Abstract
Stripe (yellow) rust, caused by Puccinia striiformis Westend. f. sp. tritici Eriks. (Pst), is an important disease of wheat (Triticum aestivum L.) globally. Use of host resistance is an important strategy to manage the disease. The cultivar Flinor has temperature-sensitive resistance to stripe rust. To map quantitative trait loci (QTLs) for these temperature-sensitive resistances, Flinor was crossed with susceptible cultivar Ming Xian 169. The seedlings of the parents, and F1, F3 progeny were screened against Chinese yellow rust race CYR32 in controlled-temperature growth chambers under different temperature regimes. Genetic analysis confirmed two genes for temperature-sensitive stripe rust resistance. A linkage map of SSR markers was constructed using 130 F3 families derived from the cross. Two temperature-sensitive resistance QTLs were detected on chromosome 5B, designated QYr-tem-5B.1 and QYr-tem-5B.2, respectively, and are separated by a genetic distance of over 50 cM. The loci contributed 33.12 and 37.33% of the total phenotypic variation for infection type, respectively, and up to 70.45% collectively. Favorable alleles of these two QTLs came from Flinor. These two QTLs are temperature-sensitive resistance loci and different from previously reported QTLs for resistance to stripe rust.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat stripe (yellow) rust, caused by Puccinia striiformis f. sp. tritici (Pst), is a major disease that can cause tremendous wheat production losses worldwide (Stubbs 1988). Using resistant cultivars is the major method for controlling this disease (Johnson 1988; Line 2002). Stripe rust resistance is controlled by race-specific or non-race-specific resistance. However, the race-specific resistance is easily overcome and cultivars having only such resistance become susceptible soon after they are released (about 3–5 years) because of the rapid evolution/selection of new races capable of overcoming the newly deployed race-specific resistances. In contrast, some non-race-specific resistance displays quantitative inheritance and provides durable resistance to stripe rust. It is worthwhile to mention that the resistance in some wheat cultivars is temperature-sensitive. It has also been reported that temperature-sensitive resistances to stripe rust are expressed at higher-temperatures both in adult-plant (Qayoum and Line 1985; Milus and Line 1986a, b; Park et al. 1992; Chen 2005) and at seedling growth-stages (Brown and Sharp 1969; Park et al. 1992). Also, temperature-sensitive genes for stripe rust resistance have been documental in T. dicoccoides (Gerechter-Amitai and Van Silfhout 1989). Several authors have expressed the opinion that temperature-sensitive resistance could prove to be durable (Qayoum and Line 1985; Kaul and Shaner 1989), and if used in conjunction with other types of resistance, could provide a long-term means of control of stripe rust. The intrinsically durable resistant cultivars are important resistance sources for use in breeding new varieties (Johnson 1984; Line 2002; Singh et al. 2005).
Temperature-sensitive resistance genes are effective either in the seedling-stage or in the adult-plant stage, in the field. Currently, a few genes, Yr36 on 6BS in Triticum turgidum ssp. dicoccoides (Uauy et al. 2005) and Yr39 on 7BL in Alpowa (Lin and Chen 2007), conferring high-temperature adult-plant (HTAP) resistance have been identified. Similarly, several QTLs for HTAP resistance have been identified, including QYrst.wgp-6BS.1 and QYrst.wgp-6BS.2 in Stephens (Santra et al. 2008), three QTLs (QYrex.wgp-6AS, QYrex.wgp-3BL and QYrex.wgp-1BL) in Express (Lin and Chen 2009), and QYrlo.wpg-2BS in Louise (Carter et al. 2009). HTAP is a type of field resistance induced by an increase in temperature during the growing season and is not expressed in the seedling-stage. HTAP resistance is assessed in adult-plants with races virulent to the seedlings at standard high diurnal temperature cycle. In contrast, little emphasis has been placed upon seedling temperature-sensitive resistance. The temperature-sensitive stripe rust resistance gene YrCK, the only known temperature-sensitive stripe rust resistance gene, characterized for the seedling-stage (Navabi et al. 2005), does contribute to overall resistance to stripe rust in the adult-plant stage as well. YrCK is located on chromosome arm 2DS in Cook (Park et al. 1992; Bariana et al. 2001). It is clear that the presence of adult-plant resistance in the absence of effective seedling resistance genes does not guarantee durability of resistance. Thus temperature-sensitive resistance to stripe rust for all host growth-stages might need more attention.
