Abstract
Thirty-nine sea beet [Beta vulgaris L. ssp. maritima (L.) Arcang.] accessions of the Adriatic coast were screened genetically and for their adaptive morpho-functional root traits in order to identify new sources of abiotic resistances for sugar beet breeding programs. Genetic diversity was evaluated with 21 microsatellites markers that identified 44 polymorphic alleles. Sea beets grouped into two main clusters: the West and the East Adriatic coast groups, with the latter showing higher genetic diversity. Among sea beet accessions with desirable root traits, four accessions have proved to be interesting for sugar beet [B. vulgaris (L.) ssp. vulgaris] breeding aimed to improve tolerance to nutritional stresses. Lastovo (ID 29) and Zut (ID 34) accessions were characterized by the highest values of RER, TRL, FRL and RSA still maintaining a high value of RTD, while Grado (ID 21) an Portic (ID 23) accessions were characterized by the highest RTD, but with low values of RER, TRL, FRL and RSA parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, crop yields have been enhanced by the increase of fertilizers and irrigation inputs, without taking into account adaptive traits improving water and soil nutrients use efficiencies (Cacco et al. 1983; Tilman 1999; Wissuwa et al. 2009). Improvement of these efficiencies in plants is strongly associated with the structure and performance of the root system (Lynch and St.Clair 2004, Lynch 2007). Selection for high yields under high-input agricultural systems has resulted in cultivars with a shallower root apparatus which hampers the acquisition of deep soil resources (Jackson 1995). Wild progenitors of crop plants tend to have root systems that can exploit more efficiently the soil environment than their cultivated relatives (Seiler 1994; Gallardo et al. 1996). Similar results were observed comparing wild and cultivated accessions of Triticum ssp., Zea mays L. and Beta vulgaris L. with the identification of specific adaptive morpho-functional traits of the root system related to stress avoidance under limited water and nutrient availability (Vamerali et al. 2003; Reynolds et al. 2007; Waines and Ehdaie 2007; Saccomani et al. 2009). In B. vulgaris L., close and positive relationships were found among the root parameters root elongation rate (RER), total root length (TRL), root surface area (RSA), number of root tips (NT), root length density (RLD), leaf water content (RWC) and sugar yield (Stevanato et al. 2010). These root parameters are the most important root system morpho-functional components related to drought-resistance and nutrient use efficiency (Marcum et al. 1995; Kell 2011). These traits determine the overall potential of the root system to quickly and thoroughly explore the soil for water and nutrients uptake. Among the root traits influencing adaptability to the environment, root tips play an important role in perceiving exogenous stress signals originating in the rhizosphere and converting those to endogenous phytohormone signals (Baluška et al. 2004; Paula and Pausas 2011).
Sugar beet is one of the most important crops supplying currently around 20 % of the world sugar consumption (Biancardi et al. 2010). Unfortunately, sugar beet varieties are characterized by a considerably narrow gene pool (McGrath et al. 2007a). Recent screening techniques based on molecular marker analysis have confirmed that modern sugar beet varieties only encompass a quarter to a third of the genetic variability present in sea beet populations (Saccomani et al. 2009). Reduced variability has mainly resulted from the introduction of cytoplasmic male sterility and monogermity for the production of commercial seed (McGrath et al. 1999). Furthermore, the introgression of genetic resistance to diseases (e.g. rhizomania and cercospora leaf spot) seems responsible for reducing root development and sugar yield in disease-free soils likely due to an increase of sugar beet sensitivity to abiotic stresses (Stevanato et al. 2006).
