Abstract
Root rot of lettuce, which is caused by Fusarium oxysporum f. sp. lactucae (FOL), is a critical problem in the production of lettuce. FOL-resistant lettuce genetic resources have been identified and used in breeding programs to produce FOL-resistant cultivars. However, the genetic characteristics of resistance genes have not been studied in depth and, therefore, no DNA markers are presently available for these genes. In this study, we analyzed the RRD2 (resistance for root rot disease race 2) locus, which confers resistance to FOL race 2. Resistance loci were analyzed using two cultivars of crisphead lettuce: VP1013 (resistant) and Patriot (susceptible). The segregation patterns of resistant phenotypes in F2 indicated a single major locus. To define the positions of resistance loci, a linkage map was constructed using amplified fragment length polymorphism and random amplified polymorphic DNA (RAPD) markers. Quantitative trait loci analysis revealed the position of the major resistance locus. A high LOD score was observed for RAPD-marker WF25-42, and this marker showed good correspondence to the phenotype in different cultivars and lines. We successfully developed a sequence characterized amplified region marker from WF25-42.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root rot of lettuce, a soil-borne disease that occurs in many countries, has become a serious problem for lettuce production (Scott et al. 2010). This disease is caused by Fusarium oxysporum f. sp. lactucae (FOL). Lettuce plants infected by FOL initially show yellowing in the leaf marginal region, then the root vascular bundles turn brown and growth of the aerial parts is inhibited. Mild symptoms lead to failure of head formation but the plant remains alive, whereas severe symptoms kill infected plants. In Japan, FOL is classified into three races, namely 1, 2, and 3 (Fujinaga et al. 2001, 2003). Lettuce genetic resources were evaluated and selected using these races by Tsuchiya et al. (2004a). The ‘Shinano Hope’ cultivar was bred to be resistant to FOL race 1 (Tsuchiya et al. 2004b), and ‘Chouya No. 37’ was bred to be resistant to FOL race 2 (Tsuchiya 2009). These breeding programs to obtain resistant lettuces were carried out using conventional selection, using resistance tests based on pathogen inoculation.
Marker-assisted selection (MAS) is a powerful technique for efficient and rapid selection of certain phenotypes in conventional breeding programs. Disease-resistant candidates can be selected without pathogen inoculation, reducing the time and expense of field work. Genetic markers that are tightly linked to resistance genes, preferably <5 centimorgans (cM) of genetic distance, are required for MAS in crop breeding (Mohan et al. 1997; Collard and Mackill 2008). Construction of a detailed genetic map can provide genetic markers that are tightly linked to the locus controlling the character of interest.
Several genetic maps of lettuce have been reported using populations raised from intra- or inter-specific crosses (Kesseli et al. 1994; Witsenboer et al. 1997; Waycott et al. 1999; Johnson et al. 2000; Jeuken et al. 2001; Syed et al. 2006). The current reference map contains 36 loci associated with resistant phenotypes for six diseases and one insect pest: downy mildew, root downy mildew, corky root rot, lettuce mosaic virus, turnip mosaic virus, lettuce dieback, and root aphids (McHale et al. 2009). To date, however, no gene loci have been identified for resistance to FOL.
The aim of this study was to obtain genetic markers for further breeding programs of FOL-resistant commercial lettuce cultivars. We constructed a linkage map using amplified fragment length polymorphism (AFLP) and random amplified polymorphic DNA (RAPD) markers and performed quantitative trait loci (QTL) analysis to identify FOL resistance loci.
Materials and methods
Plant materials
We used the crisphead lettuce cultivars VP1013 (resistant to FOL race 2) and Patriot (susceptible) in this study. VP1013 is bred by Nagano Vegetable and Ornamental Crops Experiment Station from commercial cultivar Kikugawa No. 102 bred by Yokohama Ueki Corp, with self pollination (Tsuchiya et al. 2004a, 2009). Fifty-eight individuals of the F2 progeny from a cross between VP1013 and Patriot were used for linkage analysis. To estimate the genotypes of F2 individuals, we used 14–22 plants of the selfed F3 population to test disease resistance. DNA markers were evaluated using an additional 18 fixed lines and 17 commercial cultivars (see Table 1).
