Abstract
Drought and low soil fertility are considered the most important abiotic stresses limiting maize production in sub-Saharan Africa. Knowledge of the combining ability and diversity of inbred lines with tolerance to the two stresses and for those used as testers would be beneficial in setting breeding strategies for stress and nonstress environments. We used 15 tropical maize inbred lines to (i) evaluate the combining ability for grain yield (GY), (ii) assess the genetic diversity of this set of inbred lines using RFLP, SSR, and AFLP markers, (iii) estimate heterosis and assess the relationship between F1 hybrid performance, genetic diversity and heterosis, and (iv) assess genotype × environment interaction of inbred lines and their hybrids. The F1 diallel hybrids and parental inbreds were evaluated under drought stress, low N stress, and well-watered conditions at six locations in three countries. General combining ability (GCA) effects were highly significant (P < 0.01) for GY across stresses and well-watered environments. Inbred lines CML258, CML339, CML341, and CML343 had the best GCA effects for GY across environments. Additive genetic effects were more important for GY under drought stress and well-watered conditions but not under low N stress, suggesting different gene action in control of GY. Clustering based on genetic distance (GD) calculated using combined marker data grouped lines according to pedigree. Positive correlation was found between midparent heterosis (MPH) and specific combining ability (SCA), GD and GY. Hybrid breeding program targeting stress environments would benefit from the accumulation of favorable alleles for drought tolerance in both parental lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for maize in developing countries is expected to be about 504 million tons by 2020 and this is expected to exceed the demand for both wheat and rice (IFPRI 2000). To meet this demand, there is need for increased maize production in the developing countries while maintaining the same land resources since population growth and environmental conditions limit opportunities for increasing maize area (Pingali and Pandey 2001). The need to increase maize production in developing countries is hindered by a number of constraints including both abiotic and biotic stresses. Among the major abiotic stresses limiting tropical maize production are drought and low soil fertility. Most tropical maize is produced under rain-fed conditions, in areas where drought is considered to be the most important abiotic constraint to production (CIMMYT 1999). Drought at any stage of crop development affects production, but maximum damage is inflicted when it occurs during flowering. Effects of drought stress include delayed silking and female sterility caused by abortion (Moss and Downey 1971), reduction in amount of pollen and number of kernels per plant (Hall et al. 1982), resulting into a large reduction in grain yield (GY). It was estimated that annual yield losses due to drought may approach 24 million tons, equivalent to 17% of a normal year’s production in the developing world (Edmeades et al. 1992). The incidence of stress may increase, partly due to global climate changes, displacement of maize to marginal environments by high value crops, and partly to declines in soil organic matter, reduced soil fertility and water holding capacity (Bänziger et al. 2000). Tropical soils vary greatly giving rise to differences in moisture and nitrogen at a single site within a single year (Beck et al. 1996). Nitrogen deficiency is common where nitrogen is applied at below-optimal levels because of high cost relative to economic returns, or where there are significant risks of drought and frost or of excessive leaching of nitrate (Lafitte and Edmeades 1994). Intensified land use and the rapid decline in fallow periods, coupled with the extension of agriculture into marginal lands, have contributed to a rapid decline in soil fertility, particularly in sub-Saharan Africa (Bellon 2001). In the tropics, drought and low soil fertility frequently occur in association (Bänziger et al. 1997). Maize productivity in maize-based cropping systems could be greatly improved by developing cultivars that tolerate drought stress and those that are able to utilize nitrogen more efficiently.
Using recurrent selection procedures and managed drought stress, improvement of tropical maize for drought tolerance has been achieved (Bolaños and Edmeades 1993a; Edmeades et al. 1997). Selection under managed stress was carried out for GY and secondary traits that are thought to increase adaptation to drought (Fischer et al. 1989; Bolaños and Edmeades 1993a, b). Selection under drought conditions resulted into increased yield as a result of gains in number of ears per plant, increase in kernel number, reduction in time to 50% anthesis (Bolaños and Edmeades 1993a, b), reduction in anthesis silking interval (Fischer et al. 1989; Bolaños and Edmeades 1993b) and a delay in leaf senescence (Bolaños and Edmeades 1993b). Byrne et al. (1995) reported a gain of 1.68% per cycle for GY in Tuxpeño Sequía population after eight cycles of full-sib selection. Edmeades et al. (1999) reported gains in GY, biomass, and harvest index in three maize populations that had undergone recurrent selection for drought tolerance.
Information about the genetic diversity of lines and populations is important in hybrid development programs. Maize breeders are interested in identifying inbred lines that would produce crosses exhibiting optimal levels of heterosis without making all possible crosses among the potential parents. The positive relationship between midparent heterosis (MPH) of F1 GY and parental genetic divergence was highlighted by Moll et al. (1965) and Paterniani and Lonnquist (1963). The relationship between genetic distance (GD) and hybrid performance has been investigated in maize using molecular markers (Lee et al. 1989; Godshalk et al. 1990; Smith et al. 1990; Betrán et al. 2003a). In cases where heterotic groups are not well-established, marker-based GD estimates can be used to avoid producing and testing crosses between related lines and crosses with inferior MPH could be discarded based on prediction (Melchinger 1999).
Through different breeding programs, the International Maize and Wheat Improvement Center (CIMMYT) has developed inbred lines and populations tolerant to drought and low nitrogen stress (Edmeades et al. 1992; Bänziger and Lafitte 1997; Bänziger et al. 1997, 2000; Beck et al. 1996). With several regional breeding programs at CIMMYT, it is important to know the relationship between elite lines from different programs used as testers to produce experimental hybrids, and to gain an understanding of how this facilitates flow of materials and strategies for hybrid production. Furthermore, the germplasm available as inbred lines can be used to develop either single- or three-way cross hybrids. Information on the combining ability of this germplasm would be very beneficial to the breeders in defining a breeding strategy. In addition to inbred lines with some degree of stress tolerance, other elite inbreds have been developed in breeding programs with evaluation under optimal conditions in target environments. Several of these inbred lines, which show good combining ability and yield potential, are used as testers to differentiate heterotic response of experimental lines. The objectives of this work were to (i) estimate the general and specific combining abilities for GY among tropical and sub-tropical inbred lines used as testers in different breeding programs; (ii) assess the genetic diversity of this set of inbred lines using molecular markers; (iii) estimate heterosis and assess the relationship between F1 hybrid performance, genetic diversity and heterosis; and (iv) assess genotype × environment interaction across stress conditions and testing locations and estimate stability of inbred lines and their hybrids.