The wild taxa of genus Beta are important genetic resources for the breeding of cultivated beet (Ford-Lloyd 2005, Frese 2010). Among these, the most widely employed is the sea beet [B. vulgaris L. ssp. maritima (L.) Arcang.]. Sea beet is quite common along the Mediterranean, North African and European Atlantic coasts and it is considered the closest relative of the beet crops (Biancardi et al. 2012). The sea beet of Adriatic coasts in particular, has served as a very useful source of resistance to severely damaging diseases (cercospora leaf spot, rhizomania, curly top etc.). Without such resistances, it would have been impossible to continue to grow sugar beet in the main part of the currently cultivated areas (Stevanato et al. 2001; Biancardi et al. 2002). Cultivated beet is adapted to agricultural systems based on high water and nutrients supply, while sea beet is characterized by adaptive ability to limited water and nutrient stresses, probably owing to great differences in root characteristics (Saccomani et al. 2009). The susceptibility of cultivated beet to abiotic stresses highlights the need to introgress novel stress-tolerance genes (Panella and Lewellen 2007). The knowledge of genetic diversity and phylogenetic relationships between the various beet forms is essential for an efficient utilization and genetic improvement of beet germplasm (Frese 2010). Molecular markers have been widely used for population genetic studies in beets (Biancardi et al. 2010). Among them, microsatellites (SSRs) may be useful for studying gene diversity between and within beet accessions and for breeding purposes (Smulders et al. 2010). Recently, a large set of SSR markers, distributed among the nine beet linkage groups, has been published (McGrath et al. 2007b).
The purpose of this work was to extend the study of Saccomani et al. (2009) to a larger collection of sea beet accessions, in order to evaluate their genetic diversity by means of SSR markers, and their adaptive morpho-functional root traits. The final aim was to identify new sources of abiotic resistances for sugar beet breeding programs. To our knowledge, this is the first genotypic and phenotypic extensive characterization of the sea beet populations along the Adriatic coast.
Materials and methods
Plant material
Sea beet populations were sampled along the Adriatic Sea coastline of Italy, Croatia, Montenegro, Albania and Greece (Table 1; Fig. 1). The sampling of 39 populations was performed according to Bartsch and Ellstrand (1999). Briefly, populations were considered as distinct if more than 15 km apart or separated by physical barriers. For each population, seeds were collected from randomly selected plants more than 0.5 m apart. Seeds were cleaned and stored at 7 °C and 30 % relative humidity before analysis. The total number of plants and weight of seeds collected per each population were recorded.
Genotyping analysis
DNA was extracted from 100 mg young leaves tissue of eight individuals per location using DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following manufacturer procedures. DNA quality control and quantification was estimated by spectrophotometer measurement (Biophotometer, Eppendorf, Hamburg, Germany). A total of 21 SSRs markers that were uniformly distributed across the B. vulgaris genome (McGrath et al. 2007) and selected for their high polymorphism and ease of scoring in high resolution agarose gel were scored for the first time on sea beet accessions (Table 2). This markers were previously tested for reliability on numerous sugar beet breeding lines and varieties (unpublished data). Amplification of the microsatellite markers was performed in 15 μL PCR reactions containing 100 ng genomic DNA, 1x PCR buffer (EuroClone, Paignton, UK), 0.2 μM of each SSR primers, 1.8 mM MgCl2, 150 μM dNTP and 0.8 U of Euro-Taq DNA polymerase (EuroClone, Paignton, UK). Thermal cycling (TC-512; Techne inc, Burlington, NJ, US) profiles were as follows: 94 °C for 30 s, followed by 35 cycles of 94 °C for 20 s, 54 °C for 20 s, 72 °C for 50 s, followed by a final extension at 72 °C for 5 min. PCR products were separated on 2 % agarose gels (EuroClone, Paignton, UK) at 4 V cm−1 for 2 h in 1x TBE buffer, stained with ethidium bromide, visualized with UV transilluminator and scored.
Root morphology analysis
Root morphology trait were analyzed according to Saccomani et al. (2009). Seed was surface-sterilized by immersion for 10 min in 3 % (v/v) sodium hypochlorite, rinsed several times with distilled water and imbibed in aerated, deionized water at 22 °C for 12 h. Seeds were transferred to two layers of filter paper moistened with distilled water in Petri dishes placed in a germinator at 25 °C in the dark. Three-day-old seedlings with 10 ± 2 mm long seminal roots were transferred into plastic tanks over an aerated hydroponic solution containing 200 μM Ca(NO3)2, 200 μM KNO3, 200 μM MgCl2, 40 μM KH2PO4, 10 μM FeNaEDTA, 4.6 μM H3BO3, 910 nM MnCl2, 86 nM ZnCl2, 36 nM CuCl2 and 11 nM NaMoO4 (modified from Arnon and Hoagland 1940). The nutrient solution was replaced daily. The tanks were placed in a growth chamber with a 14/10 h light (300 μmol m−2 s−1)/dark cycle at 25/18 °C and 70/90 % relative humidity. Four replicates of ten seedlings per each accession were tested.