Disease resistance test
The resistance test for FOL race 2 was performed as described by Tsuchiya et al. (2004a). Seeds (18 of both parental lines, and 14–22 of each F3 population) were sown directly into artificially inoculated soil. Approximately 1 month after germination, seedlings were examined and scored using the disease index described below, and the disease incidence and disease severity were calculated.
The disease index for leaves (including all shoots) was as follows: 0, no symptoms; 1, partial necrosis of leaf only; 2, leaf necrosis, stunting, and wilting; 3, severe wilting and death.
The disease index for roots was as follows: 0, no symptoms; 1, some brown roots; 2, many brown roots; 3, inside of crown rotten and brown.
Disease incidences and disease severities of F2 populations were calculated as follows using disease indexes of corresponding F3 lines:
where A is the number of category ‘3’ plants, B is the number of ‘2’ plants, C is the number of ‘1’ plants, and ‘N’ is the total number of plants examined.
DNA extraction
DNA was extracted from the leaves of parental lines and F2 plants using the cetyl trimethyl ammonium bromide (CTAB) method (Hayashida et al. 2008).
AFLP analysis
AFLP analysis (Vos et al. 1995) was carried out using the AFLP™ Plant Mapping Kit (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. Selective PCRs were performed using 20 selective primer combinations, 2 EcoRI + AGS (S is G or C) × 8 MseI + CWN (W is A or T and N is A, C, G, or T), EcoRI + ACA × MseI + CTG, EcoRI + ACC × MseI + CTA, EcoRI + AAG × MseI + CTT, and EcoRI + ACT × MseI + CAT with a GeneAmp® PCR System 2700 (Applied Biosystems). Fluorescence detection was performed using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems).
RAPD analysis
RAPD analysis (Williams et al. 1990) was carried out with 435 combinations of random 12-mer oligonucleotides (Wako Pure Chemicals, Osaka, Japan). PCR was performed in a 10 μL reaction solution containing 3 ng template DNA, 0.5 μM primers, 0.2 mM dNTPs, 1 μL 10× manufacturer’s reaction buffer, 4 mM MgCl2, and 0.25 U Taq DNA polymerase (Bioline, London, UK). Amplification was performed in a thermal cycler (ThermoGen, Nagano, Japan) programmed as follows: 94 °C for 1 min; 20 cycles of 94 °C for 15 s, annealing temperatures decreasing by 0.5 °C each cycle starting with 45 °C for 2 min, 72 °C for 2 min; 25 cycles of 94 °C for 15 s, 35 °C for 2 min, and 72 °C for 2 min; and 72 °C for 7 min. PCR products were electrophoresed in 2 % agarose gels and stained with ethidium bromide. Band patterns in the gels were observed using a Typhoon trio imager (GE Healthcare, UK).
Bulked segregant analysis
Based on the disease severity, resistant and susceptible bulks were constructed with eight F2 plants, respectively. The resistant bulk consisted of plants with disease severities ranging from 0 to 5; the susceptible bulk consisted of plants with disease severities ranging from 98 to 100.
Linkage analysis and QTL analysis
For individual markers, goodness-of-fit to expected segregation ratios was tested by Chi-squared tests (p > 0.05). Linkage data was analyzed using JoinMap 4.0 (van Ooijen 2006). Mapping was carried out with the following thresholds: recombination frequency < 0.4, LOD > 1, and jump = 5. Recombination frequencies were converted to map distances in centimorgans using the Kosambi function (Kosambi 1944).
Detection of QTLs was carried out using QGene 4.0 (Joehanes and Nelson 2008) using interval mapping (IM) and composite interval mapping (CIM). Cofactor of CIM was “forward cofactor selection”. IM and CIM were performed at LOD threshold values that were estimated by means of a permutation test with 1,000 permutations. The interval was 1 cM.