The wheat cultivar Flinor was introduced to China from France. It has been previously reported to possess temperature-sensitive resistance to stripe rust (Tong et al. 2006). Genetic assessment of Flinor would provide information on the nature and number of genes responsible for its stripe rust resistance, and thereby facilitate transfer of this resistance into new wheat cultivars. This study therefore aimed to identify and characterize the basis of temperature-sensitive resistance in Flinor to Pst in order to facilitate its exploitation by plant breeders.
Materials and methods
Plant materials
F3 progeny sets used for mapping QTLs were derived from a cross between Flinor and Min Xian 169. The wheat cultivar Ming Xian 169 (ZM 009379 http://icgr.caas.net.cn) (MX), the susceptible parent, is highly susceptible to all known Puccinia striiformis f. sp. tritici (Pst) races in China (Wan et al. 2004). The winter wheat cultivar Flinor, introduced from France, possesses superior resistance to wheat stripe rust. It is derived from the cross (Elite × Poncheau). All the seeds were provided by the Institute of Plant Protection, Chinese Academy of Agricultural Sciences.
Assessment of temperature-sensitive Pst resistance in seedlings
The CYR32 race of Chinese yellow rust (Pst), which is virulent against the resistance genes Yr1, Yr2, Yr3, Yr4, Yr6, Yr7, Yr9, Yr17, Yr22, Yr23, Yr27, YrA, YrCV1, YrCV2, YrCV3, YrG, YrSD, and YrSO, was used for this experiment. Because it was virulent on seedlings of Flinor, it can be used to evaluate temperature-sensitive resistance segregating among progeny of the cross Flinor × MX. Wheat seedlings were grown in 9 cm diameter plastic pots containing a soil-peat mixture with approximately 10–15 plants for each of the 130 F3 families. Seedlings were grown in a rust-free greenhouse. At the two leaf stage, the plants were uniformly inoculated with a single-spore isolate of CYR32. Following inoculation, plants were irrigated with deionized water and placed in a dew simulation chamber maintained at 10°C for 24 h in the dark. After inoculation, one set was kept under high-temperature conditions with 24°C in light and 18°C at dark, while a duplicate set was tested under normal-temperature conditions with 14°C/10°C (light/dark). Light conditions in both growth chambers were 20,000 Lux for 14 h and a dark period for 10 h. The relative humidity was 75%. Every family was divided into two groups and evaluated for infection type (IT) to stripe rust in the seedling-stage under these controlled conditions with single-replication. IT was visually rated on a 0–9 scale (Line and Qayoum 1992), when IT on susceptible check Ming Xian 169 reached a maximum level of 9.
Molecular marker analysis
Genomic DNA was isolated from the parents, F1 and more than 20 plants of each of the 130 F3 families using the cetyltrimethyl ammonium bromide (CTAB) method (Rogers and Bendich 1985). DNA concentrations were normalized. The microsatellite or simple sequence repeat (SSR) marker technique described by Röder et al. (1998) was followed. The SSR primers used in this study were from the WMC (Wheat Microsatellite Consortium), CFA (Clermont-Ferrand A genome), CFD (Clermont-Ferrand D genome), GWM (Gatersleben Wheat Microsatellite), GDM (Gatersleben D genome Microsatellite), and BARC (Beltsville Agriculture Research Center) series. The sequences of SSR primers along with their previously determined chromosomal locations were acquired from the published data (http://wheat.pw.usda.gov/). All primer sequences were synthesized by the Beijing SBS Genetech Co., Ltd. (http://www.sbsbio.com). Polymerase chain reactions (PCRs) were performed in a DNA engine Peltier thermo-cycler and a DYAD Peltier thermal cycler. The 10 μl reaction mixtures consisted of 50–100 ng of template DNA, 1.0 μl 10× PCR buffer, 0.5 units of Easy Taq DNA polymerase, 25 μM each of dCTP, dGTP, dTTP, and dATP, and 2 μM of each primer pair. PCR amplifications were performed at 50, 55 or 61°C depending on the individual primer pairs. PCR products were separated in 6% denaturing polyacrylamide gels and then visualized by silver staining (Bassam et al. 1991).
