Abstract
Malus prunifolia is an important germplasm resource for apple rootstock. However, no comprehensive and systematic evaluation has been made of its salt tolerance. We compared salt injury (SI), net photosynthetic rate (Pn), variation percentage (VP) of electrolyte leakage (EL), relative water content (RWC) and adverse resistance coefficients (ARC) in nine biotypes of Malus prunifolia when plants were exposed to 150 mM NaCl for 10 days. Two other valuable rootstock sources (M. sieversii and M. hupehensis) were also analyzed. Under high-salinity conditions, all growth parameters except EL were significantly decreased compared with the untreated control. The greatest changes were found with Pn. Values for RWC and ARC also declined under saline treatment, although the extent of that decrease varied among biotypes. Based on our results for SI, as well as ARC and cluster analysis, we deemed these biotypes to be salt-tolerant: M. prunifolia ‘fupingqiuzi’, M. prunifolia ‘dongbeihuanghaitang’, M. prunifolia ‘laoshannaizi’, M. sieversii, and M. hupehensis. By contrast, M. prunifolia ‘baihaitang’, M. prunifolia ‘wulenghaitang’ and M. prunifolia ‘neimengguhaihong’ proved to be salt-sensitive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deleterious environmental changes are widely viewed as a major challenge to global food security (Fedoroff et al. 2010). As a main abiotic factor, salt stress limits plant growth and productivity worldwide (Allakhverdiev et al. 2000). Soil salinization poses a great threat to coastal and inland agriculture, particularly in countries with very hot, dry climates (Flowers et al. 1997; Wahid and Ghazanfar 2006). However, only a few cultivars for agronomic crops have been developed that are resistant to saline soils (Flowers and Yeo 1995). Therefore, it is of paramount importance that scientists obtain more knowledge about the salt tolerance of valuable plant species (Flowers 2004).
Crops growing under high salinity show reduced photosynthesis, growth, and development, all associated with ionic/osmotic effects, a nutritional imbalance, or oxidative stress (Ahmad et al. 2008; Ashraf 2009; Ashraf and Foolad 2007; Lee et al. 2004; Ron 2002). However, the degree to which a species is susceptible can depend upon its cultivar type and the duration of salt exposure (Vigo et al. 2005). By evaluating several physiological and biochemical parameters, researchers have determined several genotype differences between tolerant and sensitive plants in an effort to develop rapid screening methods for salt tolerance (Alian et al. 2000). The capacity of a species to tolerate such stress is a function of its genetic and biochemical characteristics (Garg and Garg 1982). Our understanding of the effects of salt stress remains incomplete because of the complexity of the processes involving both ionic and osmotic components that lead to morphological, physiological, and metabolic changes (Bray 1993). Such alterations in plant properties enable them to adapt to growth under salinity stress (Bohnert et al. 1995).
Apple (Malus sp.) is one of the most economically important fruits in the world. However, the development of trees in some regions can be restricted because of environmental stresses such as high salt concentrations in the soil. China, one of the oldest breeding centers of Malus plants, has abundant germplasm resources with strong resistance to adverse environments (Ye et al. 2004). By identifying the most salt-tolerant rootstocks and determining which genes confer tolerance, researchers will be able to provide new salt-tolerance genetic resources for improved breeding and fruit productivity.
Malus prunifolia is an excellent rootstock widely used for grafting apple trees in saline and arid areas. There are many biotypes in this species, and however, no comprehensive and systematic assessment of its salt tolerance has previously been conducted. Therefore, our study objective was to evaluate trees from nine of its biotypes and compare their performance with that of two other important rootstock sources—M. sieversii Roem. and M. hupehensis Rehd. which are moderate-tolerance and high-tolerance respectively under 200 mM NaCl treatment (Yin et al. 2010). In particular, we examined their physiological responses and relative tolerance to NaCl stress.
