Abstract
One hundred sixty-one EST-SNP markers were newly developed for analysis of QTLs for resistance to black rot caused by Xanthomonas campestris pv. campestris by determining EST sequences of a resistant line obtained from cabbage ‘Early Fuji’ and a susceptible broccoli line. A linkage map consisting of nine linkage groups was constructed with a total of 209 markers, including these new SNP markers and previously reported DNA markers. F2 plants grown in a field for 1 month were inoculated by spraying bacteria of race 1, and disease severity of each plant was recorded. Three QTLs, i.e., QTL-1, QTL-2, and QTL-3, were detected on linkage group C2, C4 and C5, respectively. QTL-1, which showed the highest LOD score and additive effect, was again detected in another F2 population used the next year, suggesting QTL-1 to be a major QTL. QTL-2 and QTL-3 could be minor QTLs influenced by environmental factors. The genomic region harboring QTL-1 showed synteny with a region from 5.3 to 7.4 Mb from the short arm end of chromosome 5 of Arabidopsis thaliana, which is rich in TIR-NBS-LRR family genes. The identified SNP markers in QTL-1 are considered to be useful in marker-assisted selection for black rot resistance in Brassica oleracea lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black rot of cabbage, Brassica oleracea L. capitata group, caused by Xanthomonas campestris pv. campestris (Xcc) is a serious disease epidemic in the world. It is transmitted by contact, water splash, and also through seeds. Xcc infects through hydathodes, wounds, and rarely stomata, and is spread by rainfall. Xcc infection causes V-shaped yellowing along veins, followed by browning and black rot (Alvarez 2000). Infected cabbages lose market value and symptom development brings about complete loss of production. Therefore, control of black rot disease is important for cabbage production. No cabbage cultivar not infected by Xcc has been reported, but the level of disease resistance is different among cabbage cultivars (Williams et al. 1972).
Based on virulence in Wirosa F1 (B. oleracea), Just Right Hybrid Turnip (Brassica rapa), Seven Top Turnip (B. rapa), PI 199947 (Brassica carinata), Florida Broad Leaf Mustard (Brassica juncea), and Miracle F1 (B. oleracea), Xcc is classified into different races (Vicente et al. 2001), nine races having been reported (Fargier and Manceau 2007). Most Xcc races causing black rot in B. oleracea have been identified as race 1 and race 4 (Vicente et al. 2001). These races have also been isolated from cabbage plants infected by black rot in Japan (Ignatov et al. 1998).
Several studies for identification of black rot resistance genes in B. oleracea have been performed. Dickson and Hunter (1987) have reported that one recessive gene and two modifying genes control black rot resistance in PI 436606, a cabbage line from China. Quantitative trait locus (QTL) analysis of black rot resistance in ‘Badger Inbred-16’ using RFLP markers has revealed four QTLs on three linkage groups (Camargo et al. 1995). Analysis of resistance in ‘Reiho’ using sequence-related amplified polymorphism (SRAP) and cleaved amplified polymorphic sequence (CAPS) markers has detected two QTLs having major effects and one QTL having a minor effect (Doullah et al. 2011). Although resistance of cauliflower line SN455 from India has been reported to be determined by a recessive allele of a single gene (Jamwal and Sharma 1986), black rot resistance of most B. oleracea lines is considered to be controlled by at least three genes (Williams et al. 1972; Camargo et al. 1995). However, resistance genes have not been identified, probably because the number of mapped DNA markers has not been sufficient.
Recently, many DNA markers, especially single nucleotide polymorphism (SNP) markers, which are the most frequent DNA polymorphism in the genomes of living organisms, have become usable. Various techniques for detecting SNPs have been developed, but rapid, efficient techniques generally require special equipment or high running costs. Among them, the dot-blot-SNP technique developed by Shiokai et al. (2010) enables efficient analysis of SNPs at low cost and without high-priced equipment. In the present study, we constructed a linkage map of 161 new SNP markers in cabbage using this technique and analyzed QTL for black rot resistance.