Linkage map construction
Parental (Flinor and MX) genotypes were screened for polymorphism using 978 SSR primer pairs. SSR markers showing polymorphism between the resistant and susceptible parents were used to genotype the entire population of 130 F3 families and the resulting data set used to construct linkage maps. Linkage analysis of the entire set of marker data was performed using MAPMAKER/EXP 3.0b software. Linkage groups were assigned to wheat chromosomes as described in previous publications. Map distance was calculated using the Kosambi mapping function in centiMorgans (cM). Prior to map construction, segregation of individual markers was analyzed by a χ 2 test for goodness-of-fit to the expected 1:2:1 (A:H:B) ratio for the co-dominant markers and 3:1 (present: absent) ratio for the dominant SSRs. F1 was used to determine the dominance or co-dominance. Polymorphic marker loci that exhibited significant distortion (P < 0.05 for χ 2 test) from these expected 1:2:1, 1:3, or 3:1 segregation rations were discarded from the linkage analysis. Linkage groups were assigned to chromosomes by comparison with the International Triticeae Mapping Initiative (ITMI) map (Röder et al. 1998; Somers et al. 2004).
QTLs analyses
QTLs analysis was conducted combining linkage map genotype data and IT scores of the F3 families using composite interval mapping (CIM) and multiple interval mapping (MIM) to scan the linkage groups for the presence of temperature-sensitive resistance QTLs using Cartographer 2.0 software (Basten et al. 2003; Wang et al. 2004). For CIM, significant logarithm of odds (LOD) thresholds were estimated by conducting a permutation test with 1,000 iterations at α = 0.05. For MIM analysis, the QTL peaks above the LOD threshold value from the CIM analysis were used as the initial model. The additive and dominance effects, and the QTL × QTL interaction effects, were estimated using MIM. The proportion of phenotypic variation (R 2) explained by individual QTL and by the whole model was determined using the summary option of MIM.
Results
Stripe rust resistance at seedling-stage under high-temperature conditions
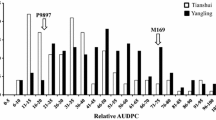
Both Flinor and MX were susceptible to the Pst race CYR32 at the seedling-stage and had an IT 9 under normal-temperature conditions. In contrast, Flinor was highly resistant at the seedling-stage under high-temperature conditions with lower IT of two or three, whereas the susceptible parent MX showed no change and remained highly susceptible (IT = 9). These data indicated that there were one or more temperature-sensitive resistance gene(s) in Flinor. For all 130 F3 progenies from the Flinor/MX cross were susceptible under normal conditions (data not shown). The IT frequency distribution of F3 families under high-temperature conditions is exhibited in Fig. 1. Under these conditions, the 130 F3 families were comprised of 54 uniformly resistant (R), 70 segregating (seg) and 6 uniformly susceptible (S) lines, which segregated in 7R: 8seg: 1 ratio (χ 2 = 1.09, ns for 7:8:1 ratio expected of digenic complementary recessive epistasis). The results of the Mendelian genetic analysis of seedling screens conducted under two temperature profiles demonstrated that the stripe rust resistance in Flinor is conferred by recessively inherited alleles with temperature-sensitive characteristics at two genes. All 130 F3 families were susceptible, with IT values of 8–9 under normal-temperature conditions. F1 was susceptible to the race under high-temperature, indicating the gene from Flinor was recessively.
Detection of QTLs for temperature-sensitive resistance
Of the 978 SSR primer pairs used to screen for polymorphism between the parents, 282 were polymorphic. The polymorphic markers were then used for genotyping the entire set of 130 F3 families. A molecular marker linkage map was constructed using 229 marker loci exhibiting non-distorted segregation that were distributed over the 21 wheat chromosomes with 6–16 loci per chromosome. Linkage groups were assigned by referring to the ITMI maps.