The root elongation rate (RER; mm d−1) was calculated from the difference of primary root lengths of 11-day-old compared to three-day-old seedlings. Root morphological traits were evaluated on 11-day-old seedlings using a scanner-based image analysis system (WINRHIZO Pro, Regent Instruments, QC, Canada). Before measurements, root systems were stained with 0.1 % (w/w) of toluidine blue (Sigma-Aldrich, Montréal, QC) for 15 min to increase contrast. The stained root systems were placed in 3 mm-deep water in a plexiglas tray and lateral roots were spread to minimize root overlap. The tray was scanned (STD-1600 EPSON) at 1,200 dpi resolution. Images were utilized to determine total root length (TRL; cm plant−1), fine root length of roots with diameter <0.5 mm (FRL; cm plant−1), root surface area (RSA; cm2 plant−1) and root tip density (RTD; cm−1).
Data analysis
Root trait data was subjected to ANOVA using Statistica 8.0 (StatSoft, Inc. Tulsa, OK, US). Least significant difference (LSD) test at the 0.01 probability level was used to compare data from different factors. Genotypic and morphological data was used to carry out similarity analyzes among accessions using Numerical Taxonomy System software, Version 2.1 (NTSYSpc, Exeter Software, Setauket, New York, USA) (Rohlf 2000). Genotypic data was also used for estimates of heterozygosity and genetic differentiation (F ST) among accessions by means of FSTAT software version 2.9.3.2 (Goudet 1995). Similarity matrix for SSR data was calculated using Jaccard coefficients while matrix for phenotypic data was calculated using Euclidean distances (Jaccard 1908). To visualize the genotypic and phenotypic relationships between accessions, the similarity matrixes based on the Jaccard coefficient and the Euclidean distances were used for the construction of dendrograms and principal coordinate analysis (PCO) using Sequential Agglomerative Hierarichal Nested (SAHN) and Unweighted Pair-Group Method Arithmetic (UPGMA) average procedures.
Results
Genotyping analysis
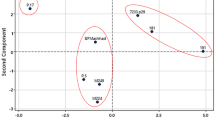
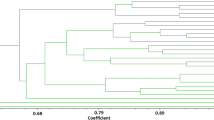
Genetic diversity was determined in 39 sea beet accessions by using 21 SSRs. SSR markers generated a total of 44 alleles among the accessions considered. The results showed that 81 % of SSRs were polymorphic. The number of alleles per SSR ranged from 1 to 4 with an average of 2.1. The average heterozygosity and PIC of all SSRs were found to be high: 0.569 and 0.608 values, respectively (Table 2). Cluster and PCO analysis revealed two distinct groups of accessions that were located respectively in the West and East Adriatic coast. The East Adriatic coast group (EA) revealed a higher genetic diversity with respect to the West Adriatic coast group (WA) (0.62 vs. 0.83 Jaccard similarity) (Fig. 2a). PCO showed that the first two main components were responsible for a total of 36 % and 23 % of the genetic differences, respectively (Fig. 2b). Genetic differentiation between these two groups was confirmed by F ST analysis (F ST = 0.473; P < 0.001).
Root morphological analysis
The morphological variation of root traits among the 39 accessions and between the two accessions groups, EA vs. WA group, deduced from SSR analysis is reported in Fig. 3. A high range of phenotypic variability for all traits considered was observed with the following percentage variations: RER (261 %), TRL (275 %), FRL (561 %), RSA (470 %) and RTD (164 %). The EA accessions showed the highest percentage variations for all traits evaluated with the exception of RTD (Table 3). Within the EA accessions, Lastovo (ID 34), followed by Zut (ID 29), was characterized by the highest values of RER, TRL, FRL and RSA still maintaining a high value of RTD (Fig. 3). Grado (ID 21) and Portic (ID 23) accessions were characterized by exceptionally high values of RTD (respectively, 7.3 and 6.7). Differently, the Vlorë accession (ID 38) showed the lowest values of all root traits with respect to the other accessions.