Materials and methods
Plant material and source of pathogen
A cabbage inbred line CY resistant to black rot, developed from black rot resistant cultivar ‘Early Fuji’ (Kaneko Seeds Co. Ltd), was crossed with a broccoli inbred line BB susceptible to black rot, derived from ‘Green Dome 115’ (Kaneko Seeds Co. Ltd). One F1 plant was self-pollinated, and obtained F2 plants were used for inoculation tests and QTL analysis.
Xanthomonas campestris pv. campestris (Xcc) used for inoculation tests was isolated from a black rot infected cabbage in Isesaki, Gunma in 2008. Isolated Xcc was identified to be race 1 by the method of Vicente et al. (2001) (data not shown).
Inoculation test
Inoculation tests using 140 and 142 F2 plants were performed in October 2009 (09Au) and October 2010 (10Au), respectively. F2 plants were grown on a 128-cell tray for 1 month and transplanted to an isolated field. The tests were performed in Isesaki, Gunma, Japan. The average temperature and total precipitation in a period from inoculation to recording were 17.7 and 110 mm in 09Au test, and 17.0 °C and 176 mm in 10Au test. Xcc was grown on potato sucrose agar medium for 48 h at 28 °C. Xcc culture from the surface of the medium was suspended in distilled water with 0.03 % spreader (Mix Power, Syngenta Japan) and the concentration was adjusted to about 107 cfu/ml by serial dilution method. Xcc was inoculated into plants about 1 month after transplanting using an engine power sprayer. The severity of the black rot symptoms was recorded by visual scale taking in account the entire plant about 1 month after inoculation. Disease indices were as follows: (1) <25 % of the leaf showing black rot symptoms, (2) 25–49 % of leaf edge having black rot symptoms, (3) 50–74 %, (4) more than 76 % of leaf edge having the black rot symptom. In 09Au test, two susceptible checks (‘Wirosa F1’ and ‘Miracle F1’) (Vicente et al. 2001) were also inoculated and both had black rot symptoms. In 09Au and 10Au tests, there was no plant without black rot symptoms.
DNA polymorphism analysis
DNA was extracted from leaves using the CTAB method (Murray and Thompson 1980). Primers were designed from expressed sequence tag (EST) sequences of radish, which belongs to the same family as cabbage and broccoli. Polymerase chain reaction (PCR) was performed in a 20 μl reaction mixture containing about 10 ng of DNA, 0.5 mM of forward and reverse primers, 1×Ex Taq buffer, 4 nmol of dNTP, and 1 unit of Ex Taq DNA polymerase (TAKARA BIO INC., Japan). The PCR conditions were initial denaturation at 94 °C for 30 s followed by 45 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min, and final extension at 72 °C for 3 min. PCR products amplified as a single fragment were sequenced by the Sanger method and sequences were analyzed to find SNPs using SEQUENCHER software (Gene Codes Cooperation, MI, USA). The sequences having SNPs between CY and BB were used for producing probes for dot-blot-SNP analysis according to Shiokai et al. (2010). In case of SNP at a recognition site of a restriction enzyme, primer pairs were used as CAPS markers. If the sizes of PCR products were clearly different between CY and BB, primer pairs were used as sequence characterized amplified region (SCAR) markers.
Simple sequence repeat (SSR) markers (Brassica info (http://www.brassica.info/); Piquemal et al. 2005; Okazaki et al. 2007; Iniguez-Luy et al. 2009; Nagaoka et al. 2010) and CAPS markers (Okazaki et al. 2007; Nagaoka et al. 2010) were used to assign a linkage group according to the internationally agreed nomenclature of the B. oleracea reference linkage group.
F2 genotyping, linkage map construction, and QTL analysis
For F2 genotyping, PCR was performed in a 10 μl reaction mixture containing about 5 ng of DNA, 0.5 μM of forward and reverse primers, 1× reaction buffer, 2 nmol of dNTP, and 0.5 units of KAPA Taq Extra (Nippon Genetics Co. Ltd., Japan) or HybriPol (BIOLINE, UK). Dot-blot-SNP analysis was carried out according to Shiokai et al. (2010). SCAR, CAPS, and SSR markers were electrophoresed using agarose gel or polyacrylamide gel and visualized by ethidium bromide staining. From F2 genotyping data, a linkage map was constructed using the JoinMap 4.0 software (van Ooijen 2006). The marker order was determined by a regression mapping algorithm and nine linkage groups were made on the basis of a minimum LOD score of 2.5. Kosambi mapping function was used to convert recombination values to genetic distances. QTL analysis was performed using QTL Cartographer ver. 2.5 by composite interval mapping (Wang et al. 2007). The 1,000 times permutation tests at 5 % significant level were performed to determine LOD thresholds. LOD threshold values for 09Au and 10Au tests were 3.9 and 3.5, respectively.