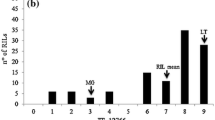
A LOD threshold of 3.01 to declare QTLs as significant was established empirically with 1,000 permutations. Based on this LOD, there were two QTLs detected for seedling-stage temperature-sensitive resistance to stripe rust, for the F3 families were scored susceptible under normal-temperature conditions. They were both located on chromosome 5B (Table 1, Fig. 2), and are referred to as QYr-tem-5B.1 and QYr-tem-5B.2, respectively. QYr-tem-5B.1 was flanked by SSR markers Xwms67 and XBarc89, and QYr-tem-5B.2 was flanked by SSR markers Xwmc235 and Xwms604 (Fig. 2). QYr-tem-5B.1 and QYr-tem-5B.2 explained up to 33.12 and 37.33% of the phenotypic variation of infection type, respectively, and together explained up to 70.45% of the observed phenotypic variation in reaction to stripe rust. The two QTLs are separated by a genetic distance over 50 cM and behave in an additive fashion without significant dominance and epistatic effects. The favorable alleles at both of the two QTLs for temperature-sensitive seedling-stage resistance to stripe (yellow) rust came from Flinor. Quantitative trait locus is attributable to two genes, which also confirmed the patterns of Mendelian inheritance.
Discussion
The expression of host resistance from the Flinor donor parent is greatly influenced by temperature conditions, and resistance is better expressed at higher-temperatures. Temperature-sensitive, recessive, and additive ‘minor gene’ resistances effective against Pst have been described for some spring and winter wheat cultivars (Krupinsky and Sharp 1978, 1979). Such resistances appeared to have durable effects (Qayoum and Line 1985; Kaul and Shaner 1989). Temperature-sensitive resistance can be expressed when temperatures are high in either the seedling-stage or adult stage. Not all temperature-sensitive resistances observed in the fields are detected in seedling screens. Chen (2005) described HTAP resistance, in which the genetically resistant host expresses a susceptible phenotype in the seedling-stage under both normal- and high-temperature conditions, and in the adult-plant stage at normal-temperatures, but expresses resistance in the adult-plant stages at high-temperature. Such environmentally differential expression might be caused by other environmental conditions, such as light intensity, and not just temperature itself. The resistance of durably resistant cultivars such as Nugaines and Luke was temperature-sensitive, being more effective in adult stage at high-temperatures. Seedling resistance to yellow rust was also broadly categorized as all growth-stage resistance, which can be detected at the seedling-stage, but is also expressed at all-stages of plant growth. In the present study, the temperature-sensitive resistance was expressed in the seedlings, so it would also be expressed in all growth-stage. The evaluation of temperature-sensitive resistance in the test material was carried out in growth chambers, programmed at different temperature regimes. Pre-inoculation of the seedlings were done under identical environmental conditions, and the differential temperature treatments were imposed immediately after inoculation. After inoculation the plants were divided into two sets, which were maintained at 10/14°C and 18/24°C dark/light, respectively. The 18/24°C treatment was effective for the detection of temperature-sensitivity (Sharp and Fuchs 1982). Pathogen isolates with a wide spectrum of virulence were used to select the race which was virulent to the cultivars at the seedling-stage, but whose resistance would increase when the temperature increased. The shift toward resistance was observed in the seedling tests at the higher-temperature regime, but not under the normal, lower-temperature regime.
This investigation demonstrated the presence of temperature-sensitive resistance genes in Flinor. IT of Flinor was lower at high-temperatures than at lower-temperatures, as observed in previous studies (Tong et al. 2006). Comparing the high- and normal-temperature tests, the resistance in Flinor was expressed well only at higher-temperature, with variation of the IT score for Flinor ranging from 9 (at normal-temperatures) down to 2–3 at higher-temperatures. The frequency distribution of IT data of F3 families suggested there were two loci controlling temperature-sensitive resistance to stripe rust in Flinor, and the fit of these F3 families to a Mendelian segregation ratio of seven uniformly resistant to eight segregating to one uniformly susceptible suggested the involvement of two recessively inherited resistance genes, with homozygosity for the recessive resistance allele at either of these loci resulting in uniform expression of resistance in that F3 families.