Based on the considered root morphological traits, the origin of the populations from either the West or the East Adriatic coasts did not influence the clustering of the wild accessions (Fig. 4). PCO revealed that the first two main components accounted for a total of 85 and 12 % of the observed phenotypic variance, respectively (Fig. 4).
Discussion
The majority of crops have been developed by a mixture of adaptive traits selected from their wild relatives during domestication processes (Goodrich and Wiener 2005; Ross-Ibarra et al. 2007). Analysis of the extent of the genotypic and phenotypic variability among breeding lines and their wild relatives is critical for the exploitation of favourable allelic combinations (Fernie et al. 2006; Panella and Lewellen 2007).
The genetic analysis highlighted the high levels of genetic polymorphism among the sea beet accessions according to previous studies (Desplanque et al. 1999; McGrath et al. 1999; Richard et al. 2004). Our results identified two distinct groups within the Adriatic Sea populations, one genetically more homogeneous along the Italian West coast and another one more variable along the East coast. Similarly, distinct groups of sea beet based on their locations were revealed by Bartsch et al. (1999). The genetic homogeneity of the sea beets of the West coast could be explained considering the vicinity of several collection sites to sugar beet crops. This could have generated stable wild beet populations after the hybridization between sugar beet and sea beet, as reported by Driessen et al. (2001) and Fénart et al. (2008). Pollen and seed mediated gene flow from cultivated to sea beet populations has been demonstrated in several areas including France (Arnaud et al. 2003, Cuguen et al. 2003; Viard et al. 2004) and north-eastern Italy (Wehres 2007). Wehres (2007) found that two of 28 examined populations along Italian Adriatic coast showed some degree of Owen’s CMS plasma presence in sea beet, indicating the occurrence of casual crosses (Driessen 2003).
The differences in the root system observed among wild accessions could depend on selective pressure exerted by the coastline soils characterized by different resources availability (Ahmad et al. 2010). Significant adjustments of root morphology to variability and scarcity of nutrient resources were observed in pasture species (Hill et al. 2006) and also in wild and cultivated beet (Saccomani et al. 2009). Wild accessions usually grow in more stressful soil environments than the cultivated plants and, therefore, they could be important source of phenotypic variation for root traits (Fita et al. 2008). The most interesting wild accession is Lastovo (ID 34) which is characterized by high RTD and showed the highest root adaptive traits (RER, TRL, FRL and RSA) among the evaluated sea beets. These key root traits are related to a rapid adaptation to soil environments (Stevanato et al. 2010). Therefore, it would be plausible to assume that the Lastovo (ID 34) accession, for its morphological similarity with the cultivated beets (Saccomani et al. 2009; Stevanato et al. 2010), could have been derived by pollen and seed mediated gene flow. However, the absence of sugar beet cultivation in the vicinity of the Lastovo Island evidenced the true wild origin of this population and further strengthened its importance in breeding programs. Furthermore, this accession should be particularly suitable to sugar beet breeders because the presence of rhizomania (Rz1) resistance, revealed by molecular analysis (unpublished results), did not lower root development as frequently observed in the cultivated varieties (Stevanato et al. 2006). Among sea beet accessions with desirable root traits, Zut (ID 29) also showed high values of root adaptive traits which enable plants to maintain relatively high water and nutrient acquisition at low availability of these soil resources (Ryser and Lambers 1995; Vamerali et al. 2003). Furthermore, Grado (ID 21) and Portic (ID 23) are characterized by the highest RTD. This character is one of the most important adaptive traits to maximize resources exploitation in low-inputs agronomic systems (Saccomani et al. 2009; Stevanato et al. 2010) since root tips are important sites of hormones biosynthesis involved in determining the behaviour and adaptability of plants to adverse environmental conditions (Barlow 2010; Baluška et al. 2010, Calvo Garzón and Keijzer 2011). In addition, Farzad Haerizadeh et al. (2011) and Kyndt et al. (2012) respectively reported in soybean and maize that many genes involved in defense signaling were upregulated in root tips and Rellán-Álvarez et al. (Rellán-Alvarez et al. 2010) have evidenced significant changes induced in the root tip proteome and metabolome of sugar beet plants in response to iron deficiency. A previous study evidenced particularly high general and specific combining ability effects for RTD (Stevanato et al. 2006). Thus, these accessions appeared to be ideal candidates for breeding programs. The accession Vlorë (ID 38), living in the saltern of Vlorë and characterized by the lowest root growth parameters, has certainly developed a high tolerance to salinity stress appearing to be of great breeding interest.