Results
Inoculation test
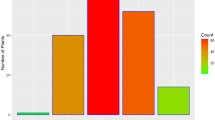
In 09Au and 10Au tests, 142 and 140 F2 plants, respectively, were inoculated with Xcc. In each test, five plants of each CY, BB and F1 were tested simultaneously. Results of inoculation tests are shown in Fig. 1. Disease severities of F2 plants were distributed continuously.
Linkage map construction
Out of 1,907 primer pairs designed from radish EST sequences, 690 primer pairs amplified single DNA fragments from both CY and BB, and the amplified fragments were sequenced. In 537,024 sequenced bases, SNP sites between CY and BB were 606 (1/886 bases) containing 762 SNP bases (1/704 bases) and Indel sites were 69 (1/7,783 bases) containing 409 Indel bases (1/1,013 bases). Polymorphic DNA fragments were 245 (35.5 %). To construct a linkage map, new markers of 161 SNPs, 7 CAPS, and 2 SCAR were developed in this study (Supplementary Table 1). Nine SNP markers (Ashutosh et al. 2012), 24 SSR markers (Brassica info; Piquemal et al. 2005; Iniguez-Luy et al. 2009), and six CAPS markers (Okazaki et al. 2007; Nagaoka et al. 2010) were also used for construction of a linkage map (Fig. 2). The linkage map had nine linkage groups with a total of 209 markers. The total length was 928.7 cM and the average marker interval was 4.4 cM. The chromosome of each linkage group was determined according to Brassica info or Piquemal et al. (2005). All the linkage groups except for one were assigned to the B. oleracea reference linkage groups. The remaining one linkage group did not have markers corresponding to ones in the reference linkage groups. Assembling the present map with our previously reported map of B. oleracea (Ashutosh et al. 2012) using JoinMap 4.0 software revealed this linkage group to be C4 (Fig. 2).
Linkage map and detected QTLs for a Brassica oleracea F2 population derived from a cross between CY and BB lines. Detected QTLs in 09Au and 10Au are shown by black bars and gray bar, respectively. The arrow heads indicate the peak of LOD score in the QTLs. The last letters of s, c, a, and r represent dot-blot-SNP markers, CAPS markers, SCAR markers, and SSR markers, respectively
QTL analysis
QTL analyses were performed using the data of disease indices of 09Au and 10Au and the genotyping data of F2 plants. In 09Au analysis, a major QTL was detected on C2 and named QTL-1. QTL-1 had 6.04 of the maximum LOD (logarithm of the odd) score, −0.46 of the additive effect by CY, and 15.05 % of variance explained (Table 1). QTL-2 and QTL-3 were detected on C4 and C5, respectively, but with smaller LOD scores, additive effects, and variances explained than those of QTL-1. In 10Au, one QTL was detected near the QTL-1 of 09Au. The regions of QTL-2 and QTL-3 showed 2.67 and 1.06 of the maximum LOD score, −0.25 and −0.31 of the additive effect, −0.26 and −0.04 of the dominance effect by CY, and 7.0 and 2.7 % variance explained, respectively, in 10 Au. Although these regions had higher LOD scores than other regions, they did not reach a threshold value.
Discussion
Several studies on genetics of black rot resistance in cabbage have been reported, and multiple genes have been considered to be responsible for the resistance (Camargo et al. 1995; Doullah et al. 2011). In the present study, disease severities of F2 plants showed a continuous distribution, indicating participation of multiple genes in disease resistance, and various QTLs were detected. QTL-1 on C2 was detected in both 09Au and 10Au populations, and is considered to be a major QTL. On the other hand, QTL-2 and QTL-3 on C4 and C5, respectively, were detected in 09Au, but the LOD scores were lower than the threshold value in 10Au, suggesting that QTL-2 and QTL-3 were largely influenced by environmental factors.