Flinor was first commercially grown in France in 1974. It was bred from the cross ‘Elite × Poncheau’, whereas the former was developed from Bellevue × Hybride de Bersee. French winter wheats cultivars Hybride de Bersee, Cappelle Desprez and Camp Remy, each maintained good levels of field resistance to stripe (yellow) rust for more than 20 years, and therefore can be characterized as having durable resistance to this disease. Using euploid and aneuploid stocks of Bersee it was shown that a large part of this resistance was controlled by chromosome 5BS–7BS and expressed in seedlings (Johnson and Law 1975). The higher level of resistance in Bersee was controlled by four genes (Bariana and McIntosh 1995). This information has made a major contribution to the control of wheat stripe (yellow) rust. Pedigree information suggests that Hybride de Bersee may have contributed to the durable resistance expressed in Flinor. Chromosome 5B may contain crucial loci for yellow rust resistance. In our study, resistance alleles from Flinor at two QTLs for temperature-sensitive resistance expressed in the seedling-stage, designated as QYr-tem-5B.1 and QYr-tem-5B.2, respectively, were identified on chromosome 5B. Each has major effects, conferring temperature-sensitive resistance to yellow rust that is expressed at the seedling-stage of host plant growth. These two QTLs are separated by a genetic distance of over 50 cM. They independently reduced IT in an additive fashion. QYr-tem-5B.1 and QYr-tem-5B.2, explained 33.12 and 37.33% of the observed phenotypic variation of IT, respectively, and up to 70.45% collectively. There were no interaction effects between them.
Several resistance genes for stripe (yellow) rust, including Yr19 (Compair) and YrDru (Druchamp) (Chen et al. 1995, 1996) had previously been reported on chromosome 5B. Compair is a UK cultivar with the pedigree ‘Chinese Spring × Aegilops comosa’. Compair and Flinor are of different origins suggesting that the Pst resistance genes in them may well be different. Druchamp is the French cultivar derived from cross ‘Vilmorin 27 × Fleche d’Or’. Based on pedigree information analysis (Fig. 3), Druchamp and Flinor share the parents, such as Parsel, Japhet and Hatif Inversable (http://www.ars-grin.gov). In addition, Q.Dru.htap-1 associated with HTAP resistance in Druchamp was detected on chromosome 5B within a 14.3 cM interval flanked by molecular markers Xgwm335 and Xbarc004 (http://www.reeis.usda.gov/web/crisprojectpages/203894.html). More studies should be performed to determine (1) Whether YrDru is a temperature-sensitive resistance gene, (2) Whether YrDru is the same as or allelic with Q.Dru.htap-1, and (3) To test allelism between Q.Dru.htap-1 and the two QTLs detected in the present study.
In addition to these Pst resistance genes/QTLs, there have been several other QTLs identified on 5B: a QTL for stripe rust severity in the cultivar Oligoculm near marker locus Xwmc415 (Suenaga et al. 2003), QYr.inra-5BL.1 and QYr.inra-5BL.2 in the French cultivar Camp Remy within the marker interval Xgwm499-Xgwm639 (Mallard et al. 2005), QYr.caas-5BL.1 in Strampelli within the interval Xwmc415–Xwmc537 and QYr.caas-5BL.2 in Libellula within the interval Xbarc142–Xgwm604 (Lu et al. 2009). They are all adult-plant resistance QTLs for stripe rust. However, most adult-plant resistance might be classified as HTAP resistance because temperatures become higher in the most of wheat-growing areas in the world during the growing season. HTAP resistance is related to both temperature conditions and to the growth-stage of the wheat, whereas the seedling temperature-sensitive resistance is only related to the former. Therefore, although the QTLs, QYr-tem-5B.1 and QYr-tem-5B.2, identified in this study were mapped on the same chromosome as those of the adult-plant resistance QTLs for Pst reported previously, they had different characteristic. QYr-tem-5BL.1 and QYr-tem-5BL.2 from Flinor are likely different from previously reported QTLs for controlling temperature-sensitive resistance to stripe (yellow) rust at any growth-stages.
Comparisons should be made between the quantitative resistance loci controlling host plant resistance to stripe (yellow) rust that have been detected from difference cultivars, no matter whether the resistance was expressed differentially with temperature variation at seedling or adult stage. QTLs have been detected on wheat chromosome 5B in Libellula, Strampelli, Camp Remy, Oligoculm (the pedigree is unknown), Druchamp and Flinor for stripe (yellow) rust resistance. Moreover, pedigree analyses did show that most of these resistance donors share common ancestors (Fig. 3). For example, Camp Remy and Druchamp share common ancestor Vilmorin 27 and its ancestors including Japhet and Parsel that in turn are shared with Flinor. Camp Remy and Flinor share common ancestor Vilmorin 23, and Libellula shares with Stampelli ancestors such as Ardito and Reiti; whereas Hatif Inersable is included in the pedigrees of Flinor, Camp Remy, Druchamp and Libellula. Hatif Inersable is an intersection, which maybe an important resistance resource that could be referred to as a nucleus resistance resource.
