Abstract
Firmness is an indicator of fruit freshness and a main component of tomato (Solanum lycopersicum) fruit texture. In this work, the genetic variability in fruit firmness and stiffness was analyzed in pre- and postharvest periods and underlying anatomical and biochemical traits were identified. Three tomato contrasted parental lines and six derived quantitative trait loci (QTL)-NILs harboring texture QTL on chromosome 4 (QTL4) and 9 (QTL9) were analyzed; the seasonal variability was assessed on two distant trusses. Firmness and stiffness were measured by compression and puncture tests at harvest and after 7-day storage at 20 °C. QTL4 poorly influenced the textural variables, on the contrary to QTL9 which increased firmness measured by puncture test and had similar effects in the two genetic backgrounds. According to this test, firmness increased along the season, but ranks among genotypes and QTL effects were hardly affected. Only some of the QTL effects were still significant after storage and firmness losses were not predicted by firmness at harvest. Fruit firmness and stiffness measured by puncture tests correlated with both morphological (locular number, R = −0.89), histological (cell size, R < −0.80) and biochemical (dry matter (R > 0.82) and soluble sugar content (R < −0.74)) fruit traits. In contrast, compression test values hardly correlated with any of the measured traits. This work provided an original comprehensive approach to analyse fleshy fruit firmness and paves the way for a future predictive model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding and controlling the texture of tomato (Solanum lycopersicum L.) fruit is crucial for consumer acceptance, shelf life, and transportability. After flavour, texture is the first criteria of quality perception by consumers (Causse et al. 2003; Sinesio et al. 2010; Szczesniak 2002), which impacts on the whole organoleptic quality (Seymour et al. 2002), and strongly influences flavour and aroma perception (Causse et al. 2003). Texture is a complex trait that includes several components, such as firmness, meltiness, mealiness, juiciness and crunchiness (Harker et al. 2002). Due to this complexity, quantifying texture through measurements is not straightforward. It can be evaluated by sensory analysis (Szczesniak 2002), or it can be objectively measured by instrumental methods, including mechanical measurements (Abotts 1999; Desmet et al. 2002), magnetic resonance imaging, or sonic and ultrasonic techniques (Abotts 1999; Musse et al. 2009; Kim et al. 2009). The most common mechanical methods are compression and puncture tests (Barrett et al. 2010), which mainly evaluate fruit or tissue firmness and elasticity, and usually correlate well with sensory evaluation (Bourne 1979; Barrett et al. 1998; Camps et al. 2005; Causse et al. 2002; Mehinagic et al. 2004).
Fruit firmness depends on several factors, such as genotype (Saliba-Colombani et al. 2001), growth conditions (Sams 1999; Rosales et al. 2009), harvest and postharvest conditions (Ketelaere et al. 2004; Moneruzzaman et al. 2008; Page et al. 2010). For instance, water supply plays a major role, although conflicting results have been reported (Van Hooijdonk et al. 2007; Perez-Pastor et al. 2007). From the genetic point of view, few studies have been directly oriented towards fruit textural traits. Some of them revealed several quantitative trait loci (QTL) of textural traits (review by Labate et al. 2007 for tomato fruit), but most of them have focussed on the characterization of mutants affected in ripening related genes. For instance, the tomato ripening inhibitor (rin) gene, non-ripening (nor) gene, delayed-fruit deterioration (DFD) gene or colorless non-ripening (Cnr) gene (Giovannoni 2004; Saladié et al. 2007; Seymour et al. 2002). Also, QTL detection for manual or instrumental firmness has been performed on apple fruit (King et al. 2000; Longhi et al. 2012), melon (Obando et al. 2008), strawberry (Lerceteau-Kohler et al. 2004) and peach (Ogundiwin et al. 2007). In most cases, several QTL were detected, which were spread out over the whole genome. In the case of tomato, a few genome regions appeared as particularly important for the control of fruit firmness (Chaib et al. 2007; Labate et al. 2007). A subtractive cDNA bank was produced between near isogenic lines (NILs) differing for QTL regions, which allowed the isolation of genes putatively related to tomato fruit quality, but full coincidence between functional genes and QTL has been rarely shown (Page et al. 2008).
Mechanisms underlying fruit texture are complex and partly species dependent (Goulao and Oliveira 2008). A great deal of work has focussed on the molecular and biochemical mechanisms that lead to fruit softening during ripening. In particular its relation to the increased expression of numerous cell wall modifying enzymes (Brummell and Harpster 2001; Toivonen and Brummell 2008; Goulao and Oliveira 2008). However, fruit texture might already be determined during the fruit growth period (Chaib et al. 2007) and, thus, likely involves various mechanisms. Several works have focused on the importance of fruit anatomical and histological properties (Barrett et al. 1998). At the fruit scale, the proportion and thickness of the different tissues determine fruit texture (Bourne 2002). At the tissue scale, the cellular structure is likely involved in fruit mechanical properties, such as firmness (Allende et al. 2004; Chaib et al. 2007; Legland et al. 2009; Mann et al. 2005). Moreover, cell turgor (Shackel et al. 1991; Vicente et al. 2007), transport of solutes among cell compartments (Almeida and Huber 1999), transpiration water loss and cuticle structure (Saladié et al. 2007) contribute to textural properties.
As during the expansion phase, several mechanisms influence the postharvest evolution of fruit texture as natural senescence continues after harvest. The effects of temperature storage and atmospheric composition have been primarily investigated. In particular, low temperatures, used by retailers or consumers to extend fruit shelf life, may trigger physiological disorders and loss of quality, ultimately resulting in chilling injury (Lyons 1973) and relative symptoms such as a rubbery texture, watery flesh and irregular ripening (Stevens et al. 2008). A recent study, which compared two genotypes with contrasting firmness, outlined that storage ability is not necessarily linked to fruit firmness measured at harvest (Page et al. 2010).
In order to hierarchize and identify interactions among key-factors of fruit textural traits, the present study investigated tomato firmness and stiffness at harvest and in the postharvest period in response to genetic variations and to genetic, annual and seasonal interactions. Variations in textural variables were analyzed in relation to several anatomical and biochemical parameters that are linked to growth processes, such as cell division and expansion, carbon and water accumulation, carbon partitioning among soluble and insoluble compounds, soluble sugar accumulation, fruit conductivity and tissue proportions. Firmness was measured by compression and puncture methods. Nine genotypes were investigated, including three parental lines presenting contrasted textural properties in pre- and postharvest periods (Lecomte et al. 2004; Chaib et al. 2007) and six NILs carrying QTL for fruit texture. These QTL, involved in physical and sensory components of tomato fruit texture, are located on chromosomes 4 and 9, in two genome regions where texture QTL have been also detected in several other tomato crosses involving S. peruvianum and S. pimpinellifolium (reviewed by Labate et al. 2007). Finally, the present work provides several important insights and perspectives for the control of fruit texture variations, because it establishes a comprehensive network of the traits involved.