Materials and methods
Germplasm, environments and stress management
Fifteen inbred lines of tropical origin with a range of response to abiotic stresses were used in this study (Table 1). The inbred lines were from the sub-tropical, tropical and stress breeding programs at CIMMYT México and from the mid-altitude maize breeding program at CIMMYT Zimbabwe. These inbred lines have been used as testers by the different maize programs to evaluate new experimental lines and classify them in potential heterotic groups. Diallel crosses were made among the fifteen inbred lines in 1996–1997 at CIMMYT México. Seeds from each reciprocal cross were bulked to form a set of 105 F1 hybrids. The F1 hybrids and 5 experimental single cross hybrids were evaluated at two locations each in México, USA, and Zimbabwe (Table 2). Two trials were conducted each under managed drought stress and low N stress, while four trials were conducted under well-watered and optimal fertilization conditions. Trials under managed drought stress were conducted during the dry (winter) season which is rain free, thus allowing the control of drought stress by withdrawing irrigation water during flowering. Water stress was achieved by withholding water from 2 weeks before silking to the end of the flowering period. Low nitrogen stress conditions were achieved at the two sites by continuous cropping of maize without N fertilizer application, and removal of all stover biomass after harvest. In the well-watered experiment, irrigation water was supplied to avoid moisture stress. There were four well-watered, two managed drought stress, and two low N stress environments (Table 2). The 15 parental inbred lines were also evaluated under well-watered, drought stress, and low N stress at Harare and Chiredzi (Zimbabwe), and Poza Rica (Mexico) in separate experiments adjacent to the hybrid trials. Standard cultural and agronomic practices were followed as recommended for each location.
Experimental design and field measurements
The experiments were planted in 1999 at all locations. All experiments were planted in an alpha-(0, 1) lattice design (Patterson and Williams 1976) with two replicates for the hybrids and three replicates for the parental inbred lines. Incomplete blocks were comprised of ten plots for the hybrids and five plots for the parental inbred lines. Hybrids and lines were oversown with two seeds per hill in single rows of different sizes depending on the location (Table 2) and were later thinned to the desired plant densities. At harvest, plants at each end of row were not included when measuring field weight. Measurements on plot basis were recorded for grain weight. Grain moisture (g kg−1 moisture) of grain at harvest was measured either by combine mounted equipment or measured using a moisture meter. Grain weight per plot was adjusted to 12.5% grain moisture and used to calculate grain yield (expressed in Mg ha−1).
DNA finger printing
Maize genomic DNA was extracted from the 15 inbred lines according to CIMMYT’s Applied Biotechnology Center Manual of Laboratory Protocols (CIMMYT 2001). For RFLP analyses, DNA was purified, quantified, digested with the restriction enzyme (EcoRI), separated in agarose gels (0.7%, w/v) and transferred to nylon membranes by Southern blotting. Labeled probes (digoxigenin-dUTP) were used to detect polymorphism with antidigoxigenin-alkaline phosphatase-AMPPD chemiluminescent reaction. A set of 80 restriction fragment length polymorphism probes spread across the genome were used to screen the plant material. RFLP patterns were binary coded by 1 for presence or 0 for absence of bands in each inbred line.
AFLP marker analyses were performed as described by Vos et al. (1995). Genomic DNA of the maize inbred lines was digested with enzymes EcoRI and MseI in a buffer. Double-stranded adapter sequences were ligated to the restricted DNA fragment ends. Six primer combinations used for amplification were ACA-CAT, ACA-CAC, ACA-CAG, ACA-CGA, ACA-CGG, and ACA-CGT. The polymerase chain reaction (PCR) products were separated by electrophoresis on a denaturing polyacrylamide gel. After drying, the gels were exposed to phosphor-imager plates for 16 h. The imager plates were scanned with a phosphor-imager and polymorphic bands were binary scored by 1 or 0 for presence or absence in each inbred line respectively.
For SSR analyses, the procedure and PCR conditions described in detail by Warburton et al. (2002) were followed. Thirty-two SSR markers were chosen from the MaizeDB database to genotype the 15 maize inbred lines. Fragments were separated using acrylamide gels run on an ABI377 automatic DNA sequencer. Fragment sizes were calculated with GeneScan 3.1 (Perkin Elmer/Applied Biosystems) using the Local Southern sizing method. Allele identity was assigned using Genotyper 2.1 (Perkin Elmer/Applied Biosystems). Simple sequence repeat bands were binary coded by 1 or 0 for their presence or absence in each inbred line, respectively.
Statistical analyses
Analysis of variance for each environment was conducted with the PROC MIXED procedure of SAS (SAS Institute 2001). Genotypes were considered fixed effects while replications, blocks within replications and environments were considered random effects. Combined analyses of variance across stress and optimal environments were computed using PROC GLM in SAS (SAS Institute 2001). For diallel analysis, the experimental single cross hybrids were not included. The sums of squares for hybrids and hybrids × environments were partitioned into sources of variation due to general combining ability (GCA) and specific combining ability (SCA) and their interaction with environments (GCA × environment and SCA × environment), respectively, following Griffing’s Method 4 Model I of diallel analysis (Griffing 1956) using the DIALLEL-SAS program (Zhang and Kang 1997). The significance of GCA and SCA sources of variation was determined using the corresponding interaction with the environments as the error term. The significance of GCA × environment and SCA × environment interactions was determined using the pooled error. Adjusted means from PROC MIXED procedure were used to estimate GCA effects of the parents and SCA effects for the crosses using the DIALLEL-SAS program. The predicted performance of hybrids based on GCA was calculated by adding the GCA of both parents to the overall mean GY of the diallel hybrids. GCA and SCA equivalent variance components of mean squares were calculated by a fixed model for the diallel design. The relative importance of GCA and SCA was estimated as the ratio
where \( \hat{\sigma }^{2}_{\text{GCA}} \) and \( \hat{\sigma }^{2}_{\text{SCA}} \) are the variance components for GCA and SCA, respectively (Baker 1978). MPH and highparent heterosis (HPH) were calculated using the adjusted means of the hybrids and inbred lines. MPH was calculated as
where F1 is the mean of the F1 hybrid performance and MP = (P1 + P2)/2 where P1 and P2 are the means of the two inbred parents. HPH was calculated as
where HP is mean of the best parent. Simple linear regression was computed to determine the relationship between GY, SCA, and MPH.
Additive Main Effects and Multiplicative Interaction (AMMI) analysis of GY of inbred lines in hybrid combination and inbred lines per se was carried out to assess the relationship among inbreds and environments. A biplot (Gabriel 1971) of the first two principal components was used to visualize the relationships among inbreds and environments. Stability analysis of hybrids and parental inbreds across locations and stresses was conducted using the joint linear regression method (Eberhart and Russell 1966). AMMI analysis, singular value decomposition and stability analysis were carried out using IRRISTAT (IRRI 1998) and Biplot v1.1 (Lipkovich and Smith 2002).
Polymorphism information content (PIC) for the SSR and RFLP markers was calculated as
where p i is the frequency of the ith allele in a locus for individual p (Smith et al. 1997). GD between a pair of lines based on marker data was estimated according to the method of Nei and Li (1979) as
where N ij is the number of bands common to lines i and j, and N i and N j are the total number of bands for lines i and j, respectively. Cluster analysis of GDs using the Unweighted Pair-Group Method using Arithmetic Averages (UPGMA) was used to identify relationships among the inbred lines using GD estimates between all pairs of inbred lines calculated from the combined genotypic data across all markers (i.e., AFLP, RFLP, and SSR). GD and cluster analysis were carried out using NTSYSpc software (Rohlf 1997). GD between pairs of inbred lines was correlated with F1 hybrid GY, SCA, MPH and HPH.