QTLs for black rot resistance have been detected on LG2 and LG9 of B. oleracea by Doullah et al. (2011). Since Bo13 marker (=BOHM13) on LG9 of Doullah et al. (2011) was mapped on C3 in the present study, their LG9 is considered to correspond to C3. CAM1, CO, DGAT1, GSA, and GA1 on LG2 of Doullah et al. (2011) have been mapped on O9 (=C9) (Okazaki et al. 2007). LG1 of Camargo et al. (1995), which contains QTL for black rot resistance, was regarded as LG9 by Doullah et al. (2011). QTLs on C3 and C9 were not detected in the present study, and other QTLs were found. The difference of these results is probably due to the difference of disease resistant lines used in these studies. In the present study, race 1 was used, while a used race was not described by Camargo et al. (1995) and Doullah et al. (2011). The difference of detected QTLs might be also due to difference of used races.
BoCL5989 and BoCL5545 near QTL-1 on C2 had high homology with At5g16360 and At5g22400 of Arabidopsis thaliana L., respectively. These sequences are on 5.3 and 7.4 Mb, respectively, from the end of the short arm of chromosome 5 of A. thaliana. Synteny of a long region between C2 and A. thaliana chromosome 5 has been reported (Ashutosh et al. 2012). The region between At5g16360 and At5g22400 is a region rich in TIR-NBS-LRR family genes (Mayers et al. 2003). Analysis of The Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org/index.jsp) revealed the presence of nine TIR-NBS-LRR family genes and other disease resistance-related genes in this region. RPS4 (Gassmann et al. 1999) and RRS1-R (Deslandes et al. 2003), which are genes conferring resistance to bacteria of Pseudomonas syringae and Ralstonia solanacearum, respectively, belong to the TIR-NBS-LRR family. It has been reported that resistance of A. thaliana to Xcc is controlled by RXC1 (RXC4), RXC2, and RXC3 (Buell and Somerville 1997), the latter two having been reported to be mapped on chromosome 5. RXC2 has been located near the markers, mi138 and mi90, which are located at 7.6 and 7.9 Mb, respectively, from the top of the short arm of chromosome 5 according to PHYSICAL_KAZUSA map (TAIR; mi138, http://www.arabidopsis.org/servlets/TairObject?accession=Clone:14886; mi90, http://www.arabidopsis.org/servlets/TairObject?accession=Clone:14986). Since BoCL5545s has homology to At5g22400 near mi138, an ortholog of A. thaliana RXC2, which has not been identified at the molecular level, might be contained in the QTL-1 region. BLAST search for B. rapa genes indicated the highest homology of BoCL5989s with KBrB068I03 on A10 and also homologies with KBrB027O09 on A7 and KBrB018H04 on A3. On the other hand, BoCL5545s had the highest homology to KBrH065B20 on A2. Genome rearrangement might have occurred in this region after divergence of B. oleracea and B. rapa. It is considered to be difficult to use the B. rapa genome information for identification of a candidate gene for Xcc resistance in B. oleracea.
Brassica oleracea lines resistant to black rot disease have been selected in the field infected by Xcc or by inoculation tests. However, disease severity depends on environmental factors and plant conditions. Use of DNA markers enables reliable selection of resistant plants even at the seedling stage. DNA marker-assisted selection became popular in breeding of crops, in which marker information is rich. In tomato breeding, DNA markers for disease resistance determined by a single gene are commonly used (Barone and Frusciante 2007). Since the selection for disease resistance controlled by multiple genes requires a larger field, longer time, and higher breeding cost than selection for that controlled by a single gene, development of DNA markers is especially important. Recently, techniques for genotyping of DNA markers have rapidly advanced. For example, SNP genotyping, which had been costly or laborious, can be performed rapidly without high cost. Further analysis of the three QTLs identified in the present study will enable development of SNP markers useful in B. oleracea breeding for black rot resistance.