Conclusion
These results highlight that sea beet populations collected along the Adriatic Sea coastline clustered into two main geographical groups: the West and the East Adriatic coast groups, with the latter showing higher genetic diversity. The morphological analysis identified some interesting accessions characterized by stress-tolerant root adaptive traits. These accessions will be further evaluated for their use as parental lines in breeding programs aimed to develop sugar beet varieties with greater adaptability to abiotic stresses.
Abbreviations
- RER:
-
Root elongation rate
- TRL:
-
Total root length
- FRL:
-
Fine root length
- RSA:
-
Root surface area
- RTD:
-
Root tips density
References
Ahmad MSA, Ashraf M, Ali Q (2010) Soil salinity as a selection pressure is a key determinant for the evolution of salt tolerance in Blue Panicgrass (Panicum antidotale Retz.). Flora 205:37–45
Arnaud JF, Viard F, Delescluse M, Cuguen J (2003) Evidence for gene flow via seed dispersal from crop to wild relatives in Beta vulgaris (Chenopodiaceae): consequences for the release of genetically modified crop species with weedy lineages. Proc R Soc Lond B Biol Sci 270:1565–1571
Arnon DI, Hoagland DR (1940) Crop production in artificial culture solution and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci 50:463–483
Baluška F, Mancuso S, Volkmann D, Barlow P (2004) Root apices as plant command centres: the unique ‘brain-like’ status of the root apex transition zone. Biologia (Bratisl) 13:1–13
Baluška F, Mancuso S, Volkmann D, Barlow P (2010) Root apex transition zone: a signalling-response nexus in the root. Trends in Plant Sci 15:402–408
Barlow P (2010) Plant roots: autopoietic and cognitive constructions. Plant Root 4:40–52
Bartsch D, Ellstrand NC (1999) Genetic evidence for the origin of Californian wild beets (genus Beta). Theor Appl Genet 99:1120–1130
Bartsch D, Lehnen M, Clegg J, Pohl-Orf M, Schuphan I, Ellstrand NC (1999) Impact of gene flow from cultivated beet on genetic diversity of wild sea beet populations. Mol Ecol 8:1733–1741
Biancardi E, Lewellen RT, De Biaggi M, Erichsen AW, Stevanato P (2002) The origin of rhizomania resistance in sugar beet. Euphytica 127:383–397
Biancardi E, McGrath JM, Panella LW, Lewellen RT, Stevanato P (2010) Sugar beet. In: Bradshow J (ed) Handbook of Plant Breeding, vol 4, Tuber and Root Crops. Springer, New York, pp 173–219
Biancardi E, Panella LW, Lewellen RT (2012) Beta maritima: the origin of beets. Springer, New York, pp 293
Butterfass T (1964) Die Chloroplastenzahlen in verschiedenartigen Zellen trisomer Zuckerrüben (Beta vulgaris L.). Z Bot 52:46–77
Cacco G, Saccomani M, Ferrari G (1983) Changes in the uptake and assimilation efficiency for sulfate and nitrate in maize hybrids selected during the period 1930 through 1975. Physiol Plant 58:171–174
Calvo Garzón P, Keijzer F (2011) Plants: adaptive behavior, root-brains, and minimal cognition. Adaptive Behavior 19:155–171
Cuguen J, Arnaud JF, Delescluse M, Viard F (2003) Crop-wild interaction within the Beta vulgaris complex: a comparative analysis between seabeet and weed beet populations within the French sugarbeet production area. In: Nijs HCMD, Bartsch D, Sweet J (eds) Introgression from genetically modified plants into wild relatives. CABI, Wallingford, pp 183–201
Desplanque B, Boudry P, Broomberg K, Saumitou-Laprade P, Cuguen J, van Dijk H (1999) Genetic diversity and gene Xow between wild, cultivated and weedy forms of Beta vulgaris L. (Chenopodiaceae), assessed by RFLP and PCR-based methods. Theor Appl Genet 98:1194–1201
