Abstract
It has long been proposed that white-flowered chrysanthemums (Chrysanthemum morifolium Ramat.) have a single dominant gene that inhibits carotenoid formation or accumulation in ray petals. However, the precise function of the proposed gene was unknown. We previously isolated a gene encoding carotenoid cleavage dioxygenase 4, designated CmCCD4a, which is specifically expressed in the ray petals of white-flowered chrysanthemums. Because CmCCD4a was a strong candidate for the single dominant gene, we analyzed the relationship between CmCCD4a expression and carotenoid content in two sets of petal color mutants. Here, we show that CmCCD4a represents a small gene family containing at least four members. Two of them, CmCCD4a-1 and CmCCD4a-2, were highly expressed in ray petals of two taxa with low carotenoid levels. In petal color mutants derived from these taxa, increases in carotenoid levels accompanied decreases in CmCCD4a expression levels in ray petals. Two different circumstances reduced the levels of CmCCD4a expression in the mutants: either a CmCCD4a gene was lost from the genome or the expression of a CmCCD4a gene was suppressed. In the latter case, suppression may be caused by the loss of a function that normally enhances CmCCD4a transcription. A stepwise decrease in the amount of CmCCD4a expression in either L1 or L2 resulted in a corresponding stepwise increase in the carotenoid content in ray petals. From these results, we propose that CmCCD4a expression is the key factor that controls the carotenoid content in ray petals of chrysanthemum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major pigments in the ray petals of chrysanthemum (Chrysanthemum morifolium Ramat.) are lutein derivatives (yellow carotenoids) and cyanidin derivatives (red anthocyanins) (Nakayama et al. 1997; Kishimoto et al. 2004). Combinations of these pigments produce a wide range of ray petal colors, including red, orange, yellow, pink, and white (Kawase and Tsukamoto 1976; Kishimoto et al. 2007).
Petal color mutants of outstanding chrysanthemum cultivars have sometimes been developed as new cultivars (Wasscher 1956). Petal color mutations from white to yellow occasionally occur, but mutations in the opposite direction (yellow to white) are rare (Machin and Scope 1978). It has been postulated that a single dominant gene that inhibits carotenoid formation or accumulation acts in the ray petals of white-flowered chrysanthemum (Stewart and Derman 1970; Jordan and Reinmann-Philipp 1983; Hattori 1991; Boase et al. 1997). We isolated a gene encoding carotenoid cleavage dioxygenase 4, designated CmCCD4a, that is specifically expressed in ray petals of white-flowered chrysanthemum (Ohmiya et al. 2006). Suppression of CmCCD4a expression by RNA interference (RNAi) in white-flowered chrysanthemum produced yellow-flowered transformants (Ohmiya et al. 2006, 2009). In addition, all of the F1 progeny obtained by crossing between white-flowered wild chrysanthemums containing CmCCD4a orthologs and a yellow-flowered cultivar without CmCCD4a had white ray petals, indicating that CmCCD4a is a dominant gene that was homozygous in the white-flowered wild chrysanthemums (Yoshioka et al. 2010). These results made CmCCD4a a strong candidate for the single dominant gene.
CmCCD4a may represent a small gene family rather than a single gene, because chrysanthemum cultivars are hexaploid (2n = 6x = 54; Endo 1969; Shibata et al. 1998). On the other hand, it has been proposed that carotenoid accumulation in ray petals is controlled by a single factor (Stewart and Derman 1970; Jordan and Reinmann-Philipp 1983; Hattori 1991; Boase et al. 1997). The fact that petal color mutation from white to yellow sometimes occurs in some white-flowered cultivars, such as ‘Marble’, ‘Daisy’, and ‘Indianapolis’ (Yoder Bros Inc. 1967), suggests that few copies of CmCCD4a function in such cultivars. On the other hand, there are white-flowered cultivars such as ‘Jimba’ and ‘Sei-Marine’ in which petal color mutations rarely occur, suggesting that multiple copies of CmCCD4a function in such cultivars. In addition, mutations leading to yellow flower color generally progress in a stepwise pattern from white to pale yellow to deep yellow. The copy number of functional CmCCD4a genes might explain this stepwise increase in carotenoid levels; nevertheless, little data on CmCCD4a has been available.