Materials and methods
Plant materials and salt treatments
Seeds of 11 biotypes for apple rootstocks were collected from plant germplasm resource garden (Table 1). Experiments were conducted at Northwest A & F University, Yangling, China (34 °20′N, 108 °24′E). The seeds were placed in sand for 50 days at 4 °C and 60–70 % relative humidity. After germination, three seeds each were planted in plastic pots (diameter: 12 cm; height: 12 cm) filled with sand, then moved to a greenhouse under natural light and temperature conditions. At the two-true-leaf stage, we began watering the seedlings at 2-d intervals with half-strength Hoagland’s nutrient solution (Hoagland and Arnon 1950) (pH adjusted to 6.5 ± 0.1 with diluted HCl or NaOH). When the seedlings were about 10 cm tall and had 15 leaves, they were transferred to plastic tubs (52 cm × 37 cm × 15 cm) containing 13 L of half-strength nutrient solution (54 seedlings per biotype in one tub). The tubs were wrapped with black plastic to simulate the dark environment of roots in soil, and were placed in a growth chamber (20–25/15–20 °C day/night and 13-h photoperiod from sodium lamps). The nutrient solution was aerated each hour with an air-pump (FC-680; Corporation of Super, Shanghai, China) to maintain a dissolved oxygen concentration of 8.0–8.5 mg L−1. Treatments began after 10 days of pre-cultivation, as follows: (1) Control (CK), plants were growing in half-strength Hoagland solution; or (2) salinity treatment (Tr), plants that received half-strength Hoagland solution plus 150 mM NaCl (Note: only 50 mM NaCl was applied on Day 1 to avoid osmotic shock; from Day 2 onward, 100 mM NaCl was added for a total treatment of 150 mM NaCl). Nutrient solution was replaced every 2 days. The treatment was last for 10 days.
Evaluation of salt tolerance
All leaves were evaluated for salt injury (SI). The extent of SI to leaves from each biotype was assessed on an index scale of 0–4 (Yin et al. 2010), with 0 = no injury, no lesions seen on leaves; 1 = mild injury, a few leaf tips or edges yellowing, with <10 spots that were not coalesced on a leaf; 2 = moderate injury, about 50 % of the tips or edges browning, >10 spots per leaf, and coalescence; 3 = severe injury, most tips or edges browning or dead, and leaf-drop occurring; and 4 = extremely severe injury, with the entire plant dead. SI = (0 × S0 +1×S1 +2×S2 +3×S3 +4×S4)/54, where S0, S1, S2, S3, or S4 was the number of plants with injury indices of 0, 1, 2, 3, or 4, respectively, and 54 was the total number of plants. This visual evaluation was conducted on Days 3, 6, and 10 days of the experiment.
Net photosynthetic rate
The net photosynthetic rate (Pn) was determined every 2 days during the test period with a LI-6400XT Portable Photosynthesis System (Li-Cor Inc., Lincoln, NE, USA). Leaves were selected from the same position on each sampled plant (second or third from the shoot apex). Both control and salt-treated plants were measured five times. Because all of the leaves were fairly small, we used a fluorescence leaf chamber. The photosynthetic photon flux density (PPFD) was set at 1,000 μmol m−2 s−1 (blue and red diode light source) and flow was maintained at 500 μmol s−1.
Membrane damage
Membrane damage was estimated by the electrolyte leakage (EL) method, as described by Dionisio-Sese and Tobita (1998). Briefly, ten pieces of leaf discs (0.5 cm × 0.5 cm) were placed in each test tube containing 10 ml of distilled water. They were then incubated in a water bath at room temperature for 2 h, and the initial electrical conductivity (EC1) of the medium was measured with an electrical conductivity analyzer (DDS-307; Shanghai Precision Scientific Instrument Co., Ltd., China). Samples were then autoclaved at 100 °C for 30 min to release all electrolytes before being cooled to 25 °C to obtain the final electrical conductivity (EC2). EL was calculated as: EL = (EC1/EC2) × 100. Variation percentage (VP) of EL was calculated according to Yin et al. (2010). VP = (Tr−CK)/CK × 100, where Tr is EL from NaCl-stressed trees and CK is EL from control plants that received only half-strength Hoagland solution.
Relative water content and growth measurements
Ten plants each were randomly selected from our control and treated tubs. After the shoots and roots were separately sampled, their fresh weights (Fw) were determined and the tissues were placed in distilled water to rehydrate in the dark for 5 h before recording their turgid weights (Tw). They were then oven-dried at 70 °C for at least 3 days to obtain their dry weights (Dw). Relative water content (RWC) was calculated by the following formula: RWC = (Fw−Dw)/(Tw−Dw) × 100. Adverse resistance coefficient (ARC) was calculated according to Yin et al. (2010). ARC = Tr/CK, where Tr is the weight (Fw or Dw) from stressed plants (shoot or root) and CK is the weight (Fw or Dw) from control plants (shoot or root).