Results and discussion
Performance of hybrids and inbred lines per se
Analysis of variance indicated significant differences among the F1 diallel hybrids at well-watered environments TLWW, ZBWW, and CSWW, at both drought stress environments (TLSS and ZBSS) and at one of the low N stress environments for grain yield. Highly significant (P < 0.01) environment effect across stresses was detected (Table 3), possibly a result of the varying stresses imposed on the trials. No significant differences were observed among hybrids across low N, drought stress and well-watered environments but highly significant differences were detected across environments (Table 3). Hybrid × environment interaction was highly significant (P < 0.01) across low N stress, well-watered, and environments. The best hybrids were P501 × CML247 (3.85 Mg ha−1) and CML254 × CML343 (2.68 Mg ha−1) across low N stress, CML258 × CML343 (4.53 Mg ha−1) and CML321 × CML311 (4.33 Mg ha−1) across drought stress and, CML216 × CML341 (5.35 Mg ha−1) and CML78 × SPLC-F (5.34 Mg ha−1) across well-watered environments. Across environments, the best yielding hybrids were CML258 × CML343 (4.03 Mg ha−1) and CML78 × CML339 (4.01 Mg ha−1). Hybrid CML258 × CML343 which performed well under drought stress was among the top four hybrids under managed low N stress. This hybrid is likely to be efficient at utilizing fertilizer and water (Castleberry et al. 1984). The two inbred lines that make this hybrid could be having favorable alleles for GY under drought and low N stress.
Across low N stress the best hybrid (P501 × CML247) was between an inbred line from the sub-tropical breeding program and one from the tropical breeding programs while the best hybrid across drought stress (CML258 × CML343) was between an inbred from the stress breeding program and one from tropical maize program. This suggests that flow and combination of germplasm between different breeding programs can lead to identification of outstanding hybrids. Betrán et al. (2003b) obtained results that showed lines with different selection history produced high yielding hybrids under contrasting environments, indicating favorable allele combinations between conventionally selected inbred lines and inbred lines selected for drought tolerance. In this study, some hybrids performed well across stress levels indicating that it is possible to combine stress tolerance and yield potential in tropical maize hybrids. Some of the inbred lines that were parents of the best hybrids under drought stress and low N stress are drought tolerant and this is possibly a spill-over effect of selection for drought tolerance (Bänziger et al. 1999). Selection for tolerance to midseason drought increased GY under different nitrogen stress levels by 86 kg−1 ha−1 year−1 (Bänziger et al. 1999). Decreased ear abortion and increased assimilate supply during grain filling of maize selected for tolerance to mid-season drought also provide tolerance to N stress and therefore may contribute to increased yield (Edmeades et al. 1997; Bänziger et al. 2002).
Stress at different locations resulted in varying GY reduction. At Tlaltizapán, mean hybrid yield of the severe stress trial was 58% of the well-watered trial. This yield reduction is lower than that reported by Betrán et al. (1997, 2003a) at the same location for severe stress and well-watered conditions but close to the GY reduction reported for intermediate stress and well-watered conditions (Betrán et al. 2003a). Differences in germplasm used in the two studies could account for differing results. Nitrogen stress reduced GY by 51% at Harare among the hybrids and by 65% among the inbred lines. GY reduction of 38% among S2 lines and 68% among topcrosses (Lafitte and Edmeades 1995) and 20–50% among populations (Bänziger et al. 1999) relative to high N environment was reported in studies in Mexico. The low N yield reduction for inbred lines obtained in this study is similar to that reported by Betrán et al. (2003a) for inbred lines at Poza Rica. Due to heterosis, yield reduction among topcrossses and hybrids is smaller compared to that observed among inbred lines when grown under stress. Combined over environments, analysis of variance showed significant differences among inbreds and significant inbred × environment interaction. Mean GY of the inbred lines was 0.7 Mg ha−1 at ZBLN, 1.0 Mg ha−1 at ZBDR, 2.0 Mg ha−1 at ZBWW and 0.4 Mg ha−1 at PRLN. Average GY of inbred lines across environments was 0.7 Mg ha−1 (range 0.59–1.43 Mg ha−1).
Combining ability analysis
GCA was highly significant (P < 0.01) across low N, drought stressed, and well-watered environments (Table 3), indicating that there were differences in the performance of the inbred lines as parents in hybrid combinations. The GCA × environment (GCA × E) interaction was highly significant (P < 0.01) for GY across drought stress, well-watered, and environments. Significant GCA × environment effect for GY indicates that GCA effects associated with parents were not consistent over environments. Therefore, using different parents at the different locations for hybrid development and selection would be more effective when based on performance across environments. The SCA × environment interaction was highly significant (P < 0.01) for GY across low N stress and across environments (Table 3). GCA mean squares were 3.8, 4.2, 4.0 and 4.8 times larger than SCA mean squares across low N, drought, well-watered and environments. This suggests that additive gene action could be more important than non-additive gene action for GY in this set of materials. The importance of additive gene effects for GY has been reported in several studies (Beck et al. 1991; Vasal et al. 1992; Welcker et al. 2005; Malvar et al. 2008). In a study among tropical white endosperm populations, San Vicente et al. (1998) reported greater relative importance of non-additive genetic effects than additive genetic effects for GY. These differences indicate the importance of both additive and non-additive gene action in control of GY. With predominance of GCA over SCA variance, early testing may be more effective and promising hybrids can be identified and selected mainly based on the prediction from GCA effects and the best hybrids could be obtained by crossing the parents with the highest GCA (Baker 1978). In this study the best hybrids across stresses had at least one parent with high significant positive GCA.
GCA effects for GY varied significantly among lines and between environments (Table 4). Inbred line P501 showed significant positive GCA effects for GY at the two low N stress environments and also the highest positive significant GCA effects (0.35 Mg ha−1) across low N environments. Inbred line CML258 had the highest positive significant GCA effects across drought stress environments (0.70 Mg ha−1) and across environments (0.33 Mg ha−1). This inbred line contributed to higher GY of its hybrids under both well-watered and drought stress environments. At each environment and across environments, inbred line CML341 showed consistently positive GCA effects for GY. Inbred lines showing favorable GCA effects for GY are likely to contribute favorable alleles in a recurrent selection program and could be used as parents to form a synthetic population that would be improved for drought and low N tolerance. Inbred lines CML341 and CML343 which had positive GCA effects for GY under managed drought stress and well-watered conditions are from population La Posta Sequía which was improved for drought tolerance through recurrent selection. Lines extracted from this population showed a higher frequency of dominant alleles for GY (Betrán et al. 2003b), which makes them good candidates for recycling of inbred lines. Hybrid CML78 × SPLC-F had the highest SCA effect (0.89 Mg ha−1) for GY across environments (Table 5). SCA effects were negative for crosses between lines with similar background (CML339 × CML341, CML339 × CML343) (Table 5). The best yielding hybrid across environments (CML258 × CML343) had a small SCA effect (0.30 Mg ha−1) but the same hybrid had a higher SCA effect across drought and low N stress environments (0.76 and 0.54 Mg ha−1, respectively).