Materials and methods
Plant material
This study investigated nine genotypes which were the following: three parental lines (Cervil, Levovil and VilB) on which QTL of fruit quality traits have been previously detected (Causse et al. 2001, 2002) and six NILs that carry homozygous alleles from Cervil at QTL bearing introgressed regions into the Levovil (Lev) and VilB genetic backgrounds (Online Resource 1). Lev is a large-fruited line with low firmness, and VilB is also a large-fruited line, but firmer and with better postharvest performance than Lev. The initial QTL analysis was performed on a population of recombinant inbred lines (RILs) developed from an intraspecific cross, between Cervil (cherry tomato) and Levovil (large fruited tomato) (Causse et al. 2002). Based on the QTL map, two regions (located on chromosomes 4 and 9) harboring QTL for fruit texture (firmness, elasticity, mealiness, and meltiness) were introgressed into the two genetic backgrounds (Chaib et al. 2007). The introgressed lines were produced as described in Chaib et al. (2006). NIL-L4, NIL-L9 and NIL-B9 were obtained after three backcrosses on the recipient line followed by three selfing generations (Lecomte et al. 2004), where L and B indicate the genetic background (Lev and VilB, respectively) and the number, 4 or 9, indicates chromosome number. Introgressed segments in NIL-L4, NIL-L9 and NIL-B9 span over respectively, 19.7, 51.6 and 51.6 cM. NIL-L4-1, NIL-L4-2 and NIL-L9-1 are sub-NILS bearing shorter introgressed regions (10.9, 8.9 and 36.3 cM, respectively), which were obtained from a fourth backcross with the recurrent parent followed by a fourth selfing.
Cultural conditions
Two experiments were performed. The first one (Exp.1) was carried out over 2 years in 2008 and 2009 with all genotypes. Its aims were as follows: (i) studying QTL × genetic background interaction effects on fruit firmness, and (ii) evaluating QTL stability during fruit conservation. The second experiment (Exp.2) performed in 2009, had the following intentions: (i) evaluate QTL by season interactions, and (ii) shed light on the correlations between fruit firmness and several physical and biochemical traits. Exp.2 focussed on the three parental lines and four QTL-NILs (NIL-L4, NIL-L9, NIL-L9-1, and NIL-B9).
The two experiments were carried out in the same greenhouse in Avignon, Southern France. Plants were grown during the spring season (from January to July) at a density of 2.5 plants m−2. Day/night temperature in the greenhouse was set-up to 20/18 °C. Nutrients were provided by fertigation (EC 2.0, pH 6.5) and no water stress occurred. Side shoots were removed once a week. Flowers were shaken three times a week for pollination and inflorescence size was naturally regulated by the plant (around five to eight flowers on all genotypes except Cervil which can bear more than 20 flowers per inflorescence). Each genotype was represented by 10 plants.
Fruit sampling
In Exp.1, 15–35 red-ripe fruits from each genotype were harvested on the second, third and fourth trusses, avoiding the first and last fruits from each truss. Six harvest dates were necessary over 2 weeks and firmness was immediately measured at harvest (T0). In 2009, at each harvest date half of the fruits were stored at room temperature (20 °C) during 7 days, after which postharvest firmness (T7) was determined.
In Exp.2, the seasonal variations in fruit firmness and other physiological traits were investigated by comparing fruits harvested on the first and fifth truss in May and June, respectively. Seasonal effects included climatic factors and plant factors, such as the competition among growing fruits which increased during plant development. Red-ripe fruits from each genotype were individually harvested on both trusses, avoiding the first and last fruits. Firmness was immediately evaluated on at least 10 fruits (20 fruits for Cervil) that were randomly selected, and, then, anatomical and biochemical measurements were performed on the same and/or on other fruits of the same truss. Each fruit was cut into two halves (except Cervil) in order to measure several traits on a given individual fruit (see below).
Biological measurements
Firmness evaluation
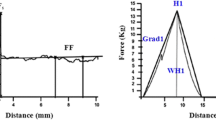
Firmness was measured with a texturometer (Texture analyser TAplus: Ametek, Lloyd Instruments Ltd., Fareham, UK) through three rheological tests applied either on whole fruits or on fruit slices. First, the uniaxial compression test (CP test) measures the force required to compress the fruit between two steel plates until its diameter decreases by 3 % of its initial diameter. This test evaluates the global fruit properties, including its hardness, elasticity and plasticity. The resulting force/displacement curves were analyzed and two parameters were recorded: the maximum force (CPmax in N) associated with fruit hardness, and the slope of the compression (CPslope in N m−1) associated with fruit stiffness (Young’s modulus), which has been often assimilated to fruit elasticity in past studies. Second, a fruit puncture test (FP test) was applied on the whole fruit using a cylindrical probe of 2 mm diameter, which was placed perpendicular to the main axis of the fruit (avoiding septum) and displaced 7 mm. Data obtained from force/displacement curves were expressed as the maximum force (FPmax in N), the slope (FPslope in N m−1) and the maximum deformation (FPdef in m) necessary to punch the skin (cuticle and epidermis layer) which are linked to, respectively, firmness, stiffness and elasticity (Camps et al. 2005). Third, a slice puncture test (SP test) was performed on fruit radial slices (1 cm thick). For each slice, four replicate tests were performed in the outer pericarp avoiding the areas where the outer pericap joins the radial pericarp. The average maximum force (SPmax in N) run into the slice was recorded as an indicator of flesh firmness.
Anatomical and biochemical traits
In Exp.2, morphological and chemical fruit properties were measured on individual fruits picked on the first and fifth trusses. For each genotype, the locule number was recorded, and the individual fruit fresh weight, dry weight, soluble sugar (glucose, fructose, sucrose) and alcohol insoluble solid (AIS) contents were measured on six to eight fruits. On eight other fruits from each truss, the water conductance was estimated by weight loss of detached fruits stored in a controlled climatic chamber, as described in Leonardi et al. (2000). Proportions of the different tissues were assessed on six fruits from each truss. The pericarp, gel, columella and seeds were separated and their fresh weight and dry weight were measured. The number and mean size of pericarp cells were measured according to the method described in Bertin et al. (2002). Finally, the pericarp content in AIS and soluble sugars were analysed on eight individual fruits.
Fruit or tissue dry weight was obtained after 72 h desiccation in a ventilated oven at 80 °C. For sugar analysis, tissues or fruits were immediately frozen in liquid nitrogen after sampling, ground, and stored at −20 °C. Soluble sugars (sucrose, fructose, glucose) were extracted and measured by HPLC (Gomez et al. 2002). AIS content was measured on lyophilized powder by extraction and elimination of soluble molecules in an 80 % ethanol solution mix after starch hydrolysis by amyloglucosidase (Quemener et al. 2007).
Statistical analysis
The genetic, QTL, year, conservation and seasonal effects on textural traits were evaluated by two-way ANOVA using Sigmastat Jandel Scientific software (V2.03, SPSS Inc., Chicago, IL, USA). These ANOVA were first performed to compare the three parental lines (Cervil, Levovil and VilB) among themselves. Then, the QTL effects were analyzed in each genetic background (L or B). When significant effects were detected, Tukey or Dunnett’s tests were applied to compare, respectively, the three parental lines and the QTL-NILs with their parent in each background. Significant effects were reported when p values were lower than 0.05. On all of reported figures, the data are presented as mean values with vertical bars indicating standard errors.
A principal component analysis was performed (STATISTICA software) to track the genetic and seasonal variability among anatomical, biochemical and textural fruit traits measured in Exp.2. Then, correlations among fruit traits were visualized and analyzed by means of a correlation network. Pearson product-moment correlations were calculated between the genetic means of pairs of variables. For all pairwise correlations, P-values were calculated and only significant correlations (P < 1 %, |r| > 0.7, tested with cor.test R-function) were plotted. The network was constructed using the Pajek graph drawing software version 2.02 (Batagelj et al. 2003), based on the Fruchterman–Reingold algorithm. The network relied on the correlation matrix resulting from four textural variables and 30 morphological, histological and biochemical variables, which were measured on six genotypes (Lev, VilB, NIL-L4, NIL-L9, NIL-L9-1, and NIL-B9) and two different trusses (Tr1 and Tr5). Cervil was discarded to avoid bias, because of its huge difference in some traits, such as fruit size. The topological properties of the network were defined by the betweenness centrality. Betweenness is a centrality measure of a vertex within a graph, which represents, for each node, the number of shortest paths between all pairs of nodes that pass through this node (Freeman 1977). Betweenness centrality values were used to identify some important nodes in the network. Nodes that occur on many shortest paths between other vertices have higher betweenness than those that do not. Then, in order to better describe the dependence of textural variables on anatomical and biochemical variables, forward stepwise regressions were performed (Sigmastat Jandel Scientific software (V2.03, SPSS Inc., Chicago, IL, USA)).