References
Alvarez AM (2000) Black rot of crucifers. In: Slusarenko AJ, Fraser RSS, van Loon LC (eds) Mechanisms of resistance to plant diseases. Kluwer Academic Publishers, Dordrecht, pp 21–52
Ashutosh, Sharma B, Shinada T, Kifuji Y, Kitashiba H, Nishio T (2012) Molecular mapping of a male fertility restorer locus of Brassica oleracea using EST-based SNP markers and analysis of a syntenic region in Arabidopsis thaliana for identification of genes encoding PPR proteins. Mol Breed (in press). doi:10.1007/s11032-012-9761-4
Barone AL, Frusciante L (2007) Molecular marker-assisted selection for resistance to pathogens in tomato. In: Guimarães EP, Ruane J, Scherf BD, Sonnino A, Dargie JDD (eds) Marker assisted selection. FAO, Rome, pp 151–164
Buell CR, Somerville SC (1997) Use of Arabidopsis recombinant inbred lines reveals a monogenic and a novel digenic resistance mechanism to Xanthomonas campestris pv campestris. Plant J 12:21–29
Camargo LEA, Williams PH, Osborn TC (1995) Mapping of quantitative trait loci controlling resistance of Brassica oleracea to Xanthomonas campestris pv. campetris in the field and greenhouse. Phytopathology 85:1296–1300
Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, Boucher C, Somssich I, Genin S, Marco Y (2003) Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci USA 100:8024–8029
Dickson MD, Hunter JE (1987) Inheritance of resistance in cabbage seedlings to black rot. HortScience 22:108–109
Doullah MAU, Mohsin GM, Ishikawa K, Hori H, Okazaki K (2011) Construction of a linkage map and QTL analysis for black rot resistance in Brassica oleracea L. Int J Nat Sci 1:1–6
Fargier E, Manceau C (2007) Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol 56:805–818
Gassmann W, Hinsch ME, Staskawicz BJ (1999) The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant J 20:265–277
Ignatov A, Hida K, Kuginuki Y (1998) Black rot of crucifers and sources of resistance in Brassica crops. Jpn Agric Res Q 32:167–172
Iniguez-Luy FL, Lukens L, Farnham MW, Amasino RM, Osborn TC (2009) Development of public immortal mapping populations, molecular markers and linkage maps for rapid cycling Brassica rapa and B. oleracea. Theor Appl Genet 120:31–43
Jamwal RS, Sharma PP (1986) Inheritance of resistance to black rot (Xanthomonas campestris pv. campestris) in cauliflower (Brassica oleracea var. botrytis). Euphytica 35:941–943
Mayers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nagaoka T, Doullah MAU, Matsumoto S, Kawasaki S, Ishikawa T, Hori H, Okazaki K (2010) Identification of QTLs that control clubroot resistance in Brassica oleracea and comparative analysis of clubroot resistance genes between B. rapa and B. oleracea. Theor Appl Genet 120:1335–1346
Okazaki K, Sakamoto K, Kikuchi R, Saito A, Togashi E, Kuginuki Y, Matsumoto S, Hirai M (2007) Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea. Theor Appl Genet 114:595–608
Piquemal J, Cinquin E, Couton F, Rondeau C, Seignoret E, Doucet I, Perret D, Villeger M-J, Vincourt P, Blanchard P (2005) Construction of an oilseed rape (Brassica napus L.) genetic map with SSR markers. Theor Appl Genet 111:1514–1523
Shiokai S, Shirasawa K, Sato Y, Nishio T (2010) Improvement of the dot-blot-SNP technique for efficient and cost-effective genotyping. Mol Breed 25:179–185
van Ooijen JW (2006) JoinMap 4.0: Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, The Netherlands
Vicente JG, Conway J, Roberts SJ, Taylor JD (2001) Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Phytopathology 91:492–499
Wang S, Basten CJ, Zeng Z-B (2007) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh
Williams PH, Staub T, Sutton JC (1972) Inheritance of resistance in cabbage to black rot. Phytopathology 62:247–252
Acknowledgments
This work was supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (BRAIN), Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kifuji, Y., Hanzawa, H., Terasawa, Y. et al. QTL analysis of black rot resistance in cabbage using newly developed EST-SNP markers. Euphytica 190, 289–295 (2013). https://doi.org/10.1007/s10681-012-0847-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0847-1