Development of SCAR marker
A sequence characterized amplified region (SCAR) marker WF25-42-SCAR was developed based on the RAPD marker WF25-42. The DNA sequence of the WF25-42 fragment was analyzed and a primer set was designed (5′-GAGCAATTGATACATACCTGCAACATTGTT-3′ and 5′-GGAGGGGCAATAAGAACATTTGCTCTT-3′). For detection of WF25-42-SCAR, PCR was performed in a 10 μL reaction solution containing 3 ng template DNA, 0.5 μM primers, 0.2 mM dNTPs, 1 μL 10× manufacturer’s reaction buffer, 4 mM MgCl2, and 0.25 U Taq DNA polymerase (Bioline). Amplification was performed in a thermal cycler (ThermoGen) programmed as follows: 94 °C for 1 min; 35 cycles of 94 °C for 15 s, 56 °C for 5 s, and 72 °C for 30 s; and 72 °C for 5 min. PCR products were electrophoresed in 2 % agarose gels and stained with ethidium bromide. Band patterns in the gels were observed using a Printgraph photo image instrument (ATTO, Tokyo, Japan).
Results
Segregation of resistance to FOL race 2
The disease severity of the F2 progenies derived from the cross between the resistant and susceptible cultivars was used to understand the pattern of inheritance of resistance to FOL race 2. The disease severities of VP1013 and Patriot were reported previously by Fujinaga et al. (2001, 2003). The disease severities of F2 populations were evaluated according to the results of disease resistance tests of F3 lines raised from selfed F2 individuals. Of the 58 F2 individuals, the disease severities of leaves of their progenies were distributed as follows: 12 lines, 0–20; 32 lines, 20–80; 14 lines, 80–100. The disease severities of roots were distributed as follows: 11 lines, 0–20; 32 lines, 20–80; 15 lines, 80–100. Regardless of tissue, resistance to FOL race 2 as scored by the disease severity seemed to segregate at the expected ratio of 1:2:1 in the F2 population (Fig. 1). Results shown in Fig. 1 suggested resistance to FOL race 2 might be determined by a single major dominant locus, but with minor loci that contribute some modifications. We designated the major locus as RRD2 (resistance for root rot disease race 2).
Disease severities among F2 plants. Histogram shows the number of F2 plants showing each disease severity score. Disease severity was calculated as described in the “Materials and methods”. a Disease severity scores for leaves. b Disease severity scores for roots. The horizontal axis indicates disease severity in 5-point increments
Establishment of molecular markers
We carried out AFLP analysis to estimate the position of RRD2. The marker candidates that showed polymorphism in the parental lines were tested using individual F2 plants, and 63 markers showed almost 3:1 segregation. RAPD analysis using 432 primer combinations was carried out with resistant and susceptible bulks to identify polymorphic amplified bands that were tightly linked to RRD2. The candidate bands were subsequently validated on individual F2 plants. Finally, 16 markers were selected that showed almost 3:1 segregation. The segregation of all 16 RAPD-markers in 58 F2 plants was tested with the Chi-squared test (p > 0.05) and did not deviate from expected ratios of 3:1.
Construction of linkage map and QTL analysis
Linkage analysis was carried out with 63 AFLP markers and 16 RAPD markers, and in total these 61 markers formed five linkage groups (numbered LG1 to LG5 in descending order of length), and 18 markers were independent of these groups (Fig. 2). More than half of the AFLP markers were located in two major linkage groups, namely LG1 and LG2 (65.6 and 32.7 cM, and 29 and 32 % of all markers are incorporated, respectively).
Linkage map, LOD scores, and identification of the RRD2 locus. Join Map 4.0 and QGene software were used for the analysis as described in the “Materials and methods”. The marker distances and LOD scores are illustrated at the same scale. LOD scores less than thresholds were omitted. Locus names and map distances (cM) are indicated on the left of the linkage groups (LGs). AFLP markers are named as XXX NN–NN format, whereas RAPD markers are WFXX-XX format (where X means numeric, and N means G, A, T, or C). Roman letters indicate AFLP markers and italic letters indicate RAPD markers. In the QTL chart, a solid line indicates the LOD score calculated from the leaves data set, and a broken line indicates the LOD score calculated from the roots data set. Vertical lines indicate LOD thresholds. LOD thresholds in SIM were 2.9 (leaves) and 2.8 (roots). LOD thresholds in CIM were 2.6 (leaves) and 2.8 (roots)
QTL analysis of resistance to FOL race 2 was performed on the constructed linkage map with the data sets of disease severities from both leaves and roots (Fig. 2). Only the LOD score on LG2 showed large peaks that exceeded the LOD thresholds, as calculated by QGene in both tissues. The strongest QTL peak was located in the region between the two markers WF11-12 and WF09-23.