Results
QTL and genetic background effects on several indicators of fruit texture
Three rheological tests (compression and puncture tests) were performed over 2 years (2008, 2009) on nine tomato genotypes. Maximum forces and slopes of the force/displacement curves were used to analyze the genetic variation over 2 years (Fig. 1). The most significant effects detected by ANOVA are summarized in Table 1.
Fruit textural parameters evaluated through maximum forces, slopes and distance of the force–displacement curves measured over 2 years by compression (CP) and puncture (FP and SP) tests on red ripe tomato fruits. Compression test (a–d) and fruit puncture test (b, e, f) were performed on whole fruit, while slice fruit puncture test (c) was performed on 1 cm thick tomato slice (not applied to Cervil because of the small fruits size). Nine tomato lines were investigated in 2008 (grey bars) and 2009 (black bars). Cervil was measured only in 2009. Data are means of 15–20 fruits with vertical bars indicating standard errors
The three parental lines (Cervil, Levovil, VilB) could be significantly (P < 0.01, df = 65) discriminated with the three tests over the 2 years (Fig. 1). Maximum forces and slopes measured on the VilB fruits were the highest in contrast to those measured on the Cervil fruits. On average, CPslope was higher (P = 0.015, df = 65) and SPmax was lower (P = 0.001, df = 65) in 2008 than in 2009, whereas the FP test did not significantly discriminate between the 2 years.
Within the L background, both year and QTL effects on the textural variables were significant (P < 0.01, df = 254), but the significant interactions between the two factors suggested that some of the QTL effects were unstable (Table 1). Compared to Lev, CPmax and CPslope were significantly decreased in NIL-L9 fruits on both years, whereas CPslope was significantly increased in NIL-L4-1 fruits in 2009 only. In contrast, FPmax was significantly higher for all NILs except NIL-L4 and NIL-L4-1, independent of the year. FPslope measured on NIL-L9 and NIL-L9-1 fruits were, respectively, significantly lower and higher than on Lev fruits. On fruit slices, a significant increase in SPmax was measured on NIL-L9-1 fruits compared to Lev fruits on both years. In contrast, SPmax was increased in NIL-L9 fruits and decreased in NIL-L4 and NIL-L4-1 fruits, but only in 2009.
Within the B genetic background, FPmax and SPmax were higher for NIL-B9 than for VilB (P < 0.01, df = 70; no significant interaction with the year effect), whereas the opposite was observed for CPmax. Whatever the year, a significant decrease in CPslope and FPslope was measured in NIL-B9 compared to VilB. No significant difference was measured between the 2 years.
The comparison of NIL-L9 and NIL-B9 (Table 1) indicated that the QTL9 had similar effects on the textural variables in the two genetic backgrounds. Over the 2 years, this QTL increased the SPmax value by 24 % in the L background and by 64 % in the B background.
Effects of QTL and genetic background on fruit firmness loss in the postharvest period
In 2009, the three tests (CP, FP, SP) have been performed on red-ripe fruit at harvest (T0), and then after a 7-day period of fruit storage at 20 °C (T7) to quantify the relative loss of firmness in the postharvest period (Fig. 2). Negative values resulted from the fact that different fruit batches were used at T0 and T7, as puncture tests are destructive. CPmax decreased much more (from 28 to 41 %) than FPmax (from 2.0 to 22 %), whereas changes in SPmax were the most heterogeneous (from −2 to 50 %). CPslope and FPslope also decreased in the storage period (data not shown). Two-way ANOVAs were run to evaluate absolute variations in textural variables between T0 and T7 and to see whether ranks among genotypes and QTL effects were affected by storage (Table 1; Fig. 3).
Fruit firmness loss during postharvest storage measured on the three parental lines and six QTL-NILs. Fruits were harvested at red ripe stage in 2009 and stored at 20 °C during 7 days. Firmness was assessed at harvest (T0) and after 7 days (T7) by fruit compression test (a), whole fruit puncture test (b) and slice puncture test (c). The loss of firmness was calculated as (T0–T7)/T0 from data measured on 35 (CP tests) and 17 (FP and SP tests) fruits
Relationship between the relative losses of firmness during postharvest storage and firmness measured at harvest by puncture test applied at the fruit (a) and pericarp (b) level. Cervil, Levovil and VilB are indicated by black symbols, respectively, square, circle and triangle. The L and B backgrounds are represented, respectively, by circles and triangles
After a 7-day period at 20 °C, fruits from the parental lines lost on average 6 mg of fresh weight (not significant). During this period, none of the variables measured on Cervil fruits significantly varied. On the other hand, significant decreases were observed, for VilB fruits, in CPmax, FPmax and SPmax, and, for Lev fruits, in CPmax, and SPmax (P < 0.001, df = 100). For both genotypes, CPslope and FPslope decreased significantly during storage. Ranks among genotypes were similar at T0 and T7. Despite contrasted rates of evolution, maximum forces and slopes were still the highest in VilB fruits after 7 days of storage at 20 °C (Table 1).
In the L and B backgrounds, fruits lost on average 9 and 4 mg of fresh weight, respectively (not significant). Regardless of the test, absolute variations in textural variables during storage were significant for all genotypes (P < 0.01, df = 394), except variations in FPmax and SPmax measured on NIL-L4-1 fruits and SPmax measured on NIL-L4 and NIL-L9-1 fruits. Similarly, CPslope and FPslope significantly decreased in the postharvest period for all genotypes. According to the compression test (CP), ranks among genotypes and QTL effects hardly changed during the storage period, irrespective of the genetic background (Table 1). In contrast, all QTL effects measured with puncture tests (FP or SP) were lost in the postharvest period, except in two cases: NIL-L9-1 and NIL-B9 for which positive QTL effects on, respectively, SPmax and FPmax remained significant. The relative decreases in FPmax and SPmax during the storage period were independent of the values measured at harvest (Fig. 3a: R 2 = 0.05 and Fig. 3b: R 2 = 0.453).
Relationships between fruit fresh weight and pericarp cell number (a) and mean cell size (b). Each point represents one genotype and one truss position (first or fifth truss on the stem) and is a mean of six fruits. The determination coefficient of the linear relationship between fruit fresh weight and cell number (a) was R 2 = 0.89 considering all genotypes and R 2 = 0.80 excluding Cervil. The determination coefficient of the linear relationship between fruit fresh weight and cell volume (b) was R 2 = 0.69 considering all genotypes and R 2 = 0.41 excluding Cervil
Genetic and seasonal variability of textural, anatomical and biochemical fruit traits
The seasonal variability in textural variables was assessed by comparing fruits sampled on the first (Tr1) and fifth (Tr5) trusses from seven genotypes (Cervil, Lev, VilB, NIL-L9, NIL-L9-1, NIL-L4, NIL-B9). In addition, several anatomical and biochemical fruit traits were measured on the two trusses (Table 2 and Online Resource 2) in order to analyze their genetic variability and involvement in fruit textural properties.
