliar diseases and their control by selective fungicides on a range of groundnut (Arachis hypogaea L.) genotypes. Ann Appl Biol 104:813–819
Subrahmanyam P, McDonald D, Waliyar F, Reddy LJ, Nigam SN, Gibbons RW, Rao VR, Singh AK, Pande S, Reddy PM, Subba Rao PV (1995) Screening methods and sources of resistance to rust and late leaf spot of groundnut. Information Bulletin no. 47. International Crops Research Institute for the Semi-Arid Tropics, Hyderabad, p 24
Tallury SP, Isleib TG, Stalker HT (2009) Comparison of virginia-type peanut cultivars and interspecific hybrid derived breeding lines for leaf spot resistance, yield, and grade. Peanut Sci 36:144–149
Tiwari SP, Ghewande MP, Misra DP (1984) Inheritance of resistance to rust and late leaf spot in groundnut (Arachis hypogaea L.). J Cytol Genet 19:97–101
Waliyar F (1991) Evaluation of yield losses due to groundnut leaf diseases in West Africa. Summary proceedings of the second ICRISAT regional groundnut meeting for west Africa, 11–14 Sep 1990, ICRISAT Sahelian Center, Niamey, Niger, pp 32–33
Waliyar F, Bosc JP, Bonkoungou S (1993) Sources of resistance to foliar diseases of groundnut and their stability in West Africa. Oleagineux 48:283–287
Walls SB, Wynne JC, Beute MK (1985) Resistance to late leafspot peanut of progenies selected for resistance to early leafspot. Peanut Sci 12:17–22
Williams JH, Ramraj VM, Pal M (1984) Physiological studies on foliar diseases: varietal differences in response to use of fungicides. In: Proceedings of a discussion group meeting on groundnut rust diseases, ICRISAT Center, Patancheru, pp 49–53
Zhu J, Weir BS (1994) Analysis of cytoplasmic and maternal effects I. A genetic model for diploid plant seeds and animals. Theor Appl Genet 89:153–159
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pasupuleti, J., Ramaiah, V., Rathore, A. et al. Genetic analysis of resistance to late leaf spot in interspecific groundnuts. Euphytica 193, 13–25 (2013). https://doi.org/10.1007/s10681-013-0881-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-013-0881-7