Statistical analysis
All data were statistically analyzed by one-way ANOVA, using the SPSS-16 for Windows statistical software package. Differences between treatments were separated by LSD tests, with P-values ≤0.05 considered significant. All graphs were generated by Sigmaplot-10 or SAS-V8 software.
Results
Salt injury index and salt tolerance
The index of salt injury can reflect the extent of tissue damage under salt stress. Symptoms were apparent on the leaves of Malus plants after 3 days of exposure to 150 mM NaCl (Table 2). Four biotypes of M. prunifolia—‘honghaitang’, ‘baihaitang’, ‘dongbaiguo’, and ‘neimengguhaihong’ were more susceptible than other biotypes after 3 days of exposure (SI ≥ 0.10). Values for SI continued to rise after 6 and 10 days of treatment, although the biotypes varied in their response to NaCl. For example, M. prunifolia ‘fupingqiuzi’, M. prunifolia ‘dongbeihuanghaitang’, and M. prunifolia ‘laoshannaizi’, as well as plants of M. sieversii and M. hupehensis, had relatively low SI indices, demonstrating their greater tolerance compared with three more sensitive biotypes of M. prunifolia—‘baihaitang’, ‘wulenghaitang’, and ‘neimengguhaihong’—that had higher SI values of 0.519, 0.509, and 0.625, respectively. All genotypes responded similarly to time of stress.
Net photosynthesis and salt tolerance
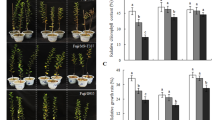
Compared with the control plants, rates of net photosynthesis showed obvious declines over the first 2 days in all treated biotypes (average Pn of 3–6 mmol m−2 s−2). Although ‘baihaitang’ and ‘neimengguhaihong’ had relatively low Pn values (3.032 and 3.213 mmol m−2 s−2, respectively) those rates were higher for M. prunifolia ‘laoshannaizi’ (5.697 mmol m−2 s−2) and M. sieversii (5.371 mmol m−2 s−2). After 4 days of salt stress, the highest rates were found with M. prunifolia ‘fupingqiuzi’, M. sieversii, and M. hupehensis. Moreover, M. sieversii showed the least change in photosynthetic activity among the three biotypes under salinity treatment, only down by 28.92 % when compared with the control. By contrast, stressed plants of ‘baihaitang’ had the lowest rate—1.307 mmol m−2 s−2 (Fig. 1), down by 44.59 % compared with the control.
Rate of net photosynthesis (Pn) measured on Days 2 and 4 from 11 Malus biotypes. For 10 days, stressed trees were exposed to NaCl while control plants were untreated. Data are mean ± SD (n = 5). Rootstock codes are defined in Table 1
Electrolyte leakage and relative water content
All treated plants had elevated EL readings after 10 days of stress. Calculations of the variation percentage (VP) revealed the range of tolerance among biotypes. For example, those less salt-tolerant had values between 26.662 and 34.727, indicating that NaCl stress led to serious damage to the integrity of cell membranes (Fig. 2). Such damage was not as severe among highly salt-tolerant biotypes. Such as M. prunifolia ‘fupingqiuzi’, M. prunifolia ‘laoshannaizi’, M. sieversii and M. hupehensis. Values for RWC in the shoots declined to varying degrees, i.e., down by 1.00–3.34 % for highly tolerant biotypes, 6.22–9.36 % for moderately tolerant, and 11.15–23.79 % for low-tolerant. The differences in root RWC showed a trend similar to that of the shoots but not very great (Fig. 3).