The fruit fresh weight was significantly lower on Tr5 than on Tr1 for all genotypes (−48 % in average) except Cervil (+40 %), indicating that the competition for assimilates increased during plant development. Except for Cervil, fruits sampled on Tr5 contained less and smaller pericarp cells than fruits picked on Tr1, though differences were not significant for NIL-L4 (cell number and size), NIL-L9-1 and NIL-B9 (cell number). The final fruit fresh weight and the pericarp cell number were significantly smaller in the QTL-NILs than in their parents. A unique linear relationship between the fruit fresh weight and cell number was observed (Fig. 4a) accounting for both genotype and seasonal effects, while the fruit fresh weight was less correlated to the mean cell volume (Fig. 4b) than to the cell number. Variations in dm, AIS and soluble sugar contents were highly dependent on the genotype and no clear trends could be drawn (Table 2). Similarly, the fruit conductance was highly variable. On Tr1, this might result from the high fruit growth rate leading to micro-cracks at the fruit surface (Gibert et al. 2010). For that reason, genetic differences in fruit conductance were considered on Tr5 only in the following analysis. Among the three parental lines, the proportion of pericarp was significantly higher on Tr5 than on Tr1 (Online Resource 2). On the contrary, the seed number (Lev only) and proportions of columella (VilB only) and gel were higher on Tr1 than on Tr5. The proportion of pericarp was the lowest in Cervil (50 %) and the highest in VilB (75 %). In contrast, the proportions of columella and gel were significantly higher in Cervil fruits. Within the L background, fruits sampled on Tr5 contained less seeds than fruits picked on Tr1, while the relative proportion of columella was reduced on Tr5 in each genetic background. QTL effects on tissue proportions were weak, except the seed contribution which was increased in NIL-L9 and NIL-B9 compared to their respective parent. In the B background, QTL introgression on chromosome 9 also had some negative effects on the proportion of columella.
Textural variables varied between the two trusses depending on genotype and test. The general trend shows that CPmax was higher on Tr1 than on Tr5 (+24 % for VilB and NIL-B9), whereas CPslope was lower (−18 % in the L background). On the opposite, FPmax and SPmax were higher on Tr5 than on Tr1 (+8, 13 and 26 % for VilB, Cervil and Lev respectively), whereas FPslope was lower (−15 and −23 % for VilB and NIL-B9, respectively). However, ranks among genotypes and QTL effects were not significantly dependent on the truss position, except for the B background because no significant QTL effect on SPmax could be detected on Tr1.
A principal component analysis of the anatomical, biochemical and textural (CPmax, CPslope, FPmax, FPslope, FPdef and SPmax) variables was performed to track the genetic (leaving Cervil out) and seasonal variability (Fig. 5). The first two factors explained 69 % of the variation. Both textural, anatomical and biochemical variables contributed to the first factor (46 % of the variance), which opposed pericarp cell volume, fruit or pericarp soluble sugar (glucose, fructose and total) contents and number of locular cavities to FPmax, SPmax, fruit or pericarp dry matter content and to a lesser extend FPslope. Four textural, biochemical and anatomical variables mainly contributed to the second factor (23 % of the variance), which opposed CPmax and the pericarp cell number to the fruit sucrose content. The third factor explained 11 % more of the global variation; it was positively correlated to fruit CPmax and CPslope and negatively correlated to FPdef and fruit sucrose content (not shown). Projection of the individuals separated both the genotypes and truss position. The genetic background was mainly separated on the first axis, while, within each background, the truss position was separated on the second axis. Within the B background, NIL-B9 negatively contributed to the first axis and was thus characterized by high FPmax and SPmax, high dm content, but low soluble sugar content (in % dm), small cells, and low number of locular cavities. Fruits picked on Tr1 positively contributed to the second factor. This was especially true for VilB fruits with high CPmax values, numerous cells and low sucrose content. Within the L background, NIL-L4 and Lev (Tr1) had the highest positive contribution to the first axis and were associated with low FPmax and SPmax values, low dm content, large pericarp cells, high soluble sugar content, and number of locular cavities. Fruits picked on Tr5 negatively contributed to the second factor; in particular NIL-L9 and NIL-L9-1 fruits picked on Tr5 could be characterized by low CPmax values, low cell number and high sucrose content. The third factor mostly opposed NIL-L9-1 Tr5 to NIL-L9 Tr1 (not shown).
Principal component analysis of anatomical (blue), biochemical (black) and textural (red) fruit traits performed on six genotypes and two truss positions. Projections of traits (upper graph) and individuals (lower graph) on the plane defined by the first two principal components. Genotype names are in italics when they refer to truss 1 and in bold characters when they refer to truss 5. (Color figure online)
Correlation network among textural, anatomical and biochemical fruit traits
To better visualize and analyze the complex interactions among fruit traits, a correlation network was drawn based on mean data from the two trusses for each genotype, except Cervil (Fig. 6). All textural variables associated with the force/displacement curves from compression and puncture tests were included, as well as biochemical data (dm and sugar content) measured at both fruit and tissue levels. In this network only significant correlations (P-value <0.01) were drawn using solid lines when positive and dotted lines when negative, while the size of each node reflected its centrality. Three nodes were highly central in the network: the pericarp cell volume, FPmax, and the fruit diameter, which indicated that variations in other fruit traits were mainly correlated to these traits. One cluster of high connection density was observed involving FPmax and SPmax, which were positively correlated to one another (R = 0.81). In this cluster, FPmax and SPmax were positively and significantly correlated to the fruit or pericarp dry matter content (R > 0.82), whereas it was negatively and significantly correlated to the mean pericarp cell volume (R < −0.80), to the fruit or pericarp soluble sugar (glucose, fructose or sum of all soluble sugars) contents (−0.87 < R < −0.74), and to the number of locular cavities (R = −0.89 for the FP test only). A second cluster of lower connection density included FPdef, which was uncorrelated from all other textural indicators measured in this work. FPdef was positively correlated to the % dry matter in the columella (R = 0.91) and to the contribution of seeds to the fruit fresh weight (R = 0.74). It was negatively correlated to the fruit fresh weight and diameter (R < −0.71).
Correlation network of textural, anatomical and biochemical fruit traits. Data were measured at red ripe stage on two trusses (1 and 5) from two parental lines (Lev and VilB) and four QTL-NILs (NIL-L9, NIL-L9-1, NIL-L4, and NIL-B9). All textural parameters described in “Materials and methods” section and associated with the force/displacement curves from compression (CP) and puncture tests (FP and SP) were taken into account. Anatomical and biochemical data were measured at the fruit or tissue level. In this network solid and dotted lines represent respectively, positive and negative correlations among variables (nodes). Only the significant correlations (P value <0.01) were drawn. Line widths are relative to the correlation coefficient values. The size of each node indicates the centrality within the graph (betweenness). Nodes that occur on many shortest paths between other nodes have higher betweenness than those that do not. List of variables: maximum forces (N) and slopes (N m−1) of the CP, FP and SP test: CPmax, CPslope, FPmax, FPslope, SPmax. Maximum deformation in FP test (m): FPdef. Fruit fresh weight (g) and diameter (mm): FWfr, Diamfr. Fruit locule number: Locul. Fruit seed number: NbSeed. Fruit conductance (cm h−1): Cond. Proportions of pericarp, columella, locular gel and seeds (g 100 g−1 fruit fresh weight): peri, colum, gel, seed. Pericarp cell number (106) and mean volume (nl): NbCell, VolCell. Fruit, pericarp and columella contents in dm and AIS (g 100 g−1 dm): DMfr, AISfr, DMper, AISper, DMcol. Fruit and pericarp contents in glucose, fructose, sucrose and total soluble sugars (g 100 g−1 dm): GLUfr, FRUfr, SUCfr, SSfr, GLUper, FRUper, SUCper, SSper
Other textural parameters were marginal to these clusters. FPslope and CPslope were positively correlated to FPmax or CPmax (0.74 < R < 0.88). FPslope was negatively correlated to the number of locular cavities (R = −0.79) and to the contribution of locular gel to the fruit fresh weight (R = −0.74). Interestingly, CPmax was not significantly correlated to any biochemical or anatomical fruit variables, and no significant correlation was observed among variables measured by compression and puncture tests.