The relative importance of GCA and SCA variance was examined by expressing it as the ratio of additive to total genetic variance. The closer this ratio is to unity, the greater the predictability based on GCA alone (Baker 1978). The ratio ranged from 51 to 79% for GY under well-watered conditions, and was generally higher under optimal conditions compared to stress environments (Fig. 1). Under drought stress environments TLSS and ZBSS, additive genetic variance accounted for 40 and 64% of the total genetic variance for GY, respectively. Under low N stress environments PRLN and ZBLN, additive variance accounted for 53 and 37% of the total genetic variance for GY, respectively. Additive variance accounted for 42, 67, and 71% of total genetic variance for GY across low N stress, drought stress and well-watered environments, respectively. Additive genetic effects appear to be more important under drought and well-watered conditions, but non-additive genetic effects seem to be more important under low N stress conditions in this set of maize inbred lines. This is consistent with the findings reported by Betrán et al. (2003b). Thus, it is beneficial to have drought tolerance in both parental lines to obtain acceptable hybrid performance under severe drought stress that coincides with flowering (Betrán et al. 2003b).
Inbred-hybrid correlation and prediction of hybrid performance
Inbred line information indicative of hybrid performance is desirable to reduce costs associated with hybrid evaluation. The relationship between inbred line and hybrid performance was investigated by simple linear correlation between inbred line per se and hybrid performance at the same environment. Inbred line GY was significantly correlated with hybrid GY at PRLN (r = 0.55, P < 0.05) and ZBLN (r = 0.53, P < 0.05) (Fig. 2). These results agree with reports by Betrán et al. (1997) but are contrary to observations by Lafitte and Edmeades (1995) who reported no significant correlation between S2 line GY and topcross GY under low N. The correlation between inbred line GY and hybrid GY was not significant at ZBDR and ZBWW. In other studies significant correlations between inbred line and hybrid GY have been reported under drought stress and optimum conditions (Betrán et al. 1997, 2003b). Inbred line traits under severe stress are more strongly correlated with topcross performance under severe drought stress than are line traits under optimal conditions (Betrán et al. 1997) but this was not observed in this study. The degree of inbreeding could cause the low correlation between inbred and hybrid performance under stress as early generation lines can tolerate better severe drought intensity than fully inbred lines (Betrán et al. 2003b). The inbred lines used in this study are fixed and this may partly explain the low correlation observed. A low correlation between line per se and testcross performance may indicate that large amounts of non-additive gene action are affecting testcross performance (Smith 1986). Lack of any definitive correlation between yields of parent inbred lines and their crosses indicates that selection for combining ability in the final analysis must be based on the performance of the lines in crosses rather than on the inbred lines (Hallauer and Miranda 1988). The low correlations observed in this study emphasize the need to evaluate hybrids under stress to identify superior hybrids for stress environments.
Methods that can predict F1 hybrid performance with some accuracy prior to field evaluation are of particular interest to plant breeders. Hybrid GY performance predicted from the sum of the two parental GCAs and overall GY was strongly correlated with observed hybrid performance across stresses and environments (Fig. 3). Similar results were obtained for GY performance of maize under acid soils (Welcker et al. 2005) and for seed yield in oilseed rape (Diers et al. 1996). Of the 20 crosses predicted to have the highest GY based on the sum of parental GCAs and overall mean yield, twelve and ten crosses ranked in the top 20 for hybrid GY across drought stress and low N environments, respectively. Inbred line CML258 which had the highest GCA effects for GY across managed drought stress environments was a parent to ten of the predicted top 20 hybrids under managed drought stress. Under low N stress, inbred line P501 was a parent to eleven of the predicted top 20 hybrids. Inbred line P501 had the highest GCA effects for GY across low N environments. These results suggest that parents producing high yielding F1 hybrids could be selected based on GCA effects of the parents. In practical plant breeding combining abilities have found their principle use in predicting the performance of hybrid populations of outbreeders, often in form of testcrosses or polycrosses (Simmonds 1979). A strong correlation between observed and predicted values indicates that GCA accounts for a large share of the differences between crosses, i.e., a small SCA contribution. In this study, the prediction efficiency ranged from 31 to 46% and this could have been improved by removing the related lines from this set (Charcosset et al. 1998). The additive model used for prediction reported in this study is more efficient at predicting hybrid performance when the parents are unrelated (Burstin et al. 1995; Charcosset et al. 1998).
Heterosis for grain yeild
MPH and HPH were estimated at four environments where the hybrids and inbreds per se were evaluated in adjacent experiments. MPH and HPH for GY were highest in the drought stressed environment (ZBSS) (Table 6). MPH ranged from 74 to 1119 (average 367) in the drought stress environment, with the largest MPH being recorded for cross CML216 × SPLC-F (1119, SCA = 2.15, P < 0.01). At well-watered environment ZBWW, MPH averaged 168 and ranged from 14 to 448. In the low N stress environments, average MPH for GY was 110 for PRLN and 283 for ZBLN. The hybrids showing the highest MPH at low N stress environments were P501 × CML258 (MPH = 892, SCA = 3.46, P < 0.01) and CML216 × CML339 (MPH = 625, SCA = 0.34) at PRLN and ZBLN, respectively. We recorded higher MPH for GY in stressed environments compared to the well-watered environment. These results are consistent with the findings of Betrán et al. (2003a) and Welcker et al. (2005) who reported higher average MPH for GY with increasing intensity of stress. The higher heterosis values under drought stress are due to the poor performance of inbreds under severe drought stress. The observed heterosis in this germplasm could be exploited through development of synthetic varieties. Since synthetics mainly exploit that part of heterosis contributed by GCA, the predominance of GCA variance observed in this study also encourages synthetic variety development. Synthetic varieties are not expected to experience a decrease in heterosis after the F2 generation (Bernardo 2002). Elite inbred lines within the same heterotic group and having good GCA for GY can be used to form a narrow-based synthetic. The improved GCA of the lines, being based on additive variance is retained in the synthetic population. Synthetics can be used as non-inbred parents to form topcross or double-topcross hybrids and can also be used to extract new inbred lines. Segregation in the F2 and later generations then allows a new set of inbred lines to be made with gene combinations different from those of the lines used to make the population. This process can lead to further improvement of combining abilities (Falconer and Mackay 1996). Topcross and double-topcross hybrids can be a catalyst for the development of the seed industry in many countries of sub-Saharan Africa where the seed industry is not well-developed as the seed of these types of hybrids is easier to produce. Alternatively the synthetics can be used as open-pollinated varieties, and these are expected to show yield stability over environments due to their heterogeneous genetic constitution.