In this study, we sought to clarify the involvement of CmCCD4a in regulating carotenoid accumulation in ray petals of chrysanthemum. Two types of petal color mutants leading to increases in carotenoid content were used for the analysis: one was obtained by somatic variation during tissue culture, the other by bud-sport mutation. We analyzed the relationship between CmCCD4a transcript level and carotenoid content in ray petals of these mutants. From the results, we propose a mechanism that could cause an increase in carotenoid content in chrysanthemum ray petals during the process of petal color mutation.
Materials and methods
Plant materials
The chrysanthemum cultivars used in this study were obtained from the genetic resource stocks of the National Institute of Floricultural Science (Tsukuba, Ibaraki, Japan). The ‘Marble’ cultivars are a series of bud sports with various petal colors; the ones used in this study were ‘Pink Marble (PM)’, ‘Apricot Marble (AM)’, ‘Bronze Marble (BrM)’, ‘Blue Marble (BM)’, ‘Coral Marble (CM)’, ‘Orange Marble (OM)’, ‘White Marble (WM)’, ‘Polished Marble (PoM)’, and ‘Florida Marble (FM)’. ‘Paragon’ is a white-flowered cultivar. Six independent petal color mutants (cam1–cam6) were found among regenerants derived from ‘94-765’, a tissue culture line obtained from Seikoen Co., Ltd., Japan (Fig. 1).
RT-PCR-mediated cloning of CmCCD4a homologs
Total RNA was isolated from ray petals of fully opened flowers by using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and then treated with DNase I (Invitrogen) according to the manufacturer’s instructions. cDNAs were synthesized from the total RNA by using the Superscript First-Strand Synthesis System (Invitrogen). For isolation of homologs of CmCCD4a, RT-PCR was performed with ray petal cDNA of each petal color mutant as the template and the primer set CCD4a-F and CCD4a-R (Table 1) using Advantage 2 DNA Polymerase (Clontech, Mountain View, CA, USA). The PCR conditions were as follows: 95°C for 2 min; and 35 cycles at 95°C for 10 s, 64°C for 20 s, and 72°C for 90 s. PCR fragments were cloned into the pCR2.1 vector (Original TA Cloning Kit, Invitrogen) and sequenced with a Big Dye Terminator v. 3.1 Cycle Sequencing Kit and an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Cloning of genomic CmCCD4a homologs by PCR
Genomic DNA was isolated from mature leaves by the cetyl trimethyl ammonium bromide method (Murray and Thompson 1980). The promoter region of CmCCD4a-1 was amplified from chrysanthemum genomic DNA by thermal asymmetric interlaced PCR (Liu et al. 1995). Three nested primers complementary to the coding sequence of CmCCD4a-1 (4a-1-N1, 4a-1-N2, 4a-1-N3) and an arbitrary degenerate primer (AD) were used (Liu et al. 1995). The genomic sequences including promoter and coding regions of CmCCD4a-2, -3, and -4 were isolated using primers complementary to the sequence of CmCCD4a-1. The sequences of primers used for the genomic PCR are listed in Table 1. The PCR conditions were 95°C for 2 min; and 35 cycles at 95°C for 10 s, 64°C for 20 s, and 68°C for 90 s. A predicted plastid-targeting peptide was identified by the iPSORT program (http://hc.ims.u-tokyo.ac.jp/iPSORT/).
Gene expression analyses
Levels of CmCCD4a transcripts were analyzed by reverse-transcription quantitative real-time PCR (RT-qPCR) using SYBR Premix Ex Taq II (TaKaRa, Ohtsu, Japan). Reactions were carried out with a LightCycler system (Roche Diagnostics, Basel, Switzerland). The RT-qPCR conditions were as follows: 95°C for 20 s; and 45 cycles at 95°C for 4 s, 64°C for 8 s, and 68°C for 20 s. Known concentrations of plasmid DNAs were used for drawing standard curves. The expression level of actin (GenBank accession no. AB205087) was used to normalize the transcript level in each sample. The sequences of primers used for RT-qPCR are listed in Table 1.