Other variables such as AIS content, cell or seed numbers, fruit conductance and proportions of pericarp or columella were not directly correlated to fruit textural traits as evaluated in this work with the three tests. However, within the L genetic background, positive linear correlations could be observed between the pericarp AIS content and SPmax (R = 0.81) or FPmax (R = 0.77) (not shown).
Forward stepwise regressions were performed in order to better describe the dependence of textural variables on the anatomical and biochemical variables. The proportion of pericarp, the cell number and cell volume, the pericarp contents in soluble sugar, dm and AIS, and the number of locular cavities were used as independent variables. FPmax could be predicted from a linear combination of the pericarp soluble sugar content, locule number and cell volume (P < 0.001, R 2 = 0.99), whereas the pericarp dm content was sufficient to predict SPmax (P < 0.001, R 2 = 0.74). CPmax could be predicted from a linear combination of cell number and cell volume (P = 0.01, R 2 = 0.56), whereas CPslope and FPslope could be predicted from the locule number alone (P = 0.02, R 2 = 0.44 and P = 0.002, R 2 = 0.63, respectively).
Discussion
The understanding and control of fruit texture is crucial to ensure postharvest handling and consumer acceptance. However, large fluctuations of texture occur during crop production and in the postharvest period, which may be undesirable. Many studies have investigated the genetic variability in fruit firmness. Some genes involved in textural traits have been identified (reviewed in Causse et al. 2011) and several QTL have been detected (reviewed in Labate et al. 2007). Most biochemical studies on fruit texture were devoted to maturation mechanisms and changes in fruit firmness were linked to cell wall degradation processes (Crookes and Grierson 1983; Rose et al. 2003). However, genotypic differences in fruit firmness have been demonstrated at early stages of fruit development far before the red ripe stage (Chaib et al. 2007). Indeed several mechanisms controlled at the molecular, cell, tissue and organ levels likely interact in the pre- and postharvest periods (Barrett et al. 1998; Saladié et al. 2007). In this respect, global approaches are required to better understand the genetic and environmental variability in textural traits and to identify interactions among key-determinant factors, which could be used in an integrated fruit model (Génard et al. 2007). This was a main objective of the present work.
Compression and puncture tests evaluate different textural properties
A main difficulty of textural studies is to select objective quantitative criteria, in part due to the numerous physical or sensory properties underlying the term texture (Barrett et al. 1998). This is particularly true in the case of tomato fruits that have a specific organ structure with a complex anatomy and various tissues. Sensorial assessment by trained panelists certainly provides the most integrative evaluation of fruit texture. However, instrumental measurements are more objective and they can detect small variations, for instance, among genotypes or between cultural conditions. In this work the genetic and seasonal variability in fruit textural variables have been assessed by compression and puncture tests. These tests have been shown to correlate well with sensory evaluations of different fruit species (Bourne 1979; Causse et al. 2002; Harker et al. 2002; Mehinagic et al. 2004; Ross et al. 2009). In tomato, studies based on compression tests, reported that fruit elasticity (associated to CPslope) correlates negatively with fruit juiciness (Causse et al. 2002), while firmness (associated to CPmax) correlates positively with sensory firmness and skin thoughness, and negatively with juiciness (Chaib et al. 2007). The present work clearly confirms that compression and puncture tests point to different fruit properties and provide complementary information. Indeed, firmness assessed by maximum forces of the force/displacement curves (CPmax, FPmax, SPmax) varied in opposite way according to the compression and puncture tests (Table 1), whereas stiffness measured by the slopes of these curves (CPslope, FPslope) showed similar variations for both compression and puncture tests. This suggested that fruit stiffness can be well assessed by compression test, while fruit firmness is better assessed by puncture tests which account for local tissue properties. The compression test has already been reported as rather insensitive to tissue properties and more influenced by fruit shape, size and proportion of different tissues (Barrett et al. 1998). It is also less sensitive than puncture tests to determine small differences in textural properties of processed tomatoes (Barrett et al. 1998).
QTL on chromosome 9 had major and stable effects on tomato fruit texture
The present study investigated nine contrasted genotypes, including three parental lines and six derived QTL NILs. It completes previous results obtained on part of this plant material (Causse et al. 2002; Chaib et al. 2007; Zanor et al. 2009; Bertin et al. 2009; Page et al. 2010; Quemener et al. 2007). It is well known that textural traits strongly depend on environmental factors (Sams 1999; Chaib et al. 2006; Chaib et al. 2007). In this work, the genetic effects on firmness were, to a certain extent, conserved over 2 years, although some interactions between year and QTL effects could be detected within the L background (Table 1, SPmax). In addition, ranks among genotypes were hardly affected during the season (except the absence of QTL effect on Tr1 in the B background), while a recent study demonstrated that only a low proportion of QTL for tomato fruit traits is stable at two contrasted plant fruit loads (Prudent et al. 2009). This supports the hypothesis that genotypic differences affect tomato fruit firmness more than growing conditions (Carli et al. 2011).
The effect of QTL introgression on fruit textural properties varied according to the region and the genetic background. The texture QTL on chromosome 4 was initially detected in a RIL population within a region of 20 cM, in the upper part of the chromosome. It controls several textural traits, such as instrumental firmness and stiffness, and also sensory traits, e.g. skin thoughness and mealiness. In the same genome area, a QTL for firmness was also detected by hand evaluation in an advanced backcross population between S. lycopersisum and S. peruvianum (Fulton et al. 1997). Introgression of the QTL region located on chromosome 4 (NIL-L4) did not allow any improvements in firmness (Table 1) and this is in accordance with a previous study (Chaib et al. 2007). This suggested some strong interactions between QTL and the genetic background. The two sub-NILs were complementary lines, as NIL-L4-1 introgression corresponded to the upper part of the QTL region and NIL-L4-2 to the lower part. According to the FP test, NIL-L4-2 fruits were firmer compared to Lev fruits, suggesting that genes underlying the firmness QTL effect could be located in this basal part of the genome region and that antagonist epistatic interactions with genes located in the upper part of the region could exist.
In contrast, the QTL on chromosome 9 strongly improved firmness as measured by FPmax and SPmax. The comparison of Lev, NIL-L9 and NIL-L9-1 (Table 1) suggested that the chromosome region lacking in NIL-L9-1, was involved in the decrease of fruit firmness and stiffness measured by compression test (CPmax and CPslope). The increase of CPslope measured on NIL-L9-1 fruits even suggested the presence of several QTL with antagonistic effects in the 50 cM region. Effects of the QTL introgressed on chromosome 9 were very similar in the two genetic backgrounds. Moreover, this QTL for firmness colocalized with other firmness QTL detected in S. lycopersicum × S. peruvianum (Fulton et al. 1997) and S. lycopersicum × S. neorickii (Fulton et al. 2000) populations. However, as the introgressed genome region represents up to about 50 cM for NIL-L9 and NIL-B9, and about 36 cM for NIL-L9-1, many genes alleles may vary between parental lines and NILs. It is thus, now impossible to look for candidate genes using genome information, but comparative analysis of NILs differential expression has already established some relations between the introgressed QTL bearing regions and pericarp cell size and repartition (Chaib et al. 2007), cell wall polysaccharides content (Quemener et al. 2007) protein expression (Page et al. 2010), and metabolite content (Zanor et al. 2009).