Within this major QTL region, three RAPD markers were located (WF25-42 (11.0 cM), WF43-50 (11.7 cM) and WF09-23 (14.1 cM)). According to the genotyping data, WF25-42 and WF43-50 were associated with the susceptible phenotype, whereas WF09-23 was associated with the resistant phenotype (Fig. 3).
Relationship between RAPD markers and resistance phenotypes. Disease severities and genotypes of RAPD markers of individual F2 plants derived from VP1013 × Patriot cross were plotted. The RAPD markers used were: a WF25-42, b WF43-WF45 and c WF09-WF23. Dots represent each F2 plant, and indicate disease severity of roots and positive/negative signals of the marker
RRD2 genotypes of different cultivars and lines
The three markers in the largest QTL peak and four linked markers were used to genotype 17 cultivars and 18 lines. Of these 35 cultivars/lines, 21 were resistant and 14 were susceptible. The genotypes of RRD2 and markers were considered to be homozygous in these cultivars/lines because lettuce is a self-pollinator. The resultant genotypes of the DNA markers correlated strongly with the phenotypes of resistance to FOL race 2 (Table 1). In particular, WF25-42 closely predicted the susceptible phenotype.
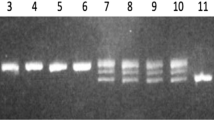
For six of the DNA markers, some exceptions in the correlations between phenotypes and genotypes were observed. WF10-46-2, WF42-43, WF41-45, and WF43-50 were associated with the susceptible phenotype, but signals of these markers were detected in some resistant cultivars/lines (Chouya No. 37, Kikugawa No. 102, UC-016, UC-023, and YL216). Signals of the markers WF11-12 and WF09-23, which were associated with the resistant phenotype, were inconsistent with the phenotypes of Banchu Red Fire, N8-19, UC-016, Shinano hope, Ster Ray, Success, and VI59. Because of its good predictability, the amplified fragment of WF25-42 was sequenced in order to design a specific primer set so that it could be utilized as a SCAR marker. The resultant WF25-42-SCAR showed completely identical signals with the RAPD WF25-42 (Fig. 4).
Detection of WF25-42 RAPD marker and WF25-42-SCAR SCAR marker. Agarose gel electrophoresis of the PCR product using RAPD primers for WF25-42 visualized using an image scanner. The specific band at 260 bp marked by an arrow indicates the position of the WF25-42 marker. Photo images of the WF25-42-SCAR PCR product stained with ethidium bromide are presented. Lanes 1–21 are samples from resistant plants. Lanes 22–35 are samples from susceptible plants. The names of the cultivar/line in each individual lane are listed in Table 1. V VP1013, lane P Patriot
Discussion
We constructed a linkage map of lettuce using F2 plants from the cross between VP1013 and Patriot, and identified the major root rot resistance locus RRD2, which was positioned between WF11-12 and WF09-23 in LG2. Particularly the RAPD marker WF25-42 was tightly associated with the susceptible allele of RRD2. Thus, this marker will provide efficient selection of candidates that are resistant to FOL race 2 in breeding programs. FOL is categorized into three races, namely 1, 2, and 3 (Fujinaga et al. 2001, 2003). To date, only single races of FOL have been found in infected areas, but it is possible that fields will be contaminated by multiple races in the near future. In lettuce, it is presumed that individual resistance genes exist for each of the FOL races. Pyramiding those resistance genes is desirable to produce new cultivars that are resistant to several races. The DNA markers identified in this study could help to achieve this goal.