Genetic diversity among the inbred lines
The 32 SSR primers produced 114 alleles with an average of 3.6 alleles per locus. The average number of alleles in this study, however, was relatively smaller than the number reported in studies by Senior et al. (1998) and Pejic et al. (1998) who detected an average of 5 and 6.8 alleles per locus among temperate maize inbred lines, respectively. Warburton et al. (2002), Xia et al. (2004, 2005) found a higher level of diversity among tropical maize inbred lines, with average number of alleles per locus ranging from 4.9 to 7.7. However, Garcia et al. (2004) reported a lower number of alleles per locus (2.9) in a study with tropical maize inbred lines. The number of alleles for RFLP markers ranged from 2 to 28, with an average of 10, a result similar to that reported by Warburton et al. (2005) but different than that reported by Betrán et al. (2003a) and Garcia et al. (2004). The total number of alleles in diversity studies is usually proportional to sample size and this could explain the differences in the average number of alleles reported among these studies (Xia et al. 2004). This study involved 15 tropical maize inbred lines while the studies of Warburton et al. (2002) and Xia et al. (2004), all had 57–155 tropical maize inbred lines. The PIC, which is a measure of allele diversity at a locus, ranged from 0.38 to 0.78 with an average of 0.59 for SSR markers. The results obtained in this study are similar to those reported in temperate maize (Senior et al. 1998; Reif et al. 2003a; Smith et al. 1997) and tropical maize (Xia et al. 2005). The average PIC value for RFLP markers was 0.73 with a range of 0.12–0.94, a result similar to that reported by Betrán et al. (2003a) but lower than that reported by Garcia et al. (2004) in tropical maize inbred lines. Average GD among the fifteen maize inbred lines using pooled marker data was 0.57 with a range of 0.45 to 0.63. The most genetically distant lines were CML247 and CML339 (GD = 0.63) while the most closely related line based on GD were CML202 and CML216 (GD = 0.45). Cluster analysis revealed four clusters that had most lines grouped together in accordance with known pedigree and origin (Fig. 4). Drought tolerant inbred lines CML339, CML341, and CML343 that were developed from the same source population clustered together as did inbred lines CML254 and CML258 originating from Population 21. The agreement between origin and GD is consistent with other studies in tropical maize (Warburton et al. 2002; Betrán et al. 2003a) and temperate maize (Lee et al. 1989; Boppenmaier et al. 1992; Senior et al. 1998). Some inbred lines, for example CML78 and P501, CML202 and CML216, not related by pedigree, clustered together. Xia et al. (2005) reported clustering among subtropical maize inbred lines that did not correspond to origin. Lines that cluster together but are not related by pedigree could be a result of adaptive selection, markers that are identical in state but not identical by descent (Mumm and Dudley 1994; Warburton et al. 2005). The clusters contained lines that are assigned to both heterotic groups A and B used at CIMMYT. For example, cluster 1 contained two lines in group B (P502 and CML202), one line in group A (CML247), and one in group AB (CML216). The second cluster contained two lines in group A (CML78 and P502), and one line in group B (CML311). The third cluster had one line each in group A (CML311) and B (CML339), and two lines in group AB. This suggests that lines in the two heterotic groups were not very well differentiated by markers in this germplasm. Similar results were reported by Warburton et al. (2005), who analyzed a larger set of inbred lines. Heterotic grouping of tropical maize lines should be carried out using both molecular marker information and combining ability studies. In tropical maize breeding programs with limited resources use of markers for heterotic grouping may not be cost effective and therefore heterotic grouping should be carried out based on combining ability studies.
Relationship between F1 hybrid performance, specific combining ability, heterosis and genetic distance
Simple linear correlation coefficients were used to study the relationship between heterosis, F1 hybrid performance, SCA, and GD. MPH was significantly (P < 0.01) correlated with F1 hybrid GY under drought stress, low N stress and well-watered conditions (Table 6) although the predictive power was low for well-watered conditions (R 2 = 0.27) and low N stress environments (R 2 = 0.23 and 0.17 for ZBLN and PRLN, respectively). The strong correlation between MPH and GY under drought stress (r = 0.65, P < 0.01; R 2 = 0.43, P < 0.05) suggests that MPH could be used to predict performance of F1 hybrids under drought stress. Across environments, MPH was significantly correlated with F1 hybrid GY (r = 0.64, P < 0.01) and SCA (r = 0.53, P < 0.05), results similar to those reported by Betrán et al. (2003a). GD and F1 hybrid GY were significantly correlated (r = 0.24, P < 0.05) across environments. Other studies have demonstrated significant correlations between molecular marker-based GD and F1 hybrid GY in tropical maize (Benchimol et al. 2000; Betrán et al. 2003a; Barbosa et al. 2003) and temperate maize (Lee et al. 1989; Melchinger et al. 1990a; Smith et al. 1990; Ajmone Marsan et al. 1998). In contrast, Godshalk et al. (1990) reported no significant correlation between GD and F1 hybrid GY in temperate maize. Efficiency of prediction of hybrid performance based on GD would be greater with crosses between inbred lines from the same heterotic group than in crosses between inbred lines from different heterotic groups (Melchinger 1999). The inbred lines used in this study are in different heterotic groups and this could be the cause of the low correlation. Poor correlation between F1 performance and GD can be due to lack of linkage between genes controlling the trait and markers used to estimate GD, inadequate genome coverage and different levels of dominance among hybrids (Bernardo 1992). Molecular marker heterozygosity would be predictive of hybrid performance when dominance effects are strong, allele frequencies are negatively correlated between parents, heritability is high, and there is linkage between most markers and quantitative trait loci (Bernardo 1992). The existence of epistasis could also cause the low correlation between GD and F1 hybrid performance (Moll et al. 1965).
GD and average MPH showed a positive and significant (P < 0.05) correlation at ZBLN and across environments (Table 6). Similar results were reported by Melchinger et al. (1990a, b), Smith et al. (1990), Benchimol et al. (2000), and Reif et al. (2003b). Melchinger (1999) indicated that high estimates of r(GD, MPH) can be expected if correlations are calculated across different types of crosses because GD and MPH are expected to increase from crosses among related lines to intra-group crosses and further into inter-group crosses. We could not establish whether the range of GD obtained in this study (0.45–0.63) is within the range of GDs for crosses among unrelated lines in which the correlation between marker-estimated GD and MPH is expected to be weak. SCA had a weak correlation with GD (Table 6). Betrán et al. (2003a) reported a highly significant correlation (r = 0.80) between GD and SCA in tropical maize inbreds grown under stress and non-stress environments while Parentoni et al. (2001) reported a weak and positive correlation (r = 0.16) between GD and SCA in tropical maize inbreds. Lee et al. (1989) and Dudley et al. (1991) reported a strong correlation between SCA and GD in temperate maize germplasm. The weak correlation between GD and F1 hybrid GY, MPH and GD suggests that GD in this set of maize inbred lines is of limited value in predicting F1 hybrid GY and high heterotic performance among these materials. Differences in correlations could be a result of evaluating different types of materials and marker-based GD is not sufficiently associated with GY, heterosis, and SCA to identify superior single crosses (Melchinger et al. 1990a).
AMMI and stability analysis
Pattern analysis was used to investigate genotype × environment interaction in this study. The lattice adjusted mean GY and the GCA effect for GY for each line were added to create a new variable that was used in this analysis. A dendrogram was constructed to examine similarities among environments. The clustering based on GY revealed three groups of environments, with the well-watered and different stressed environments clustering separately (Fig. 5). Thus the grouping of the environments was based on growing conditions and stress levels prevailing at the different locations. Similar results have been reported in maize (Chapman et al. 1997; Betrán et al. 2003b). There is need to develop hybrids specific for each type of stress as hybrids respond differently to the stress levels (Betrán et al. 2003b). In a study with wheat, Yau et al. (1991) reported that clustering patterns observed were based on moisture supply, and their results confirmed differential responses of genotypes to irrigated/high rainfall and rain fed conditions low rainfall areas.