Postharvest changes in fruit texture were disconnected from texture at harvest
The evolution of texture in the postharvest period may dramatically impact the transportability and distribution of fresh fruits. Since most fleshy fruits soften as they mature, the stage of maturity at harvest directly affects texture of the product to be purchased and consumed. In apple, there is a significant correlation between firmness at harvest and after storage (Knee and Smith 1989), which was not observed in other fruits, such as tomato (Ahrens and Huber 1990). The absence of relationship between tomato fruit firmness measured at harvest and its evolution during storage was confirmed in the present work.
Due to contrasted rates of texture changes, positive effects of QTL9 (NIL-L9 and NIL-B9) on fruit firmness (FPmax and SPmax) were partially lost in the postharvest period in both backgrounds (Table 1). In agreement, a recent study showed that cold storage reduces NIL-B9 firmness more dramatically and rapidly than that of the parent VilB (Page et al. 2010). This loss of firmness was correlated with the lower expression of genotype specific proteins, among which some were heat shock proteins. More generally, the fact that the relative loss of firmness strongly differed according to the test and strongly varied among genotypes suggested that several mechanisms were involved at the fruit and pericarp levels, with strong genetic variations.
Pericarp cell size and composition are strongly correlated with fruit firmness as measured by the puncture test
The contributions of tissue structure and composition to fruit textural properties have been long reported, although rare holistic approaches, including biochemical and anatomical traits at the same time, have been developed and the hierarchy among determining traits remains unknown. The present work on tomato suggested that firm fruits detected by puncture tests (high FPmax and SPmax values) have small cells, low soluble sugar content and low number of locular cavities, but high dm content. On the other hand, fruit stiffness also evaluated by puncture test (high FPslope values) was negatively correlated to the number of locular cavities and the relative proportion of gel. In contrast, compression test variables were hardly correlated to anatomical and biochemical fruit traits, except high CPmax values that were associated with high cell number. In the correlation network, the high centrality of FPmax confirmed that the puncture test accounts well for fruit textural properties related to variations in anatomical and biochemical traits.
The negative correlation observed between fruit firmness and locule number, might be spurious, since firm tomatoes with high locule number exist (Chaib et al. 2007). Indeed, in the present work, this correlation was absent within each genetic background (not shown). Moreover, the number or radial pericarp septa, which depends on the number of locular cavities, is expected to impact on the resistance to deformation. This may explain the negative correlation between fruit stiffness and locule number. At the tissue level, the impact of cell number, size, and shape on textural properties of fleshy fruits has been discussed by several authors (Abbott and Lu 1996; Mann et al. 2005; Nardozza et al. 2011). In accordance with the present study, most of these works converge to the hypothesis that small cells with few intercellular spaces would form compact texture, while large cells would result in spongy texture (Reeve 1970) and are associated with mealy fruit (Chaib et al. 2007). This hypothesis has been put forward to explain intra-plant variations in fruit texture, for instance between fruit in the interior shaded areas and those in the outer sunlit regions of an apple tree (Sams 1999). In tomato, intra-plant variations in fruit texture could be expected in relation to the competition for assimilates, which also affects several fruit traits, such as cell size, cell number or dm accumulation (Bertin et al. 2001; Bertin 2005). Accordingly, fruit firmness measured by puncture test (FPmax and SPmax) was generally greater on Tr5 than on Tr1.
From the biochemical point of view, most previous studies focussed on cell wall metabolism, in particular during ripening or in response to environmental stresses (Toivonen and Brummell 2008). In addition, fruit turgor and water status have been suggested to influence the biochemical properties of fruit tissue as well as fruit firmness (Shackel et al. 1991; Jackman et al. 1992). For instance, 25–30 % of tomato softening during ripening was attributed to changes in cell turgor (Jackman and Stanley 1995). Since cell expansion results from cell wall relaxation and concomitant decrease in cell turgor, the strong negative correlation between cell size and fruit firmness likely reflected variations in cell wall elasticity and/or turgor regulation. The cuticle properties and loss of water by transpiration undoubtedly play an important role in the regulation of cell turgor and were shown to allow higher firmness maintenance in DFD mutants (Saladié et al. 2007). However, in the present study, fruit conductance could not be linked directly to textural parameters. The accumulation of osmotically active solutes, mostly hexoses and inorganic ions, and their repartition between apoplast and symplast, also significantly contribute to the cell turgor regulation (Saladié et al. 2007). The present work provided evidence for a negative link between soluble sugar accumulation and fruit firmness, which may result from osmotically driven changes in cell turgor. Finally, the positive correlation between fruit dm content and firmness observed in this work agreed with the positive correlation between total soluble solids (TSS) and firmness reported for tomato (Saha et al. 2009) and with recent results on large-fruited kiwifruit genotypes (Nardozza et al. 2011). As an increase in dm content may result both from an increased accumulation of solids (including soluble or structural components) and from a decreased accumulation of water (balance between influx and loss by transpiration), the links with firmness is intricate. In the present dataset, negative correlations between pericarp dm content and cell volume, but also between pericarp dm content and soluble sugar content, suggested that high dm content was merely associated with smaller cells and more cell structures, and, thus, could explain higher mechanical resistance of tissues. In that case one could have expected a positive correlation between firmness and AIS content, which was not evidenced in this work. The AIS fraction of dm includes protein (8 % of fruit DM), pectic substances (7 %), hemicelluloses (4 %) and cellulose (6 %) (Davies and Hobson 1981). In tomato fruit, AIS was reported to be correlated to viscosity, but not highly associated with firmness (Barrett et al. 1998), which is in agreement with the present study.
Conclusion
This work confirmed the presence of a QTL with important and stable effects on tomato fruit firmness on top of chromosome 9. The contrasted behavior in the postharvest period and the absence of relation between firmness at harvest and its postharvest evolution, outlined the importance to combine measurements at both stages and to deeply investigate the mechanisms involved at the fruit, tissue and cell levels. Significant links between textural, anatomical and biochemical fruit traits were emphasized in this study. Altogether, the results suggested that the compression test reflected an integrative fruit property poorly correlated to the individual fruit traits, in contrast to the puncture test. In this work, cell volume, locule number and dm or soluble sugar contents appeared as main factors underlying genetic and seasonal variability in fruit firmness and stiffness, as measured by the puncture test. They may be good screening indicators for a breeding program on fruit texture.
Identifying functional links among traits or mechanisms is a challenge in several biological fields to find the structure of potential regulatory networks. Methods based on correlations as applied in this work, do not bring to light direct or functional links (Villers et al. 2008), but can reveal proximities among variables. Moreover the network topology is highly dependent on the dataset, that is the genotypic and environmental range of conditions, as illustrated for instance by Zushi and Matsuzoeb (2011). Other methods exist to emphasize functional links, however they require much larger datasets, involving either the measurements of multiple variables on a given fruit which is not possible or limited when addressing variables measured at different scales (cell, tissue, fruit), or a very high number of contrasted situations/treatments which is also difficult to achieve in practice. In the future, statistical methods adapted to such biological datasets will be necessary to improve our capacity to infer regulatory networks that involve variables measured at different scales.