Variations percentage (VP) of electrolyte leakage from leaves of 11 Malus biotypes over 10 days (five samples per control or NaCl-stressed plants). Rootstock codes are defined in Table 1
Comparisons of shoot RWC or root RWC among 11 Malus biotypes. For 10 days, plants were either exposed to 150 mM NaCl or left untreated as the control. Data are mean ± SD (n = 10). Rootstock codes are defined in Table 1
Adverse resistance coefficients and salt tolerance
The shoots and roots of biotypes undergoing salinity treatment generally had lower fresh and dry weights compared with their corresponding controls (Table 3). The extent of this reduction was reflected in significant inter-rootstock variations in salt tolerance. For example, average ARC values for ‘fupingqiuzi’ and ‘dongbeihuanghaitang’ were 0.949 and 0.902, respectively. These strong performances were further demonstrated by only slight declines in their growth rates, meaning that they had high tolerance to salinity. By comparison, the average ARC for more salt-sensitive ‘baihaitang’ and ‘wulenghaitang’ was <0.703, a phenomenon also manifested in their severe growth inhibition.
Cluster analysis of salt tolerance among biotypes
Based on Ward’s Minimum-Variance Method and the SAS-V8 system, we grouped these biotypes into three classes (Fig. 4). They included High tolerance: three for M. prunifolia—‘fupingqiuzi’, ‘dongbeihuanghaitang’ and ‘laoshannaizi’—plus M. sieversii and M. hupehensis; Moderate tolerance: M. prunifolia ‘wuqiqiuzi’ and ‘dongbaiguo’; and Low tolerance: four for M. prunifolia — ‘honghaitang’, ‘baihaitang’, ‘wulenghaitang’, and ‘neimengguhaihong’. Except for M. prunifolia ‘honghaitang’, all of these groupings followed a pattern similar to that found with SI values, further validating our classification of biotypes by their level of tolerance.
Discussion
Leaf-tip necrosis is considered the first sign of damage from high concentrations of NaCl (Wahome et al. 2001). An index based on salinity injury can be used to indicate how well apple plants respond to stress because it is related to the most sensitive of physiological processes and describes comprehensively the growth performance of exposed tissues. In our research, we first applied a leaf-necrosis rating system to obtain a preliminary classification of 11 apple biotypes based on their salt tolerance. Here, three biotypes of M. prunifolia—‘fupingqiuzi’, ‘dongbeihuanghaitang’, and ‘laoshannaizi’—plus plants of rootstocks of M. sieversii and M. hupehensis had relatively low SI values. These were identified as salt-tolerant. Biotypes with the highest SI values, i.e., M. prunifolia ‘baihaitang’, ‘wulenghaitang’, and ‘neimengguhaihong’ were categorized as the most salt-sensitive.
Suppressed plant growth is an obvious outcome of salt stress (Chartzoulakis 2005; Nazar et al. 2011; Ruiz-Carrasco et al. 2011; Verslues et al. 2006). It is a consequence of several physiological responses, including modifications to water status, photosynthetic efficiency, and carbon allocation/utilization (Zhu 2001; Ma et al. 1997; Nabil and Coudret 1995; Seemann and Critchley 1985). We compared values for Pn, RWC (in shoots and roots), and average ARC (Fw and Dw of shoots and roots) among all biotypes. Salinity treatment resulted in significantly decreased rates of photosynthesis after 2 and 4 days compared with values for their respective controls. These differences in both RWC and average ARC were very consistent with the pattern found for Pn.
Cell membranes are dynamic structures that support numerous biochemical and biophysical reactions (Campos et al. 2003). They are also major targets of environmental stress (Leshem 1992). The degree of stability in those membranes is correlated with plant tolerance to abiotic challenges (Premachandra et al. 1992). Measurement of electrolyte leakage (EL) from plant tissues is promising for assessing plant quality and physiological status. Biotypes such as ‘baihaitang’, ‘wulenghaitang’, and ‘neimengguhaihong’ had higher VP of EL values than those of the other biotypes after 10 days of salt treatment. That finding was very consistent with the patterns of SI shown here.
The genome of Malus has a complicated genetic background and high heterozygosity due to poor self-compatibility (Harris et al. 2002). Single physiological and biochemical criteria, such as the activities of antioxidant enzymes or the accumulation of osmotic adjustment substances, might explain the genetic diversity between different species. However, without a clear genetic background, it is difficult to determine which of these criteria can most accurately and conveniently describe the salt tolerance of various rootstocks. Because plants differ in their tolerance mechanisms, growth and morphology parameters may be more objective predictors of tolerance (Bai et al. 2008). Therefore, we also utilized a composite appraisal that included Pn and EL along with SI, RWC, and ARC cluster analysis to evaluate the salt tolerance of these apple biotypes.