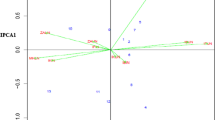
Cluster analysis based on grain yield of 105 hybrids from 15 maize inbred lines grown at eight environments (CSWW College Station well-watered, PRLN Poza Rica low N, TLSS Tlaltizapán drought stress, TLWW Tlaltizapán well-watered, WEWW Weslaco well-watered, ZBLN Harare low N, ZBSS Chiredzi drought stress, ZBWW Harare well-watered)
An AMMI biplot (Gabriel 1971) was used to show both genotypes and environments simultaneously. The biplot was generated using the principal component scores to visualize the relationship between environments and hybrids. The first two principal components explained 82.6% of the total variation in genotype × environment (G × E) sums of squares (Fig. 6). The biplot showed that environments ZBSS and ZBWW were the most discriminating for the genotypes, as indicated by their long vectors. The angle between the vectors for these two environments was large indicating they were very different in ranking genotypes. Similarly, environments ZBWW and CSWW had large angles between them suggesting they were different in discriminating genotypes. Well-watered environments CSWW and WEWW, both in Texas, had a very small angle between them showing that these two environments are expected to have a strong positive correlation of genotype GY between them and rank genotypes similarly. The two low N stress environments (PRLN and ZBLN) although different geographically, were close suggesting that these two environments are similar in genotype discrimination. Inbred line P501 had a small projection on the vector for environment WEWW indicating that hybrids of this inbred line performed well in that environment. Likewise, hybrids of inbred line CML311 performed well at ZBSS. Inbred lines CML258 and CML321 had positive projections on ZBWW and ZBSS showing that on average, they performed well in both environments. For inbred lines per se, the first two principal components accounted for 92.3% of the total variation in G × E sums of squares (Fig. 7). A biplot showed that environments ZBWW and ZBLN were the most discriminating among the inbred lines per se as shown by the long vectors. The stressed environments ZBSS and PRLN had a small angle between them, suggesting that they ranked the inbred lines similarly. Inbred line P501 had a small projection on environment ZBLN suggesting it performed well at that location. Indeed P501 had the highest yield at ZBLN (1.62 Mg ha−1). CML254 performed well at PRLN where it had the highest yield (0.70 Mg ha−1). CML216 had a small projection on environment vector for ZBWW suggesting good performance at that environment. Line CML258 had positive projection on both ZBSS and ZBWW where it was among the best performers at those environments.
Stability analysis showed that the coefficient of determination (R 2) ranged from 0.75 to 0.99 suggesting that the linear regression model was sufficient for analysis of GY for inbred lines in hybrid combination. Stability for GY as measured by the slope of linear regression b, ranged from 0.82 to 1.14 for inbred lines in hybrid combination and 0.04 to 1.84 for inbred line per se. A desirable variety is one that is stable (b = 1) and with above average performance. Inbred lines CML254, CML258, CML339, CML341 and P502 showed above average performance in hybrid combination and had a regression coefficient, b > 1 (Fig. 8). Average GY was positively correlated with the slope of regression b, for inbreds in hybrid combination (r = 0.53, P < 0.05) and inbred lines per se (r = 0.61, P < 0.05), suggesting that lines that performed well were also stable.
Conclusions
Fifteen inbred lines of various origins were evaluated in hybrid combination at six locations in three countries. Inbred lines developed by selection for stress tolerance combined well with conventionally selected lines to produce some of the best hybrids under managed drought stress and low N stress. This suggests that flow and combination of germplasm between different breeding programs can lead to identification of outstanding hybrids. Several inbred lines showed positive GCA effects for GY under drought stress. These inbred lines could be used to form synthetics that can be used as non-inbred parents or as open-pollinated varieties. Gene action controlling GY varied with type of stress, with additive gene action appearing to be more important than non-additive gene action under drought stress while non-additive gene action was more important under low N stress, consistent with findings in previous studies.
Inbred line per se performance was significantly correlated with hybrid performance under low N stress but not under drought stress, emphasizing the need to evaluate hybrids under stress to identify superior hybrids for stress environments. Predicted hybrid performance using the additive model was strongly correlated with observed hybrid performance across stresses and environments. This suggests that GCA accounts for a large proportion of the differences between crosses compared to SCA in this germplasm.
MPH for GY was highest in the drought stressed environment compared to the well-watered environment. Similar results have been reported in tropical maize. Clustering of the inbred lines using pooled marker revealed clustering of most lines in accordance with known pedigree and origin, consistent with other studies in tropical maize. MPH was significantly correlated with GY under drought and low N stress, as well as well-watered conditions. MPH was also significantly correlated with SCA at all environments. GD was weakly correlated with GY and MPH in this study, suggesting that GD is of limited value in predicting F1 hybrid GY and high heterotic performance in this germplasm.