In previous studies, linkage maps of lettuce were constructed using crosses between materials that were genetically distinct. Kesseli et al. (1994), Witsenboer et al. (1997) and Waycott et al. (1999) used a population from an intraspecific cross between crisphead and butterhead lettuce (L. sativa). Jeuken et al. (2001) used a population from the interspecific cross between L. saligna and L. sativa, and Syed et al. (2006) and Truco et al. (2007) used a population from an interspecific cross between L. serriola and L. sativa. Unfortunately, the previously reported genetic maps are not directly applicable to Japanese lettuce, as they were constructed using American/continental lines. The cultivars VP1013 and Patriot used in the present study were regarded as genetically distant materials. VP1013 is a cultivar derived from Kikugawa No. 102 (Salinas-type) and Patriot is a commercial cultivar bred from Empire-type materials in Japan. These cultivars have many different characters, such as disease resistance and flowering time.
Lactuca sativa has nine chromosomes, but only five linkage groups were constructed in this study. Markers were concentrated on a few limited linkage groups. It was thought to be due to poor polymorphisms in ice berg lettuce (Hu et al. 2005).
Many markers were identified around the RRD2 locus, which suggests partial introgression of genome regions that contain RRD2 into VP1013 from a genetically distant ancestor occurred.
Prior to the present study, it was assumed resistance to FOL race 2 was regulated by a single dominant locus in VP1013. The distribution ratio of disease severities supports this hypothesis, because they seemed to be the expected ratio of 1:2:1 (resistant:intermediate:susceptible) in the F2 population. Individuals in the intermediate class in the F2 population were supposed to be heterozygous for RRD2 locus.
The results of RAPD analysis using different cultivars and lines showed the defined RAPD markers reliably identified plants that carry the RRD2 locus, and predicted the resistant phenotype. Especially, markers WF25-42 and WF25-42-SCAR showed perfect association with the phenotype in all materials analyzed. On the other hand, markers mapped near the RRD2 locus showed some exceptions. This could be caused by chromosomal recombination accumulated during the breeding programs that produced the cultivars and lines used in this study, thus providing supportive information on the region surrounding the RRD2 locus. Thus, WF25-42 must be closely linked to RRD2, which are probably conserved among different cultivars or lines. WF25-42 will be applicable for selection of the RRD2 locus derived from a wide range of genetic resources.
It is expected that further exploration of molecular markers would facilitate identification of the RRD2 gene and elucidate molecular mechanisms of root rot resistance in lettuce. In addition, further research to develop new markers for other FOL resistance genes would be useful to increase our knowledge of FOL resistance and to exploit this trait in novel cultivars.
References
Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Phil Trans R Soc B 363:557–572. doi:10.1098/rstb.2007.2170
Fujinaga M, Ogiso H, Tsuchiya N, Saito H (2001) Physiological specialization of Fusarium oxysporum f. sp. lactucae, a causal organism of fusarium root rot of crisp head lettuce. J Gen Plant Pathol 67:205–206. doi:10.1007/PL00013012
Fujinaga M, Ogiso H, Tsuchiya N, Saito H (2003) Race 3, a new race of Fusarium oxysporum f. sp. lactucae determined by a differential system with commercial cultivars. J Gen Plant Pathol 69:23–28. doi:10.1007/s10327-002-0009-8
Hayashida N, Takabatake Y, Nakazawa N, Aruga D, Nakanishi H, Taguchi G, Sakamoto K, Matsumoto E (2008) Construction of a practical SCAR marker linked to clubroot resistance in Chinese cabbage, with intensive analysis of HC352b genes. J Jpn Soc Hortic Sci 77:150–154. doi:10.2503/jjshs1.77.150