References
Abbott JA, Lu R (1996) Anisotropic mechanical properties of apples. Trans ASAE 39:1451–1459
Abotts JA (1999) Quality measurement of fruit and vegetables. Postharvest Biol Technol 15:207–225
Ahrens MJ, Huber DJ (1990) Physiology and firmness determination of ripening tomato fruit. Physiol Plant 78:8–14
Allende A, Desmet M, Vanstreels E, Verlinden BE, Nicolai BM (2004) Micromechanical and geometrical properties of tomato skin related to differences in puncture injury susceptibility. Postharvest Biol Technol 34:131–141
Almeida DPF, Huber DJ (1999) Apoplastic pH and inorganic ion levels in tomato fruit: a potential means for regulation of cell wall metabolism during ripening. Physiol Plant 105:506–512
Barrett DM, Garcia E, Wayne JE (1998) Textural modification of processing tomatoes. Crit Rev Food Sci Nutr 38:173–258
Barrett DM, Beaulieu JC, Shewfelt R (2010) Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit Rev Food Sci Nutr 50:369–389
Batagelj V, Mrvar A (2003) Pajek—analysis and visualization of large networks. In: Jünger M, Mutzel P (eds) Graph drawing software. Springer, Berlin, pp 77–103
Bertin N (2005) Analysis of the tomato fruit growth response to temperature and plant fruit load in relation to cell division, cell expansion and DNA endoreduplication. Ann Bot 95:439–447
Bertin N, Buret M, Gary C (2001) Insights into the formation of tomato quality during fruit development. J Hortic Sci Biotechnol 76:786–792
Bertin N, Gautier H, Roche C (2002) Number of cells in tomato fruit depending on fruit position and source–sink balance during plant development. Plant Growth Regul 36:105–112
Bertin N, Causse M, Brunel B, Tricon D, Génard M (2009) Identification of growth processes involved in QTLs for tomato fruit size and composition. J Exp Bot 60:237–248
Bourne MC (1979) Fruit texture—an overview of trends and problems. J Texture Stud 10:83–94
Bourne MC (2002) Food texture and viscosity: concept and measurement, 2nd edn. Academic Press, New York
Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47:311–339
Camps C, Guillermin P, Mauget JC, Bertrand D (2005) Data analysis of penetrimetric force/displacement curves for the characterization of whole apple fruits. J Texture Stud 36:387–401
Carli P, Barone A, Fogliano V, Frusciante L, Ercolano MR (2011) Dissection of genetic and environmental factors involved in tomato organoleptic quality. BMC Plant Biol 11. doi:101186/1471-2229-11-58
Causse M, Saliba-Colombani V, Lesschaeve I, Buret M (2001) Genetic analysis of organoleptic quality in fresh market tomato 2. Mapping QTLs for sensory attributes. Theor Appl Genet 102:273–283
Causse M, Saliba-Colombani V, Lecomte L, Duffe P, Rousselle P, Buret M (2002) QTL analysis of fruit quality in fresh market tomato: a few chromosome regions control the variation of sensory and instrumental traits. J Exp Bot 53:2089–2098
Causse M, Buret M, Robini K, Verschave P (2003) Inheritance of nutritional and sensory quality traits in fresh market tomato and relation to consumer preferences. J Food Sci 68:2342–2350
Causse M, Stevens R, Ben Amor B, Faurobert M, Munos S (2011) Breeding for fruit quality in tomato. In: Jenks MA, Bebeli PJ (eds) Breeding for fruit quality. Wiley-Blackwell, Oxford, pp 279–305
Chaib J, Lecomte L, Buret M, Causse M (2006) Stability over genetic backgrounds, generations and years of quantitative trait locus (QTLs) for organoleptic quality in tomato. Theor Appl Genet 112:934–944
Chaib J, Devaux M-F, Grotte MG, Robini K, Causse M, Lahaye M, Marty I (2007) Physiological relationships among physical, sensory, and morphological attributes of texture in tomato fruits. J Exp Bot 58:1915–1925
Crookes PR, Grierson D (1983) Ultrastructure of tomato fruit ripening and the role of polygalacturonase isoenzymes in cell wall degradation. Plant Physiol 72:1088–1093
Davies JN, Hobson GE (1981) The constituents of tomato fruit—the influence of environment, nutrition and genotype. Crit Rev Food Sci Nutr 15:205–280
Desmet M, Lammertyn J, Verlinden BE, Nicolai BM (2002) Mechanical properties of tomatoes as related to puncture injury susceptibility. J Texture Stud 33:415–430
Freeman LC (1977) A set of measures of centrality based on betweenness. Sociometry 40:35–41
Fulton TM, Beck-Bunn T, Emmatty D, Eshed Y, Lopez J, Petiard V, Uhlig J, Zamir D, Tanksley SD (1997) QTL analysis of an advanced backcross of Lycopersicon peruvianum to the cultivated tomato and comparisons with QTLs found in other wild species. Theor Appl Genet 95:881–894
Fulton TM, Grandillo S, Beck-Bunn T, Fridman E, Frampton A, Lopez J, Petiard V, Uhlig J, Zamir D, Tanksley SD (2000) Advanced backcross QTL analysis of a Lycopersicon esculentum × Lycopersicon parviflorum cross. Theor Appl Genet 100:1025–1042
Génard M, Bertin N, Borel C, Bussières P, Gautier H, Habib R, Léchaudel M, Lecomte A, Lescourret F, Lobit P, Quilot B (2007) Towards a virtual fruit focusing on quality: modeling features and potential uses. J Exp Bot 58:917–928
Gibert C, Génard M, Vercambre G, Lescourret F (2010) Quantification and modelling of the stomatal, cuticular and crack components of peach fruit surface conductance. Funct Plant Biol 37:264–274
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180
Gomez L, Rubio E, Auge M (2002) A new procedure for extraction and measurement of soluble sugars in ligneous plants. J Sci Food Agric 82:360–369
Goulao LF, Oliveira CM (2008) Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends Food Sci Tech 19:4–25
Harker FR, Maindonald J, Murray SH, Gunson FA, Hallett IC, Walker SB (2002) Sensory interpretation of instrumental measurements 1: texture of apple fruit. Postharvest Biol Technol 24:225–239
Jackman RL, Stanley DW (1995) Perspectives in the textural evaluation of plant foods. Trends Food Sci Tech 6:187–194
Jackman RL, Marangoni AG, Stanley DW (1992) The effects of turgor pressure on puncture and viscoelastic properties of tomato tissue. J Texture Stud 23:491–505
Ketelaere BD, Lammertyn J, Molenberghs G, Desmet M, Nicolai B, Baerdemaeker JD (2004) Tomato cultivar grouping based on firmness change, shelf life and variance during postharvest storage. Postharvest Biol Technol 34:187–201
Kim KB, Lee S, Kim MS, Cho BK (2009) Determination of apple firmness by nondestructive ultrasonic measurement. Postharvest Biol Technol 52:44–48
King GJ, Maliepaard C, Lynn JR, Alston FH, Durel CE, Evans KM, Griffon B, Laurens F, Manganaris AG, Schrevens E, Tartarini S (2000) Quantitative genetic analysis and comparison of physical and sensory descriptors relating to fruit flesh firmness in apple (Malus pumila Mill). Theor Appl Genet 100:1074–1084
Knee M, Smith SM (1989) Variation in quality of apple fruits stored after harvest on different dates. J Hortic Sci 64:413–419
Labate JA, Grandillo S, Fulton T, Munos S, Caicedo AL, Peralta I, Ji YF, Chetelat RT, Scott JW, Gonzalo MJ, Francis D, Yang WC, Knaap E, Baldo AM, Smith-White B, Mueller LA, Prince JP, Blanchard NE, Storey DB, Stevens MR, Robbins MD, Wang JF, Liedl BE, O’Connell MA, Stommel JR, Aoki K (2007) Tomato. In: Kole C (ed) Genome mapping and molecular breeding in plants. Springer, Berlin, pp 1–125
Lecomte L, Duffe P, Buret M, Servin B, Hospital F, Causse M (2004) Marker-assisted introgression of 5 QTLs controlling fruit quality traits into three tomato lines revealed interactions between QTLs and genetic backgrounds. Theor Appl Genet 109:658–668