Carotenoid extraction, quantification, and HPLC analysis
Ray petals (0.5 g) were ground in acetone. Before grinding, the basal part of each (up to ~5 mm from the bottom) was removed, because the color was different from the rest of the petal (Fig. 1a). The extracts were partitioned between diethyl ether and aqueous NaCl. The absorbance of the diethyl ether layer at a wavelength of 450 nm was measured. Carotenoid content was calculated by using the E 1%1 cm value of lutein (Britton 1995) and expressed as micrograms of lutein equivalent per gram fresh weight (μg/g FW) of tissue. Carotenoids were extracted from petals and were analyzed by high-performance liquid chromatography (HPLC; X-LC, JASCO, Tokyo, Japan) with a YMC carotenoid column (4.6 × 250 mm2; YMC, Kyoto, Japan), according to Kishimoto et al. (2007). Peaks were identified by comparing retention times and absorbance spectra with those of previously identified compounds in ray petals of chrysanthemum (Kishimoto et al. 2004; Kishimoto and Ohmiya 2006) and with those of standard compounds.
Microscopic observation
Fresh ray petals were sliced longitudinally with paired double-edge razor blades. The sections were examined through a Provis-AX70 light microscope (Olympus, Tokyo, Japan). Photographs were taken with DP Controller software (Olympus).
Results
Cloning of CmCCD4a homologs
To isolate genes encoding CmCCD4a, we performed RT-PCR using cDNA of ray petals of ‘Paragon’, a white-flowered chrysanthemum cultivar, as a template. At least 4 types of CmCCD4a homologs with sequences >90% identical to one other were isolated: these genes were designated CmCCD4a-1, -2, -3, and -4 (Supplementary Fig. 1). This result indicates that these genes are members of a small gene family in the ‘Paragon’ genome. The protein coding sequence of CmCCD4a-1 was identical to that of CmCCD4a previously reported by Ohmiya et al. (2006). CmCCD4a-4 was determined to be a pseudogene, because in-frame stop codons caused by nucleotide deletions were found in the protein coding sequence (Supplementary Fig. 1).
Using RT-PCR–mediated cloning, we obtained 76 CmCCD4a cDNA clones from ‘Paragon’, 94 from ‘94-765’, and 50 from PM, and sequenced all of them. The nucleotide sequences of each of the four CmCCD4a homologs among these three taxa were identical. CmCCD4a-1 and CmCCD4a-2 were the main genes expressed in ‘Paragon’ and PM (Table 2). In ‘94-765’, 97% of the transcripts were CmCCD4a-2, and CmCCD4a-1 was not detected. CmCCD4a-3 was expressed at a low level in ‘94-765’ but was not detected in PM.
The genomic sequence of CmCCD4a-2 showed similar structural features to those previously reported for CmCCD4a-1 (Ohmiya et al. 2006), such as an open reading frame of 1797 bp predicted to encode a 599-amino-acid polypeptide (identity 94%; similarity 97% to CmCCD4a-1) and a 97-bp intron 1442 bp downstream from the start codon (Supplementary Fig. 1). The sequence similarity of the promoter region between CmCCD4a-1 and CmCCD4a-2 was lower than that of coding region (identity 79%; similarity 93%). As observed in CmCCD4a-1 (Ohmiya et al. 2006), a predicted plastid-targeting peptide was identified in the N-terminal region of CmCCD4a-2, suggesting that both CmCCD4a-1 and CmCCD4a-2 are localized in the chromoplast.
Analyses of petal color mutants derived from ‘94-765’
To clarify the involvement of CmCCD4a in the level of carotenoids in ray petals, we used six independent petal color mutants (cam1–cam6) found among regenerants derived from ‘94-765’ (Fig. 1a). Ray petals of the wild type of ‘94-765’ (WT) contained 4.1 ± 0.3 μg/g FW of carotenoids. Ray petals of cam1–cam4 contained 6 to 8 times that level; those of cam5 and cam6 accumulated 1.3 to 1.8 times that level (Fig. 1b). Ray petals of cam5 and cam6 had lower anthocyanin contents than the WT (Supplementary Fig. 2a).
To evaluate whether genes encoding CmCCD4a-1 and CmCCD4a-2 exist in the genomes of the WT and each mutant, we designed primers specific to each homolog and performed genomic PCR. In the WT, a fragment of appropriate length was amplified with the CmCCD4a-2 primer set (Fig. 2b), whereas no band was detected with the CmCCD4a-1 primer set (Fig. 2a), indicating that the WT genome lacks an intact copy of CmCCD4a-1. The result was consistent with that of the RT-PCR-mediated cloning, in which clones corresponding to CmCCD4a-1 were not detected among the CmCCD4a clones from the WT (Table 2). Genomic PCR analysis of cam1–cam6 showed that no intact copy of CmCCD4a-2 was present in the genome of cam2, cam3, or cam4, but CmCCD4a-2 was detected in the genomes of cam1, cam5, and cam6 (Fig. 2b). The nucleotide sequences of CmCCD4a-2 from 792 bp upstream of the start codon to the stop codon were identical among WT, cam1, cam5, and cam6 (data not shown).