References
Ajmone Marsan P, Castiglioni P, Fusari F, Kuiper M, Motto M (1998) Genetic diversity and its relationship to hybrid performance in maize as revealed by RFLP and AFLP markers. Theor Appl Genet 96:219–227
Baker RJ (1978) Issues in diallel analysis. Crop Sci 18:533–536
Bänziger M, Lafitte HR (1997) Efficiency of secondary traits for improving maize for low nitrogen target environments. Crop Sci 37:1110–1117
Bänziger M, Betrán FJ, Lafitte HR (1997) Efficiency of high-nitrogen selection environments for improving maize for low nitrogen target environments. Crop Sci 37:1103–1109
Bänziger M, Edmeades GO, Lafitte HR (1999) Selection for drought tolerance increases maize yields across a range of nitrogen levels. Crop Sci 39:1035–1040
Bänziger M, Edmeades GO, Beck D, Bellon M (2000) Breeding for drought and nitrogen stress tolerance in maize. From theory to practice. CIMMYT, Mexico, DF
Bänziger M, Edmeades GO, Lafitte HR (2002) Physiological mechanisms contributing to the increased N stress tolerance of tropical maize for drought tolerance. Field Crops Res 75:223–233
Barbosa AMM, Gerald IO, Benchimol LL, Garcia AAF, Souza CL Jr, Souza AP (2003) Relationship of intra- and interpopulation tropical maize single cross hybrid performance and genetics distances computed from AFLP and SSR markers. Euphytica 130:87–99
Beck DL, Vasal SK, Crossa J (1991) Heterosis and combining ability among subtropical and temperate intermediate-maturity maize germplasm. Crop Sci 31:68–73
Beck D, Betrán JF, Bänziger M, Edmeades GO, Ribaut J-M, Wilcox M, Vasal SK, Ortega A (1996) Progress in developing drought and low soil nitrogen tolerance in maize. In: Proceedings of the 51st annual corn and sorghum industrial research conference. Chicago, Dec 6–11. ASTA, Washington, DC, pp 85–111
Bellon MR (2001) Participatory methods in the development and dissemination of new maize technologies. In: Pingali PL (ed) CIMMYT 1999–2000 world maize facts and trends. Meeting world maize needs: technological opportunities and priorities for the public sector. CIMMYT, Mexico, DF, pp 4–20
Benchimol LL, De Souza CL, Garcia AAF Jr, Kono PMS, Mangolin CA, Barbosa AMM, Coelho ASG, De Souza AP (2000) Genetic diversity in tropical maize inbred lines: heterotic group assignment and hybrid performance. Plant Breed 119:491–496
Bernardo R (1992) Relationship between single-cross performance and molecular marker heterozygosity. Theor Appl Genet 83:628–634
Bernardo R (2002) Breeding for quantitative traits in plants. Stemma Press, Woodbury
Betrán FJ, Bänziger M, Beck DL (1997) Relationship between line and topcross performance under drought and non-stressed conditions in tropical maize. In: Edmeades GO, Bänziger M, Mickelson HR, Peña-Valdivia CB (eds) Developing drought- and low-N tolerant maize. Proceedings of a symposium, March 25–29, 1996. CIMMYT, El Batán, Mexico, DF, pp 383–386
Betrán FJ, Beck D, Bänziger M, Edmeades GO (2003a) Genetic analysis of inbred and hybrid yield under stress and nonstress environments in tropical maize. Crop Sci 43:807–817
Betrán FJ, Ribaut J-M, Beck D, Gonzalez de Leon D (2003b) Genetic diversity, specific combining ability, and heterosis in tropical maize inbreds under stress and nonstress environments. Crop Sci 43:797–806
Bolaños J, Edmeades GO (1993a) Eight cycles of selection for drought tolerance in lowland tropical maize. I. Responses in grain yield, biomass and radiation utilization. Field Crops Res 31:233–252
Bolaños J, Edmeades GO (1993b) Eight cycles of selection for drought tolerance in lowland tropical maize. II. Responses in reproductive behavior. Field Crops Res 31:253–268
Boppenmaier J, Melchinger AE, Brunklaus-Jung E, Geiger HH, Herrmann RG (1992) Genetic diversity for RFLPs in European maize inbreds. I. Relation to performance of flint 9 dent crosses for forge traits. Crop Sci 32:895–902
Burstin J, Charcosset A, Barrière Y, Hébert Y, De Vienne D, Damerval C (1995) Molecular markers and protein quantities as genetic descriptors in maize. II. Prediction of performance of hybrids for forage traits. Plant Breed 114:427–433
Byrne PF, Bolaños J, Edmeades GO, Eaton DL (1995) Gains from selection under drought versus multilocation testing in related tropical maize populations. Crop Sci 35:63–69
Castleberry RM, Crum CW, Knull CF (1984) Genetic yield improvement of U.S. maize cultivars under varying fertility and climatic environments. Crop Sci 24:33–36
Chapman SC, Crossa J, Edmeades GO (1997) Genotype by environment effects and selection for drought tolerance in tropical maize. I. Two mode pattern analysis of yield. Euphytica 95:1–9
Charcosset A, Bonnisseau B, Touchebeuf O, Burstin J, Dubreuil P, Barribre Y, Gallais A, Denis JB (1998) Prediction of maize hybrid silage performance using marker data: comparison of several models for specific combining ability. Crop Sci 38:38–44
CIMMYT (1999) 1997/98 CIMMYT world maize facts and trends. Maize production in drought-stressed environments: technical options and research resource allocation. CIMMYT, Mexico, DF
CIMMYT (2001) The applied biotechnology center’s manual of laboratory protocols, 1st edn. CIMMYT, Mexico, DF
Diers BW, McVetty PBE, Osborn TC (1996) Relationship between heterosis and genetic distance based on restriction fragment length polymorphism markers in oilseed rape (Brassica napus L.). Crop Sci 36:79–83
Dudley JW, Saghai-Maroof MA, Refener GK (1991) Molecular markers and grouping of parents in maize breeding programs. Crop Sci 31:718–723
Eberhart SA, Russell WA (1966) Stability parameters for comparing varieties. Crop Sci 6:36–40
Edmeades GO, Bolaños J, Lafitte HR (1992) Progress in breeding for drought tolerance in maize. In: Wilkinson D (ed) Proceedings of the 47th annual corn and sorghum industrial research conference. ASTA, Washington, DC, pp 93–111
Edmeades GO, Bolaños J, Bänziger M, Chapman SC, Ortega A, Lafitte HR, Fischer KS, Pandey S (1997) Recurrent selection under managed drought stress improves grain yields in tropical maize. In: Edmeades GO, Bänziger M, Mickelson HR, Peña-Valdivia CB (eds) Developing drought- and low-N tolerant maize. Proceedings of a symposium, March 25–29, 1996. CIMMYT, El Batán, Mexico, DF, pp 415–425
Edmeades GO, Bolaños J, Chapman SC, Lafitte HR, Bänziger M (1999) Selection improves drought tolerance in tropical maize populations: I. Gains in biomass, grain yield, and harvest index. Crop Sci 39:1306–1315
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman Group Ltd, Essex
Fischer KS, Edmeades GO, Johnson EC (1989) Selection for the improvement of maize yield under moisture deficits. Field Crops Res 22:227–243
Gabriel KR (1971) The biplot graphical display of matrices with application to principal component analysis. Biometrika 58:453–467
Garcia AAF, Benchimol LL, Barbosa AMM, Geraldi IO, Souza CL Jr, de Souza AP (2004) Comparison of RAPD, RFLP, AFLP and SSR markers for diversity studies in tropical maize inbred lines. Genet Mol Biol 27:579–588
Godshalk EB, Lee M, Lamkey KR (1990) Relationship of restriction fragment length polymorphisms to single-cross hybrid performance of maize. Theor Appl Genet 80:273–280
Griffing B (1956) Concept of general and specific combining ability in relation to diallel crossing systems. Aus J Biol Sci 9:463–493
Hall AJ, Vilella F, Trapani N, Chimenti C (1982) The effects of water stress and genotype on the dynamics of pollen-shedding and silking in maize. Field Crops Res 5:349–363