Legland D, Guillon F, Kiêu K, Bouchet B, Devaux MF (2009) Stereological estimation of cell wall density of DR12 tomato mutant using three-dimensional confocal imaging. Ann Bot 105:265–276
Leonardi C, Baille A, Guichard S (2000) Predicting transpiration of shaded and non-shaded tomato fruits under greenhouse environments. Sci Hort 84:297–307
Lerceteau-Kohler E, Moing A, Guerin G, Renaud C, Courlit S, Camy D, Praud K, Parisy V, Bellec F, Maucourt M, Rolin D, Roudeillac P, Denoyes-Rothan B (2004) QTL analysis for fruit quality traits in octoploid strawberry (Fragaria * ananassa). Acta Hortic 708:277–280
Longhi S, Moretto M, Viola R, Velasco R, Costa F (2012) Comprehensive QTL mapping survey dissects the complex fruit texture physiology in apple (Malus × domestica Borkh). J Exp Bot 63:1107–1121
Lyons JM (1973) Chilling injury in plants. Annu Rev Plant Physiol 24:445–466
Mann H, Bedford D, Luby J, Vickers Z, Tong C (2005) Relationship of instrumental and sensory texture measurements of fresh and stored apples to cell number and size. HortScience 40:1815–1820
Mehinagic E, Royer G, Symoneaux R, Bertand D, Jourjon F (2004) Prediction of the sensory quality of apples by physical measurements. Postharvest Biol Technol 34:257–269
Moneruzzaman KM, Hossain ABMS, Sani W, Saifuddin M (2008) Effect of stages of maturity and ripening conditions on the physical characteristics of tomato. Am J Biochem Biotechnol 4:329–335
Musse M, Quellec S, Devaux MF, Cambert M, Lahaye M, Mariette F (2009) An investigation of the structural aspects of the tomato fruit by means of quantitative nuclear magnetic resonance imaging. Magn Reson Imaging 27:709–719
Nardozza S, Gamble J, Axten LG, Wohlers MW, Clearwater MJ, Fenga J, Harkera FR (2011) Dry matter content and fruit size affect flavour and texture of novel Actinidia deliciosa genotypes. J Sci Food Agric 91:742–748
Obando J, Fernandez-Trujillo JP, Martinez JA, Alarcon AL, Eduardo I, Arus P, Monforte AJ (2008) Identification of melon fruit quality quantitative trait loci using near isogenic lines. J Am Soc Hortic Sci 133:139–151
Ogundiwin EA, Peace CP, Gradziel TM, Dandekar AM, Bliss FA, Crisosto CH (2007) Molecular genetic dissection of chilling injury in peach fruit. Acta Hortic 738:633–638
Page D, Marty I, Bouchet JP, Gouble B, Causse M (2008) Isolation of genes potentially related to fruit quality by subtractive selective hybridization in tomato. Postharvest Biol Technol 50:117–124
Page D, Gouble B, Valot B, Bouchet JP, Callot C, Kretzschmar A, Causse M, Renard CMCG, Faurobert M (2010) Protective proteins are differentially expressed in tomato genotypes differing for their tolerance to low-temperature storage. Planta 232:483–500
Perez-Pastor A, Ruiz-Sanchez MC, Martinez JA, Nortes PA, Artes F, Domingo R (2007) Effect of deficit irrigation on apricot fruit quality at harvest and during storage. J Sci Food Agric 87:2409–2415
Prudent M, Causse M, Génard M, Tripodi P, Grandillo S, Bertin N (2009) Genetic and physiological analysis of tomato fruit weight and composition: influence of carbon availability on QTL detection. J Exp Bot 60:923–937
Quemener B, Bertrand D, Marty I, Causse M, Lahaye M (2007) Fast data preprocessing for chromatographic fingerprints of tomato cell wall polysaccharides using chemometric methods. J Chromatogr 1141:41–49
Reeve RM (1970) Relationships of histological structure to texture of fresh and processed fruits and vegetables. J Texture Stud 1:247–284
Rosales MA, Cervilla LM, Rios JJ, Blasco B, Sanchez-Rodriguez E, Romero L, Ruiz JM (2009) Environmental conditions affect pectin solubilization in cherry tomato fruits grown in two experimental Mediterranean greenhouses. Environ Exp Bot 67:320–327
Rose J, Catala C, Gonzales-Carranza Z et al (2003) Plant cell wall disassembly. In: Rose JKC (ed) The plant cell wall. Blackwell, Oxford, pp 264–324
Ross CF, Chauvin MA, Whiting M (2009) Firmness evaluation of sweet cherries by a trained and consumer sensory panel. J Texture Stud 40:554–570
Saha S, Hedau NK, Mahajan V, Singh G, Gupta HS, Gahalain A (2009) Textural, nutritional and functional attributes in tomato genotypes for breeding better quality varieties. J Sci Food Agric 90:239–244
Saladié M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, Ren XL, Labavitch JM, Shackel KA, Fernie AR, Lytovchenko A, O’Neill MA, Watkins CB, Rose JKC (2007) A reevaluation of the key-factors that influence tomato fruit softening and integrity. Plant Physiol 144:1012–1028
Saliba-Colombani V, Causse M, Langlois D, Philouze J, Buret M (2001) Genetic analysis of organoleptic quality in fresh market tomato 1 Mapping QTLs for physical and chemical traits. Theor Appl Genet 102:259–272
Sams CE (1999) Preharvest factors affecting postharvest texture. Postharvest Biol Technol 15:249–254
Seymour GB, Manning K, Eriksson EM, Popovich AH, King GJ (2002) Genetic identification and genomic organization of factors affecting fruit texture. J Exp Bot 53:2065–2071
Shackel KA, Greve C, Labavitch JM, Ahmadi H (1991) Cell turgor changes associated with ripening in tomato pericarp tissue. Plant Physiol 97:814–816
Sinesio F, Cammareri M, Moneta E, Navez B, Peparaio M, Causse M, Grandillo S (2010) Sensory quality of fresh French and Dutch market tomatoes: a preference mapping study with Italian consumers. J Food Sci 75:S55–S67
Stevens R, Gouble B, Garchery C, Zamir D, Causse M (2008) Tomato fruit ascorbic acid content is linked with monodehydroascorbate reductase activity and tolerance to chilling stress. Plant Cell Environ 31:1086–1096
Szczesniak AS (2002) Texture is a sensory property. Food Qual Prefer 13:215–225
Toivonen PMA, Brummell DA (2008) Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol 48:1–14
van Hooijdonk BM, Dorji K, Behboudian MH (2007) Fruit quality of ‘Pacific Rose TM’ apple grown under partial rootzone drying and deficit irrigation. J Food Agric Environ 5:173–178
Vicente AR, Saladié M, Rose JKC, Labavitch JM (2007) The linkage between cell wall metabolism and fruit softening: looking to the future. J Sci Food Agric 87:1435–1448
Villers F, Schaeffer B, Bertin C, Huet S (2008) Assessing the validity domains of graphical Gaussian models in order to infer relationships among components of complex biological systems. SAGMB 7:14
Zanor MI, Rambla JL, Chaib J, Steppa A, Medina A, Granell A, Fernie AR, Causse M (2009) Metabolic characterization of loci affecting sensory attributes in tomato allows an assessment of the influence of the levels of primary metabolites and volatile organic contents. J Exp Bot 60:2139–2154
Zushi K, Matsuzoeb N (2011) Utilization of correlation network analysis to identify differences in sensory attributes and organoleptic compositions of tomato cultivars grown under salt stress. Sci Hort 129:18–26
Acknowledgments
The authors are grateful to Caroline Callot and Esther Pelpoir for their technical assistance. This work was supported by a joint grant from INRA and the Provence Alpes Côtes d’Azur region (FEDER fund).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aurand, R., Faurobert, M., Page, D. et al. Anatomical and biochemical trait network underlying genetic variations in tomato fruit texture. Euphytica 187, 99–116 (2012). https://doi.org/10.1007/s10681-012-0760-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0760-7