Genomic PCR and RT-qPCR analyses of CmCCD4a in WT and cam1–cam6. a Genomic PCR with CmCCD4a-1-specific primers (Pro4a-1-F and Pro4a-1-R; Table 1). b Genomic PCR with CmCCD4a-2-specific primers (Pro4a-2-F and Pro4a-2-R; Table 1). Genomic DNAs of ‘Paragon’ (P), WT, and cam1–cam6 were used as templates. An actin primer set (Actin-F and Actin-R; Table 1) was used as a PCR control. c Relative CmCCD4a-2 expression level in ray petals. Error bar: ±SEM (n = 6). A graph with expanded ordinate is shown in the inset
To investigate whether CmCCD4a-2 was involved in the increase in carotenoid content in ray petals of the cam mutants, we examined the relationship between carotenoid content and the level of CmCCD4a-2 expression. RT-qPCR analysis showed that the decrease in CmCCD4a-2 expression level was accompanied by an increase in the carotenoid content in ray petals of all six cam mutants (Figs. 1, 2c). Expression of CmCCD4a-2 was not detected in cam1–cam4. The expression level of CmCCD4a-2 in cam5 and cam6 was 0.5–1% of that in the WT. Expression of CmCCD4a-3 was extremely low in ray petals of both the WT and the cam mutants (data not shown).
Analyses of ‘Marble’ cultivars
We analyzed ‘Marble’ cultivars, another type of petal color mutants, which were obtained from bud sports all tracing back to PM. The ‘Marble’ cultivars display a variety of ray petal colors, including white, yellow, orange, and purple, derived from combinations of anthocyanins and carotenoids (Figs. 3a, Supplementary Fig. 2b). The patterns and levels of carotenoids among ‘Marble’ cultivars fell into one of three categories: PM, WM, and BM, with no yellow in the petals; AM, PoM, and CM, which had yellowish L2 layers (Shibata and Kawata 1986) and contained approximately 50 μg/g FW carotenoids; and BrM, FM, and OM, which had yellowish L1 and L2 layers and contained 100–150 μg/g FW carotenoids (Fig. 3b). The purplish petal colors of BM, CM, and OM were due to high levels of anthocyanins (Supplementary Fig. 2).
Ray petal colors and carotenoid contents of ‘Marble’ cultivars. a Flowers of ‘Marble’ cultivars. Color variants are arranged horizontally from white to yellow and vertically from white to purple. b Carotenoid contents in ray petals. Error bar ±SEM (n = 3). c Cross-sections of ray petals of WM, PoM, and FM. Illustrations show carotenoid accumulation patterns in L1 and L2 of ray petals. AM ‘Apricot Marble’; BM ‘Blue Marble’; BrM ‘Bronze Marble’; CM ‘Coral Marble’; FM ‘Florida Marble’; OM ‘Orange Marble’; PM ‘Pink Marble’; PoM ‘Polished Marble’; WM ‘White Marble’
In ray petals of PM, mainly CmCCD4a-1 and CmCCD4a-2 were expressed, and expression of CmCCD4a-3 was not detected (Table 2). To evaluate whether CmCCD4a-1 and CmCCD4a-2 were involved in the variation in carotenoid content of ‘Marble’ cultivars, we performed genomic PCR and RT-qPCR analyses. The genomic PCR analysis showed that CmCCD4a-1 was present in the genomes of PM and AM (Fig. 4a). We could not detect it in WM, BM, PoM, CM, BrM, FM, or OM, indicating that these cultivars did not contain an intact copy of CmCCD4a-1. RT-qPCR showed that only PM expressed a high level of CmCCD4a-1 among these ‘Marble’ cultivars (Fig. 4b). The expression level of CmCCD4a-1 in AM was detectable but extremely low, and the levels in WM, BM, PoM, CM, BrM, FM, and OM were below the level of detection (Fig. 4b). The genomic PCR analysis showed that the gene encoding CmCCD4a-2 existed in the genomes of all of the ‘Marble’ cultivars we examined (Fig. 4c). The RT-qPCR analysis showed that CmCCD4a-2 was expressed in PM, WM, BM, AM, PoM, and CM but was not detected in BrM, FM, or OM (Fig. 4d). Expression levels of CmCCD4a-2 in ray petals of BM and AM were higher than that in ray petals of PM (Fig. 4d).