Driessen S (2003) Beta vulgaris ssp. maritima an Deutschlands Ostseeküste. Dissertation, RWTH Aachen University
Driessen S, Pohl M, Bartsch D (2001) RAPD-PCR analysis of the genetic origin of sea beet (Beta vulgaris ssp. maritima) at Germany’s Baltic Sea coast. Basic Appl Ecol 2:341–349
Farzad Haerizadeh A, Mohan B, Singh A, Prem L, Bhalla AB (2011) Transcriptome profiling of soybean root tips. Funct Plant Biol 38:451–461
Fénart S, Arnaud JF, De Cauwer I, Cuguen J (2008) Nuclear and cytoplasmic genetic diversity in weed beet and sugar beet accessions compared to wild relatives: new insights into the genetic relationships within the Beta vulgaris complex species. Theor Appl Genet 116:1063–1077
Fernie AR, Tadmor Y, Zamir D (2006) Natural genetic variation for improving crop quality. Curr Opin Plant Biol 9:196–202
Fita A, Roig C, Picó B, Nuez F (2008) Natural variation in root structure within Cucumis melo L studied in vitro. In: Prohens J, Badenes ML (eds) Modern variety breeding for present and future needs. p 372–376
Ford-Lloyd BV (2005) Sources of genetic variation, genus Beta. In: Biancardi E, Campbell LG, Skaracis GN, De Biaggi M (eds) Genetics and breeding of sugar beet. Science Publishers Inc, Enfield, pp 25–33
Frese L (2010) Conservation and access to sugarbeet germplasm. Sugar Tech 12:207–219
Gallardo M, Jackson LE, Thompson RB (1996) Shoot and root physiological responses to localized zones of soil moisture in cultivated and wild lettuce (Lactuca spp.). Plant Cell Environ 19:1169–1178
Goodrich J, Wiener P (2005) A walk from the wild side: the genetics of domestication of livestock and crops. BioEssays 27:574–576
Goudet J (1995) FSTAT, a program for IBM PC compatibles to calculate Weir and Cokerham’s (1984) estimators of F-statistics. J Hered 86:485–486
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Jackson LE (1995) Root architecture in cultivated and wild lettuce (Lactuca spp.). Plant Cell Env 18:885–894
Kell DB (2011) Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Ann Botany 108:407–418
Kyndt T, Denil S, Haegeman A, Trooskens G, De Meyer T, Van Criekinge W, Gheysen G (2012) Transcriptome analysis of rice mature root tissue and root tips in early development by massive parallel sequencing. J Exp Bot 63:2141–2157
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512
Lynch JP, St.Clair SB (2004) Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Field Crops Res 90:101–115
Marcum KB, Engelke MC, Morton SJ, White RH (1995) Rooting characteristics and associated drought resistance of zoysiagrass. Agron J 87:534–538
McGrath JM, Derrico CA, Yu Y (1999) Genetic diversity in selected, historical US sugarbeet germplasm and Beta vulgaris ssp. maritima. Theor Appl Genet 98:968–976
McGrath JM, Saccomani M, Stevanato P, Biancardi E (2007a) Genome Mapping and Molecular Breeding in Plants. In: Kole C (ed) Vegetables, vol 5. Springer-Verlag, Berlin Heidelberg, pp 135–151
McGrath JM, Trebbi D, Fenwick A, Panella L, Schulz B, Laurent V, Steve B, Murray SC (2007b) An open-source first-generation molecular genetic map from a sugarbeet x table beet cross and its extension to physical mapping. Plant Genome 47:S27–S44
Panella L, Lewellen RT (2007) Broadening the genetic base of sugar beet: introgression from wild relatives. Euphytica 154:383–400