References
Ahmad P, Sarwat M, Sharma S (2008) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51:167–173
Alian A, Altman A, Heuer B (2000) Genotypic difference in salinity and water stress tolerance of fresh market tomato cultivars. Plant Sci 152:59–65
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123:1047–1056
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Bio Technol Adv 27:84–93
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bai TH, Li CY, Ma FW, Shu HR, Han MY (2008) Physiological responses and analysis of tolerance of apple rootstocks to root-zone hypoxia stress. Sci Agric Sinica 41:4140–4148 (In Chinese with an English abstract)
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103:1035–1040
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160:283–292
Chartzoulakis KS (2005) Salinity and olive: growth, salt tolerance, photosynthesis and yield. Agric Water Manag 78:108–121
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fedoroff NV, Battisti DS, Beachy RN, Cooper PJM, Fischhoff DA, Hodges CN, Knauf VC, Lobell D, Mazur BJ, Molden D, Reynolds MP, Ronald PC, Rosegrant MW, Sanchez PA, Vonshak A, Zhu JK (2010) Radically rethinking agriculture for the 21st century. Science 327:833–834
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol 22:875–884
Flowers TJ, Garcia A, Koyama M, Yeo AR (1997) Breeding for salt tolerance in crop plants—the role of molecular biology. Acta Physiol Plant 19:427–433
Garg B, Garg O (1982) Germination and some metabolic characteristics in green gram seedlings as affected by sodium carbonate and bicarbonate. Biol Plantarum 24:28–33
Harris SA, Robinson JP, Juniper BE (2002) Genetic clues to the origin of the apple. Trends Genet 18:426–430
Hoagland DR, Arnon DI (1950) The water-culture for growing plants without soil. Calif Agric Exp Stat Circ 347:32
Lee G, Carrow RN, Duncan RR (2004) Photosynthetic responses to salinity stress of halophytic seashore paspalum ecotypes. Plant Sci 166:1417–1425
Leshem Y (1992) Plant membranes: a biophysical approach to structure, development and senescence. Kluwer Academic Publishers, Dordrecht
Ma HC, Fung L, Wang SS, Altman A, Hüttermann A (1997) Photosynthetic response of Populus euphratica to salt stress. Forest Ecol Manag 93:55–61
Nabil M, Coudret A (1995) Effects of sodium chloride on growth, tissue elasticity and solute adjustment in two Acacia nilotica subspecies. Physiol Plantarum 93:217–224
Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87
Premachandra GS, Saneoka H, Fujita K, Ogata S (1992) Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in sorghum. J Exp Bot 43:1569–1576
Ron M (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Ruiz-Carrasco K, Antognoni F, Coulibaly AK, Lizardi S, Covarrubias A, Martínez EA, Molina-Montenegro MA, Biondi S, Zurita-Silva A (2011) Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. Plant Physiol Biochem 49:1333–1341
Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164:151–162
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 46:1092
Vigo C, Therios IN, Bosabalidis AM (2005) Plant growth, nutrient concentration, and leaf anatomy of olive plants irrigated with diluted seawater. J Plant Nutr 28:1001–1021
Wahid A, Ghazanfar A (2006) Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol 163:723–730
Wahome PK, Jesch HH, Grittne I (2001) Mechanisms of salt stress tolerance in two rose rootstocks: rosa chinensis ‘Major’ and R. rubiginosa. Scientia Hortic 87:207–216
Ye NH, Zhai H, Du ZJ, Xu H (2004) Evaluation of drought resistance of ten apple rootstocks. J Fruit Sci 21:395–398 (In Chinese with an English abstract)
Yin R, Bai TH, Ma FW, Wang XJ, Li YH, Yue ZY (2010) Physiological responses and relative tolerance by Chinese apple rootstocks to NaCl stress. Sci Hortic 126:247–252
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgments
This work was supported by the earmarked fund for China Agriculture Research System. The authors are grateful to Priscilla Licht for help in revising our English composition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, M., Li, C. & Ma, F. Physiological responses and tolerance to NaCl stress in different biotypes of Malus prunifolia . Euphytica 189, 101–109 (2013). https://doi.org/10.1007/s10681-012-0721-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0721-1