Genomic PCR and RT-qPCR analyses of CmCCD4a in ‘Marble’ cultivars. a Genomic PCR with gene-specific primers for CmCCD4a-1. Genomic DNAs of ‘Marble’ cultivars were used as templates. An actin primer set was used as a PCR control. b Relative CmCCD4a-1 expression level in ray petals. Error bars: ±SEM (n = 6). c Genomic PCR with gene-specific primers for CmCCD4a-2 (Pro4a-2-F and Pro4a-2-R; Table 1). Genomic DNAs of ‘Marble’ cultivars were used as templates. d Relative CmCCD4a-2 expression levels in ray petals. Error bars ±SEM (n = 6). Abbreviations of cultivars are defined as for Fig. 3
Discussion
We previously isolated a gene encoding CmCCD4a that was specifically expressed in white ray petals of chrysanthemum. Suppression of CmCCD4a expression by RNAi in white-flowered chrysanthemums produced yellow-flowered transformants (Ohmiya et al. 2006, 2009). In addition, experiments involving crosses between white- and yellow-flowered chrysanthemums showed that the presence of CmCCD4a led to the development of white ray petals (Yoshioka et al. 2010). From these results we hypothesized that CmCCD4a is involved in the regulation of carotenoid formation or accumulation in ray petals by cleaving carotenoids into colorless apocarotenoids. Here, we tested this hypothesis by analyzing the relationships between CmCCD4a expression and carotenoid content in petal color mutants of chrysanthemum.
Analyses of RT-PCR–mediated cloning followed by sequencing showed that CmCCD4a is actually a small gene family in the chrysanthemum genome. Four CmCCD4a homologs were identified in the clones obtained from ‘Paragon’; among these, CmCCD4a-1 and CmCCD4a-2 were the most highly expressed in ray petals. On the other hand, the laboratory line ‘94-765’ lacks an intact copy of CmCCD4a-1 in the genome, and CmCCD4a-2 was the most highly expressed in the ray petals. Using the petal color mutants cam1–cam6, which showed higher levels of carotenoid than their progenitor line ‘94-765’ (WT), we analyzed the relationship between the level of CmCCD4a-2 expression and the carotenoid content in ray petals. A remarkable decrease in CmCCD4a-2 expression occurred in ray petals of all six cam mutants (Fig. 2c), with a corresponding increased in carotenoid content (Fig. 1b). We found two different causes for the carotenoid accumulation in ray petals of cam mutants. In cam2, cam3, and cam4, CmCCD4a-2 was lost from the genome or was no longer intact (Fig. 2b). In cam1, cam5, and cam6, CmCCD4a-2 could be detected (Fig. 2b) but its expression was suppressed (Fig. 2c). Genomic sequences of CmCCD4a-2 in cam1, cam5, and cam6 were identical to that of the WT (data not shown). Therefore, this decrease in expression was not caused by any sequence changes such as nucleotide substitutions, insertions, or deletions, but may have been caused by the loss of a function that normally promotes or enhances transcription of CmCCD4a-2.
As carotenoids accumulated in ray petals of both cam5 and cam6, from 1.3 up to 1.8 times the level in the WT (Fig. 1b) even though the CmCCD4a-2 expression level in the mutants was 1/100 or less (Fig. 2c), one explanation is that excess CmCCD4a is expressed in ray petals of the WT, and the levels in cam mutants, even though greatly reduced, are still high enough to cleave carotenoids. Another possibility is that the amounts of carotenoids synthesized in ray petals of both the WT and the cam mutants are lower than in other chrysanthemum cultivars (Kishimoto et al. 2004, 2007). A similar case was observed in ‘Sei-Marine’: even though CmCCD4a expression was reduced to 2% of wild type levels, only a small amount of carotenoid accumulated in ray petals (Ohmiya et al. 2006).