Paula S, Pausas JG (2011) Root traits explain different foraging strategies between resprouting life histories. Oecologia 165:321–331
Rellán-Alvarez R, Andaluz S, Rodríguez-Celma J, Wohlgemuth G, Zocchi G, Alvarez-Fernández A, Fiehn O, López-Millán AF, Abadía J (2010) Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol 21(10):120
Reynolds M, Dreccer F, Trethowan R (2007) Drought-adaptive traits derived from wheat wild relatives and landraces. J Exp Bot 58:177–186
Richards CM, Brownson M, Mitchell SE, Kresovich S, Panella L (2004) Polymorphic microsatellite markers for inferring diversity in wild and domesticated sugar beet (Beta vulgaris). Mol Ecol Notes 4:243–245
Rohlf FJ (2000) NTSYS-pc: numerical taxonomy and multivariate analysis system. Version 2.1 manual. Applied Biostatistics Inc, New York
Ross-Ibarra J, Morell PL, Gaut BS (2007) Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc Natl Acad Sci 104:8641–8648
Ryser P, Lambers H (1995) Root and leaf attributes accounting for the performance of fast- and slow-growing grasses at different nutrient supply. Plant Soil 170:251–265
Saccomani M, Stevanato P, Trebbi D, McGrath JM, Biancardi E (2009) Molecular and morpho-physiological characterization of sea, ruderal and cultivated beets. Euphytica 169:19–29
Seiler GJ (1994) Primary and lateral root elongation of sunflower seedlings. Environ Exp Bot 34:409–418
Smulders MJM, Esselink GD, Everaert I, Riek JD, Vosman B (2010) Characterisation of sugar beet (Beta vulgaris L. ssp. vulgaris) varieties using microsatellite markers. BMC Genet 11:41
Stevanato P, De Biaggi M, Skaracis GN, Colombo M, Mandolino G, Biancardi E (2001) The sea beet (Beta vulgaris L. ssp. maritima) of the Adriatic coast as source of resistance for sugar beet. Sugar Tech 3:77–82
Stevanato P, Cagnin M, Saccomani M (2006) Meccanismi morfofisiologici di adattamento allo stress idrico-nutrizionale in barbabietola da zucchero. Agroindustria 5:131–136
Stevanato P, Trebbi D, Saccomani M (2010) Root traits and yield in sugar beet: identification of AFLP markers associated with root elongation rate. Euphytica 173:289–298
Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci USA 96:5995–6000
Vamerali T, Saccomani M, Bona S, Mosca G, Guarise M, Ganis A (2003) A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids. Plant Soil 255:157–167
Viard F, Arnaud JF, Delescluse M, Cuguen J (2004) Tracing back seed and pollen flow within the crop-wild Beta vulgaris complex: genetic distinctiveness vs. hot spots of hybridization over a regional scale. Mol Ecol 13:357–1364
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Ann Bot 100:991–999
Wehres U (2007) Untersuchungen zu potentiellen ökologischen Effekten von gentechnisch vermittelter Nematodenresistenz auf pflanzengenetische Ressourcen am Beispiel der Zuckerrüben-Wildform (Beta vulgaris ssp. maritima). Dissertation, RWTH Aachen University
Wissuwa M, Mazzola M, Picard C (2009) Novel approaches in plant breeding for rhizosphere-related traits. Plant Soil 321:409–430
Acknowledgments
The authors wish to thank Mr. Franco Bertaggia and Ms. Roberta Pellizzato for their kind help during sea beet sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stevanato, P., Trebbi, D., Biancardi, E. et al. Evaluation of genetic diversity and root traits of sea beet accessions of the Adriatic Sea coast. Euphytica 189, 135–146 (2013). https://doi.org/10.1007/s10681-012-0775-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0775-0