It is feasible to use durably resistant cultivars as resources to develop new varieties with durable resistance. HTAP resistance is only active during the adult-plant growth-stage, leaving seedlings vulnerable to infection unless adequate seedling-stage resistance genes (some of which are expresses throughout the crop life cycle) also are present. As seedling temperature-sensitive resistance exists in wheat, it will also be expressed in the adult-plant growth-stages only when the temperatures gradually rise during plant development. Because these genes could become more effective during the late growth-stages in the field, they can be expected to prove useful for controlling wheat stripe (yellow) rust. Deployment of both all stage or stage-specific and temperature-sensitive resistance genes into a single cultivar might provide a more effective and durable form of stripe (yellow) rust resistance. Combination of resistance QTL alleles from multiple resistance loci can be expected to improve the overall resistance levels, or at least contribute to enhancing durability of resistance deployed in commercial cultivars.
References
Bariana HS, McIntosh RA (1995) Genetics of adult-plant stripe rust resistance in four Australian wheats and the French cultivar ‘Hybride-de-Bersee’. Plant Breed 114:485–491
Bariana HS, Hayden MJ, Ahmed NU, Bell JA, Sharp PJ, McIntosh RA (2001) Mapping of durable adult-plant and seedling resistances to stripe rust and stem rust diseases in wheat. Aust J Agric Res 52:1247–1255
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Basten JC, Wang S, Gaffney P, Zeng ZB (2003) Windows QTL Cartographer, version 2.0, Statistical Genetics. North Carolina State University, Raleigh, N.C, U.S.A
Brown JF, Sharp EL (1969) Interactions of minor host genes for resistance to Puccinia striiformis with changing temperature regimes. Phytopathology 59:999–1001
Carter A, Chen XM, Garland-Campbell K, Kidwell KK (2009) Identifying QTL for high-temperature adult-plant resistance to stripe rust (Puccinia striiformis f. sp. tritici) in the spring wheat (Triticum aestivum L.) cultivar ‘Louise’. Theor Appl Genet 119:1119–1128
Chen XM (2005) Epidemiology and control of stripe rust on wheat. Can J Plant Pathol 27:314–337
Chen XM, Jones SS, Line RF (1995) Chromosomal location of genes for stripe rust resistance in spring wheat cultivars Compair, Fielder, Lee, and Lemhi and interactions of an euploid wheat with races of Puccinia striiformsi. Phytopathology 85:375–381
Chen XM, Jones SS, Line RF (1996) Chromosomal location of genes for resistance to Puccinia striiformis in seven wheat cultivars with resistance genes at the Yr3 and Yr4 loci. Phytopathology 86:1228–1233
Gerechter-Amitai ZK, Van Silfhout CH (1989) Race-specificity of temperature-sensitive genes for resistance to Puccinia striiformis in Triticum dicoccoides. Euphytica 43:7–14
Johnson R (1984) A critical analysis of durable resistance. Annu Rev Phytopathol 22:309–330
Johnson R (1988) Durable resistance to yellow (stripe) rust in wheat and its implications in plant breeding. In: Simmonds NW, Rajaram S (eds) Breeding strategies for resistance to the rusts of wheat. CIMMYT, Mexico, pp 63–75
Johnson R, Law CN (1975) Genetic control of durable resistance to yellow rust (Puccinia striiformis) in the wheat cultivar Hybride de Bersee. Ann Appl Biol 81:385–391
Kaul K, Shaner G (1989) Effect of temperature on adult-plant resistance to leaf rust in wheat. Phytopathology 79:391–394
Krupinsky JM, Sharp EL (1978) Additive resistance in wheat to Puccinia striiformis. Phytopathology 68:1795–1799
Krupinsky JM, Sharp EL (1979) Reselection for improved resistance to wheat stripe rust. Phytopathology 69:400–404
Lin F, Chen XM (2007) Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theor Appl Genet 114:1277–1287
Lin F, Chen XM (2009) Quantitative trait loci for non-race-specific, high-temperature adult-plant resistance to stripe rust in wheat cultivar Express. Theor Appl Genet 118:631–642