Chrysanthemum ray petals are composed of two cell layers, superficial (L1) and internal (L2) (Stewart and Derman 1970). Each layer is derived from a different somatic cell, whose genome could mutate independently. Because adventitious shoots develop from a single cell (Broertjes et al. 1976), the cam mutants obtained by somaclonal variation through tissue culture are not usually chimeras: any petal color mutation in the progenitor cell would be present in both L1 and L2. On the other hand, petal color mutants derived from bud sports, including ‘Marble’ cultivars, are often periclinal chimeras in which the color of each cell layer of ray petals may change independently (Bush et al. 1976; Boase et al. 1997; Shibata and Kawata 1986). In addition, PM, the original ‘Marble’ cultivar, contained intact copies of both CmCCD4a-1 and CmCCD4a-2. The mechanism of petal color variation due to carotenoid accumulation in ‘Marble’ cultivars is, therefore, more complicated than that in the cam mutants derived from ‘94-765’.
Shibata and Kawata (1986) reported the carotenoid accumulation patterns in ray petals of ‘Marble’ cultivars, which we have illustrated in Fig. 5. In WM, PM, and BM, carotenoids do not accumulate in either L1 or L2. In FM, BrM, and OM, in contrast, they accumulate in both layers. In PoM, AM, and CM, they accumulate only in L2. OM was isolated as a bud sport from BrM (Shibata and Kawata 1986), but the breeding processes leading to the other ‘Marble’ cultivars are unknown. We hypothesize that petal color variation is caused by the loss of enzymatic activity of CmCCD4a in L2 of PoM, AM, and CM and in both L1 and L2 of FM, BrM, and OM. On the basis of the RT-qPCR analysis using primers specific to either CmCCD4a-1 or CmCCD4a-2, we hypothesize that CmCCD4a-1 and CmCCD4a-2 are expressed in the histogenic layers of ray petals of ‘Marble’ cultivars as illustrated in Fig. 5. Because CmCCD4a-1 expression cannot be detected in WM, BM, PoM, and CM (Fig. 4b), we hypothesize that CmCCD4a-2 is expressed in the layers where carotenoids did not accumulate, i.e., in both L1 and L2 of WM and BM and in L1 of PoM and CM. Because both CmCCD4a-2 and CmCCD4a-1 were expressed in AM (Fig. 4b, d), and L2 contains carotenoid, we assume that the two CmCCD4a genes are expressed in L1. Considering the fact that PM is the original cultivar of AM and has higher expression of CmCCD4a-1 than AM, we hypothesize that both CmCCD4a-1 and CmCCD4a-2 are expressed in L1 and possibly in L2 of PM.
Schematic diagrams of the chimeric pigment distribution in ray petals of ‘Marble’ cultivars, arranged as in Fig. 3a. Layer L1 covers layer L2. Carotenoid accumulation patterns (indicated by yellow) are based on the results described by Shibata and Kawata (1986). Expression patterns of CmCCD4a-1 and CmCCD4a-2 (indicated by “4a-1” and “4a-2”) are based on the results in this study. Anthocyanins (indicated by red stripes) accumulated only in L1 (data not shown). Abbreviations of cultivars are defined as for Fig. 3
Although the typical chromosome number of chrysanthemum is 54, the actual number varies widely with cultivar, ranging from 36 to 85 (Endo 1969; Shibata et al. 1998). In addition, some cultivars have odd numbers such as 53 or 55. This variety of karyotypes suggests that chrysanthemum is able to tolerate large-scale changes in genome composition. Dowrick and Bayoumi (1966) showed that changes in chromosome number and chromosome fragmentation are sometimes responsible for changes in petal color of chrysanthemum. Here, we show that some petal color mutants of chrysanthemum lost the activity (and sometimes the actual copy) of one or more CmCCD4a genes, which resulted in carotenoid accumulation. A stepwise decrease of CmCCD4a-1 and CmCCD4a-2 activity among ‘Marble’ cultivars appears to be correlated with a corresponding stepwise increase in carotenoid levels in ray petals. It is possible that there are several copies of CmCCD4a-1 and CmCCD4a-2 in the genome of PM. The phenotypes of the ‘Marble’ cultivars (Fig. 5) indicate that fewer steps are needed to eliminate CmCCD4a activity in L2 than in L1, suggesting that fewer copies (or fewer active copies) of CmCCD4a exist in L2 than in L1. Independent mutation in each cell layer may also cause stepwise decreases in the amount of CmCCD4a.
There are some examples in which copies of CmCCD4a-1, CmCCD4a-2, or both were present in the genome but were not expressed in ray petals: for example, CmCCD4a-2 in BrM, FM, OM, and cam1. On the other hand, expression of CmCCD4a-2 was higher in BM and AM than in the progenitor (PM). An increase in expression of a gene encoding CCD4 was also reported in a fruit-color mutant of peach that showed white flesh (Brandi et al. 2011). These examples might be caused by the loss of functions that enhance or suppress transcription of CCD4. However, a transcriptional regulator that controls CmCCD4a expression has not been discovered yet. Further study will be needed to explain these expression patterns.