Hu J, Ochoa OE, Truco MJ, Vick BA (2005) Application of the TRAP technique to lettuce (Lactuca sativa L.) genotyping. Euphytica 144:225–235. doi:10.1007/s10681-005-6431-1
Jeuken M, van Wijk R, Peleman J, Lindhout P (2001) An integrated interspecific AFLP map of lettuce (Lactuca) based on two L. sativa × L. saligna F2 populations. Theor Appl Genet 103:638–647. doi:10.1007/s001220100657
Joehanes R, Nelson JC (2008) QGene 4.0, an extensible Java QTL-analysis platform. Bioinformatics 24:2788–2789
Johnson WC, Jackson LE, Ochoa O, van Wijk R, Peleman J, St. Clarir DA, Michelmore RW (2000) Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor Appl Genet 101:1066–1073. doi:10.1007/s001220051581
Kesseli RV, Paran I, Michelmore RW (1994) Analysis of a detailed genetic linkage map of Lactuca sativa (lettuce) constructed from RFLP and RAPD markers. Genetics 136:1435–1446
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
McHale LK, Truco MJ, Kozik A, Lavelle DO, Ochoa OE, Wroblewski T, Knapp SJ, Michelmore RW (2009) The genomic architecture of disease resistance in lettuce. Theor Appl Genet 118:565–580. doi:10.1007/s00122-008-0921-1
Mohan M, Nair S, Bhagwat A, Krishna TG, Yano M, Bhatia CR, Sasaki T (1997) Genome mapping, molecular markers and marker-assisted selection in crop plants. Mol Breed 3:87–103. doi:10.1023/A:1009651919792
Scott JC, Kirkpatrick SC, Gordon TR (2010) Variation in susceptibility of lettuce cultivars to fusarium wilt caused by Fusarium oxysporum f. sp. lactucae. Plant Pathol 59:139–146. doi:10.1111/j.1365-3059.2009.02179.x
Syed NH, Sorensen AP, Antonise R, Van de Wiel C, Van der Linden CG, van‘t Westende W, Hooftman D, den Nijs H, Flavell AJ (2006) A detailed linkage map of lettuce based on SSAP, AFLP, and NBS markers. Theor Appl Genet 112:517–527. doi:10.1007/s00122-005-0155-4
Truco MJ, Antonise R, Lavelle D, Ochoa O, Kozik A, Witsenboer H, Fort SB, Jeuken MJ, Kesseli RV, Lindhout P, Michelmore RW, Peleman J (2007) A high-density, integrated genetic linkage map of lettuce (Lactuca spp.). Theor Appl Genet 115:735–746
Tsuchiya N (2009) ‘Chouya No. 37’, a fusarium root rot (race 2)-resistant lettuce. J Jpn Soc Hortic Sci 78:206–210. doi:10.2503/jjshs1.78.206
Tsuchiya N, Fujinaga M, Ogiso H, Usui T, Tsukada M (2004a) Resistance tests and genetic resources for breeding fusarium root rot resistant lettuce. J Jpn Soc Hortic Sci 73:105–113
Tsuchiya N, Yoshida K, Usui T, Tsukada M (2004b) ‘Shinano Hope’, a fusarium root rot-resistant lettuce. J Jpn Soc Hortic Sci 73:429–434. doi:10.2503/jjshs.73.429
Van Ooijen JW (2006) Joinmap 4.0 manual. Kyazma, Wageningen
Vos P, Hogers R, Bleeker M, Reijans M, Van De Lee T, Hornes M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Waycott W, Fort SB, Ryder EJ, Michelmore RW (1999) Mapping morphological genes relative to molecular markers in lettuce (Lactuca sativa L.). Heredity 82:245–251. doi:10.1046/j.1365-2540.1999.00473.x
Williams JGK, Kubelik AR, Livak KL, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Witsenboer H, Vogel J, Michelmore RW (1997) Identification, genetic localization, and allelic diversity of selectively amplified microsatellite polymorphic loci in lettuce and wild relatives (Lactuca spp.). Genome 40:923–936. doi:10.1139/g97-119
Acknowledgments
This work was supported by a Grant-in-Aid from the Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology. We would like to thank Drs. Hiromitsu Nakanishi, Masayuki Hashimoto and Goro Taguchi for their valuable advice and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aruga, D., Tsuchiya, N., Matsumura, H. et al. Analysis of RAPD and AFLP markers linked to resistance to Fusarium oxysporum f. sp. lactucae race 2 in lettuce (Lactuca sativa L.). Euphytica 187, 1–9 (2012). https://doi.org/10.1007/s10681-012-0665-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-012-0665-5