Line RF (2002) Stripe rust of wheat and barley in North America: a retrospective historical review. Annu Rev Phytopathol 40:75–118
Line RF, Qayoum A (1992) Virulence aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America, 1968–87. U. S. Dep. Agric. Tech. Bull. No. 1788
Lu Y, Lan C, Liang S, Zhou X, Liu D, Zhou G, Lu Q, Jing J, Wang M, Xia X, He Z (2009) QTL mapping for adult-plant resistance to stripe rust in Italian common wheat cultivars Libellula and Strampelli. Theor Appl Genet 119:1349–1359
Mallard S, Gaudet D, Aldeia A, Abelard C, Besnard AL, Sourdille P, Dedryver F (2005) Genetic analysis of durable resistance to yellow rust in bread wheat. Theor Appl Genet 110:1401–1409
Milus EA, Line RF (1986a) Gene action for inheritance of durable, high-temperature, adult-plant resistance to stripe rust in wheat. Phytopathology 76:425–441
Milus EA, Line RF (1986b) Number of genes controlling high-temperature, adult-plant resistance to stripe rust in wheat. Phytopathology 76:93–96
Navabi A, Tewari JP, Singh RP, McCallum B, Laroche A, Briggs KG (2005) Inheritance and QTL analysis of durable resistance to stripe and leaf rusts in an Australian cultivar, Triticum aestivum ‘Cook’. Genome 48:97–106
Park RF, Ash GJ, Rees RG (1992) Effects of temperature on the response of some Australian wheat cultivars to Puccinia striiformis f.sp. tritici. Mycol Res 3:166–170
Qayoum A, Line RF (1985) High-temperature, adult-plant resistance to stripe rust of wheat. Phytopathology 75:1121–1125
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Santra DK, Chen XM, Santra M, Campbell KG, Kidwell KK (2008) Identification and mapping QTL for high-temperature adult-plant resistance to stripe rust in winter wheat (Triticum aestivum L.) cultivar Stephens. Theor Appl Genet 117:793–802
Sharp EL, Fuchs E (1982) Additive genes in wheat for resistance to stripe (yellow) rust (Puccinia striiformis Westend.). Crop Prot 1:182–189
Singh RP, Huerta Espino J, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk J Agric For 29:121–127
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Stubbs RW (1988) Pathogenicity analysis of yellow (stripe) rust of wheat and its significance in a global context. In: Simmonds NW, Rajaram S (eds) Breeding strategies for resistance to the rusts of wheat. CIMMYT, Mexico, pp 23–38
Suenaga K, Singh RP, Huerta-Espino J, William HM (2003) Microsatellite markers for genes Lr34/Yr18 and other quantitative trait loci for leaf and stripe rust resistance in bread wheat. Phytopathology 93:881–890
Tong S, Lin R, He Y, Xu S (2006) Genetic analysis of major and minor gene(s) resistant to stripe rust in resource wheat cultivars Holdfast and Flinor. Scientia Agricultura Sinica 39:2243–2249
Uauy C, Brevis JC, Chen XM, Khan I, Jackson L, Chicaiza O, Distelfeld A, Fahima T, Dubcovsky J (2005) High-temperature adult-plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor Appl Genet 112(1):97–105
Wan A, Zhao Z, Chen X, He Z, Jin S, Jia Q, Yao G, Yang J, Wang B, Li G, Bi Y, Yuan Z (2004) Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Dis 88:896–904
Wang S, Basten CJ, Zeng ZB (2004) Windows QTL Cartographer v2.0. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Acknowledgments
This research was supported by China Postdoctoral Science Foundation (No. 20080430052 and 200902156), the National Basic Research and Development Program (2011CB100403), and the National Natural Science Fund for Young Scholars of China (30700521). The authors are grateful to the various cooperators for supplying rust isolates and cultivars.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, J., Zuo, L.L., Zhang, Z.Y. et al. Quantitative trait loci for temperature-sensitive resistance to Puccinia striiformis f. sp. tritici in wheat cultivar Flinor. Euphytica 178, 321–329 (2011). https://doi.org/10.1007/s10681-010-0291-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0291-z