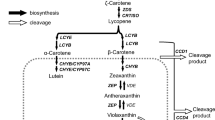
The enzymatic activities of CCDs are still a matter of debate. Floss et al. (2008) reported different substrate specificities of CCD1 between in vitro and in planta analyses. In vivo analysis using carotenoid-accumulating Escherichia coli cells showed that recombinant CmCCD4a (identical to CmCCD4a-1 in this study) specifically cleaves double bonds at the 9,10 (9′,10′) positions of β-carotene and zeaxanthin (Huang et al. 2009). It does not, however, cleave xanthophylls, including zeaxanthin and lutein, in vitro, but it does cleave the double bond at the 9,10 position of 8′-apo-β-caroten-8′-al. However, in planta activity of CCD4 has not yet been elucidated. The carotenoids in ray petals of cam2 (which lost CmCCD4a-2) consist mainly of lutein derivatives (Supplementary Fig. 3), as in other chrysanthemum cultivars previously reported (Kishimoto et al. 2004). The results suggest that CmCCD4a-2 cleaves lutein derivatives as substrates in planta.
Conclusion
We show that loss of CmCCD4a(s) or transcriptional regulator(s) occurred during the process of petal color mutation in chrysanthemum. These events resulted in decreased CmCCD4a expression and increased carotenoid levels in ray petals, indicating that members of the CmCCD4a family play a critical role in regulating carotenoid accumulation. We thereby propose that CmCCD4a genes represent the single dominant gene that inhibits carotenoid accumulation predicted in earlier studies (Stewart and Derman 1970; Jordan and Reinmann-Philipp 1983; Hattori 1991; Boase et al. 1997).
References
Boase MR, Miller R, Deroles SC (1997) Chrysanthemum systematics, genetics, and breeding. In: Janick J (ed) Plant Breeding Reviews, vol 14. Wiley, Hoboken, pp 321–361
Brandi F, Bar E, Mourgues F, Horváth G, Turcsi E, Giuliano G, Liverani A, Tartarini S, Lewinsohn E, Rosati C (2011) Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol 11:24
Britton G (1995) UV/visible spectrometry. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids, vol IB. Birkhauser Verlag, Basel, pp 13–62
Broertjes C, Roest S, Bokelmann GS (1976) Mutation breeding of Chrysanthemum morifolium using in vivo and in vitro adventitious bud techniques. Euphytica 25:11–19
Bush SR, Earle ED, Langhans RW (1976) Plantlets from petal segments, petal epidermis, and shoot tips of the periclinal chimera Chrysanthemum morifolium ‘Indianapolis’. Am J Bot 63:729–737
Dowrick GJ, Bayoumi A (1966) The origin of new form of the garden chrysanthemum. Euphytica 15:32–38
Endo N (1969) The chromosome survey on the cultivated chrysanthemums, Chrysanthemum morifolium Ramat.; II. On the chromosome numbers of cultivated chrysanthemums. J Jpn Soc Hort Sci 38:343–349
Floss DS, Schliemann W, Schmidt J, Strack D, Walter MH (2008) RNA interference-mediated repression of MtCCD1 in mycorrhizal Roots of Medicago truncatula causes accumulation of C27 apocarotenoids, shedding light on the functional role of CCD1. Plant Physiol 148:1267–1282
Hattori K (1991) Inheritance of carotenoid pigmentation in flower color of chrysanthemum. Jpn J Breed 41:1–9
Huang FC, Molnár P, Schwab W (2009) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bot 60:3011–3022
Jordan C, Reinmann-Philipp R (1983) Investigations on type and degree of polyploidy in Chrysanthemum morifolium Ramat. by genetical analysis of two flower color factors. Z Pflanzenzucht 91:111–122
Kawase K, Tsukamoto Y (1976) Studies on flower color in Chrysanthemum morifolium Ramat III: quantitative effects of major pigments on flower color variation, and measurement of color qualities of petals with a color difference meter. J Jpn Soc Hort Sci 45:65–75
Kishimoto S, Ohmiya A (2006) Regulation of carotenoid biosynthesis in petals and leaves of chrysanthemum (Chrysanthemum morifolium Ramat.). Physiol Plant 128:437–447