Hallauer AR, Miranda JB (1988) Quantitative genetics and maize breeding. Iowa State University Press, Ames
IFPRI (2000) 2020 Projections. IFPRI, Washington, DC
IRRI (1998) IRRISTAT for Windows, version 4.0. International Rice Research Institute, Manilla
Lafitte HR, Edmeades GO (1994) Improvement for tolerance to low soil nitrogen in tropical maize. I. Selection criteria. Field Crops Res 39:1–14
Lafitte HR, Edmeades GO (1995) Association between traits in tropical maize inbred lines and their hybrids under high and low soil nitrogen. Maydica 40:259–267
Lee M, Godshalk EB, Lamkey KR, Woodman WL (1989) Association of restriction fragment length polymorphisms among maize inbreds with agronomic performance of their crosses. Crop Sci 29:1067–1071
Lipkovich I, Smith E (2002) Biplot and singular value decomposition macros for Excel. J Stat Softw 7(5). http://www.jstatsoft.org/v07/i05/
Malvar RA, Revilla P, Moreno-González J, Butrón A, Sotelo J, Ordás A (2008) White maize: genetics of quality and agronomic performance. Crop Sci 48:1373–1381
Melchinger AE (1999) Genetic diversity and heterosis. In: Coors JG, Pandey S (eds) The genetics and exploitation of heterosis in crops. American Society of Agronomy/Crop Science Society of America Inc., Madison, pp 99–118
Melchinger AE, Lee M, Lamkey KR, Hallauer AR, Woodman WL (1990a) Genetic diversity for restriction fragment length polymorphisms: relation to estimated genetic effects in maize inbreds. Crop Sci 30:1033–1040
Melchinger AE, Lee M, Lamkey KR, Woodman WL (1990b) Genetic diversity for restriction fragment length polymorphisms and heterosis for two diallel sets of maize inbreds. Theor Appl Genet 80:488–496
Moll RH, Lonnquist JH, Fortuna JV, Johnson EC (1965) The relation of heterosis and genetic divergence in maize. Genetics 52:139–144
Moss GI, Downey LA (1971) Influence of drought on female gametophyte development in corn (Zea mays L.) and subsequent grain yield. Crop Sci 11:368–371
Mumm RH, Dudley JW (1994) A classification of 148 U.S. maize inbreds: I. Cluster analysis based on RFLPs. Crop Sci 34:842–851
Nei M, Li WH (1979) Mathematical models for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Parentoni SN, Magalhaes JV, Pacheco CA, Santos MX, Abadie T, Gama EEG, Guimaraes PEO, Meirelles WF, Lopes MA, Vasconcelos MJV, Paiva E (2001) Heterotic groups based on yield-specific combining ability data and phylogenetic relationship determined by RAPD markers for 28 tropical maize open pollinated varieties. Euphytica 121:197–208
Paterniani E, Lonnquist JH (1963) Heterosis in interracial crosses of maize (Zea mays L.). Crop Sci 3:504–507
Patterson HD, Williams ER (1976) A new class of resolvable incomplete block designs. Biometrika 63:83–89
Pejic I, Ajmone-Marsan P, Morgante M, Kozumplick V, Castiglioni P, Taramino G, Motto M (1998) Comparative analysis of genetic similarity among maize inbred lines detected by RFLPs, RAPDs, SSRs, and AFLPs. Theor Appl Genet 97:1248–1255
Pingali PL, Pandey S (2001) Meeting world maize needs: technological opportunities and priorities for the public sector. In: Pingali PL (ed) CIMMYT 1999–2000 world maize facts and trends. Meeting world maize needs: technological opportunities and priorities for the public sector. CIMMYT, Mexico, DF, pp 4–20
Reif JC, Melchinger AE, Xia XC, Warburton ML, Hoisington DA, Vasal SK, Beck D, Bohn M, Frisch M (2003a) Use of SSRs for establishing heterotic groups in subtropical maize. Theor Appl Genet 107:947–957
Reif JC, Melchinger AE, Xia XC, Warburton ML, Hoisington DA, Vasal SK, Srinivasan G, Bohn M, Frisch M (2003b) Genetic distance based on simple sequence repeats and heterosis in tropical maize populations. Crop Sci 43:1275–1282
Rohlf FJ (1997) NTSYS-pc. Numerical taxonomy and multivariate analysis system, version 2.02j. Exter Software, Applied Biostatistics, Inc., New York
San Vicente FM, Bejarano A, Marin C, Crossa J (1998) Analysis of diallel crosses among improved tropical white endosperm populations. Maydica 43:147–153
SAS Institute (2001) SAS system for Windows, version 8.2. SAS Institute Inc., Cary
Senior ML, Murphy JP, Goodman MM, Stuber CW (1998) Utility of SSRs for determining genetic similarities and relationships in maize using an agarose gel system. Crop Sci 38:1088–1098
Simmonds NW (1979) Principles of crop improvement. Longman, London
Smith OS (1986) Covariance between line per se and testcross performance. Crop Sci 26:540–543
Smith OS, Smith JSC, Bowen SL, Tenborg RA, Wall SJ (1990) Similarities among a group of elite maize inbreds as measured by pedigree, F1 grain yield, grain yield, heterosis, and RFLPs. Theor Appl Genet 80:833–840
Smith JSC, Chin ECL, Shu H, Smith OS, Wall SJ, Senior ML, Mitchell SL, Kresovich S, Ziegle J (1997) An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): comparisons with data from RFLPs and pedigree. Theor Appl Genet 95:163–173
Vasal SK, Srinivasan G, Beck DL, Crossa J, Pandey S, De Leon C (1992) Heterosis and combining ability of CIMMYT’s tropical late white maize germplasm. Maydica 37:217–223
Vos P, Rogers R, Bleeker M, Rejians M, Van De Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabea M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Warburton ML, Xianchun X, Crossa J, Franco J, Melchinger AE, Frisch M, Bohn M, Hoisington D (2002) Genetic characterization of CIMMYT inbred maize lines and open pollinated populations using large scale fingerprinting methods. Crop Sci 42:1832–1840
Warburton ML, Ribaut J-M, Franco J, Crossa J, Dubreuil P, Betrán FJ (2005) Genetic characterization of 218 elite CIMMYT inbred maize lines using RFLP markers. Euphytica 142:97–106
Welcker C, Thé C, Andréau B, De Leon C, Parentoni CN, Bernal J, Félicité J, Zonkeng C, Salazar F, Narro L, Charcosset A, Horst WJ (2005) Heterosis and combining ability for maize adaptation to tropical soils: implications for future breeding strategies. Crop Sci 45:2405–2413
Xia XC, Reif JC, Hoisington DA, Melchinger AE, Frisch M, Warburton ML (2004) Genetic diversity among CIMMYT maize inbred lines investigated with SSR markers: I. Lowland tropical maize. Crop Sci 44:2230–2237
Xia XC, Reif JC, Melchinger AE, Frisch M, Hoisington DA, Beck D, Pixley K, Warburton ML (2005) Genetic diversity among CIMMYT maize inbred lines investigated with SSR markers: II. Subtropical, tropical midaltitude and highland maize inbred lines and their relationships with elite U.S. and European maize. Crop Sci 45:2573–2582
Yau SK, Ortiz-Ferrara G, Srivastava JP (1991) Classification of diverse bread wheat-growing environment based on differential yield responses. Crop Sci 31:571–576
Zhang Y, Kang MS (1997) DIALLEL-SAS: a SAS program for Griffing’s diallel analyses. Agron J 89:176–182
Acknowledgments
We thank technical staff at CIMMYT research stations in Mexico and Zimbabwe for assistance with data collection. We also extend our gratitude to staff of the Texas A&M University Maize Breeding and Genetics Program for managing the trials at College Station and Weslaco. This work was funded by grants from the Rockefeller Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makumbi, D., Betrán, J.F., Bänziger, M. et al. Combining ability, heterosis and genetic diversity in tropical maize (Zea mays L.) under stress and non-stress conditions. Euphytica 180, 143–162 (2011). https://doi.org/10.1007/s10681-010-0334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0334-5