Kishimoto S, Maoka T, Nakayama M, Ohmiya A (2004) Carotenoid composition in petals of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura). Phytochemistry 65:2781–2787
Kishimoto S, Sumitomo K, Yagi M, Nakayama M, Ohmiya A (2007) Three routes to orange petal color via carotenoid components in 9 Compositae species. J Jpn Soc Hort Sci 76:250–257
Liu Y-G, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463
Machin B, Scope N (1978) Mutation breeding. In: Chrysanthemum, year-round growing. Blandford Press, Dorset, pp 34–37
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nakayama M, Koshioka M, Shibata M, Hiradate S, Sugie H, Yamaguchi MA (1997) Identification of cyanidin 3-O-(3,6-O-dimalonyl-β-glucopyranoside) as a flower pigment of chrysanthemum (Dendranthema grandiflorum). Biosci Biotechnol Biochem 61:1607–1608
Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol 142:1193–1201
Ohmiya A, Sumitomo K, Aida R (2009) “Yellow Jimba” suppression of carotenoid cleavage dioxygenase (CmCCD4a) expression turns white chrysanthemum petals yellow. J Jpn Soc Hort Sci 78:450–455
Shibata M, Kawata J (1986) Chromosomal variation of recent chrysanthemum cultivars for cut flower. In: Kitaura K, Akihama T, Kukimura H, Nakajima K, Horie M, Kozaki I (eds) Development of new technology for identification and classification of tree crops and ornamentals. Fruit Tree Research Station Ministry of Agriculture, Forestry and Fisheries, Japan, pp 41–45
Shibata M, Kishimoto S, Hirai M, Aida R, Ikeda I (1998) Analysis of the periclinal chimeric structure of chrysanthemum sport by random amplified polymorphic DNA. Acta Hortic 454:347–353
Stewart RN, Derman H (1970) Somatic genetic analysis of the apical layers of chimeral sports in chrysanthemum by experimental production of adventitious shoots. Am J Bot 57:1061–1071
Wasscher J (1956) The importance of sports in some florist’s flowers. Euphytica 5:163–170
Yoder Bros Inc. (1967) Evolution of the Indianapolis family. Grow Circ Newsl 51. Yoder Bros, Inc., Barberton, pp 1–4
Yoshioka S, Sumitomo K, Fujita Y, Yamagata A, Onozaki T, Shibata M, Ohmiya A (2010) Significance of CmCCD4a orthologs in apetalous wild chrysanthemum species, responsible for white coloration of ray petals. Euphytica 171:295–300
Acknowledgments
We thank S. Kishimoto and N. Noda for their helpful discussions and K. Sumitomo for plant propagation. This work was supported by grants from the National Agriculture and Food Research Organization (NARO) and the NIAS Genebank Project of the National Institute of Agrobiological Sciences, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Accession numbers The genomic sequences are available in the GenBank nucleotide database under the accession numbers AB627797 (CmCCD4a-1), AB627798 (CmCCD4a-2), AB627800 (CmCCD4a-3), and AB627802 (CmCCD4a-4). The cDNA sequences are available under the accession numbers AB247158 (CmCCD4a-1), AB627799 (CmCCD4a-2), AB627801 (CmCCD4a-3), and AB627803 (CmCCD4a-4).
Electronic supplementary material
Below is the link to the electronic supplementary material.
10681_2011_602_MOESM1_ESM.doc
Supplementary Fig. 1 Genomic sequence comparison of CmCCD4a homologs. Identical nucleotides are indicated with black background. CmCCD4a-2, -3, and -4 showed 95.5, 95.5, and 97.1% similarity to CmCCD4a-1, respectively (DOC 81 kb)
10681_2011_602_MOESM2_ESM.ppt
Supplementary Fig. 2 Anthocyanin contents in ray petals of (a) WT, cam1–cam6 and (b) ‘Marble’ cultivars. Supplementary Fig. 3 HPLC chromatograms of carotenoid extracts from ray petals of WT and cam2 (PPT 275 kb)
Rights and permissions
About this article
Cite this article
Yoshioka, S., Aida, R., Yamamizo, C. et al. The carotenoid cleavage dioxygenase 4 (CmCCD4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica 184, 377–387 (2012). https://doi.org/10.1007/s10681-011-0602-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-011-0602-z