Abstract
The ability to detect quantitative trait loci (QTLs) in a bi-allelic population is often limited. The power of QTL detection and identification of the most beneficial allele at each QTL could be greatly improved by comparing QTLs among different populations derived from connecting multi-parents. In this study, three sets of connected recombinant inbred lines (RILs) derived from the crosses between Zhenshan 97 and Minghui 63 (PZM), Zhenshan 97 and Teqing (PZT), and Minghui 63 and Teqing (PMT), respectively, were used. QTL analyses for the number of spikelets per panicle (SPP) and 1,000-grain weight (TGW) were performed in PZT, and five SPP QTLs on chromosomes 1, 6, and 7 and two TGW QTLs on chromosome 1 were detected. QTL for SPP was also identified in PMT, and six QTLs were detected on chromosomes 1, 2, 3, 6, and 7 in this population. In an earlier study, we identified five SPP QTLs and four TGW QTLs in PMT and nine TGW QTLs in PZM. Comparison of the QTL mapping results of these two studies showed that one QTL was common to the three populations, 11 QTLs were detected in two populations, and six QTLs were found in only one population. Comparison of genetic effect and the action direction of the QTLs detected in the three populations showed that additive effects of QTLs estimated in different populations were also expressed additively among three parental alleles. Additive effects of SPP7a estimated in three near-isogenic line F2 populations supported this finding. Based on these results, we suggest that pyramiding the most beneficial alleles among the three parents could efficiently improve rice yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex traits, such as rice yield and its components, are inherited in a quantitative manner and are typically controlled by a number of major and minor quantitative trait loci (QTLs). A good understanding of the genetic bases of yield traits is valuable for designing breeding programs aimed at the genetic improvement of quantitative traits of rice. Direct analysis of yield is cumbersome and has low efficiency, mainly because of the influence of a wide range of processes and factors involved in the life cycle of rice. Alternatively, yield components, such as 1,000-grain weight (TGW) and the number of spikelets per panicle (SPP), contribute directly to grain yield and exhibit a higher heritability than yield itself. Therefore, it is more feasible to focus on the components of yield rather than on yield as a whole, which is well illustrated by the fact that rice breeding programs designed for different eco-regions have uniquely favored combinations of yield components, with an emphasis on one or two components.
With the development of the molecular marker and QTL analysis approaches during the last two decades, substantial attention has been paid to dissecting the genetic basis of SPP and TGW (Paterson et al. 1988; Lander and Botstein 1989; Zeng 1994); as a result of such studies, a large number of QTLs have been reported (Yu et al. 1997, 2002; Zhuang et al. 1997; Septiningsih et al. 2003). To date, the results indicate that the trait values of TGW and SPP are affected simultaneously by QTLs, epistasis, and genotype × environment (G × E) interactions (Li et al. 1997; Xing et al. 2002).
QTL detection is generally based on natural allelic differences between parental lines. It is expected that when a bi-parent population is used to map QTLs, only a limited number of QTLs will be detected because the genes will present limited information about polymorphism content. An extra population derived from diverse parental materials will increase the chance of a QTL being polymorphic and, therefore, will glean systemic information pertaining to a trait-controlling QTL among different populations. Using this method, previous investigators have analyzed QTL mapping results jointly in the genetic background of independent populations (unknown pedigree relationship among the parents of different populations) (Muranty 1996; Xu 1998). Under this condition, the QTL effects are nested (in the statistical sense) within populations, and the estimated number of parameters increases with the increasing number of populations incorporated. However, global comparison of the effects of the segregation of all QTL alleles is not possible due to a lack of connection between populations. Developing connected populations (one common parent among populations) is an alternative approach in which identical allelic effects from the same QTL over populations, rather than nesting effects within populations, reduce the total number of parameters and, consequently, increase the power of QTL detection (Rebai and Goffinet 1993; Jannink and Jansen 2001; Blanc et al. 2006). In addition, the effects of segregating alleles are estimated simultaneously by executing a global comparison among several populations, which is a decidedly favorable method of exploring the most beneficial allele for improvement of several rice varieties. It has been suggested that beneficial alleles for improving a crop variety can be identified in superior and inferior varieties or wild relatives (Paterson et al. 1991; Xiong et al. 1999; Tian et al. 2006; Liu et al. 2009). By evaluating the effects and the action direction of the same QTL among several populations developed from parents with a distinct genetic background, the most beneficial alleles can be chosen for crop improvement. Therefore, it would appear that developing connected populations is an efficient and effective method for using germplasm with a distinct genetic background.
In the study reported here, three sets of recombinant inbred lines (RILs) were developed from crosses between Zhenshan 97 and Minghui 63 (PZM), Zhenshan 97 and Teqing (PZT), and Minghui 63 and Teqing (PMT), respectively. Teqing was an elite cultivar in China in the 1970s, while Zhenshan 97 and Minghui 63 are parents of the top rice hybrid Shanyou 63. These three cultivars form the fundamental rice varieties in China. QTL analysis for SPP and TGW was conducted in PZT and for SPP in PZM. The objectives of combining the QTL results of the PZT population with those of SPP and TGW in the PZM and PMT population (Xing et al. 2002; Liu et al. 2010) were to (1) uncover how many QTLs for SPP and TGW could be detected in the three populations, (2) evaluate the relationship of QTL additive effects detected in different populations, and (3) determine the most beneficial alleles from these three varieties.
Materials and methods
Experimental populations and phenotypic measurements
The PZM and PMT populations and their planting methods have been described by Xing et al. (2002) and Liu et al. (2010). The PZT population used in this study consists of 190 F7 RILs derived from a single-seed descendent of the cross between Zhenshan 97 and Teqing. For the field test, the seeds were sown in a seedling bed in May 2004 and 2006. The RILs (F7 and F8) and two parents were transplanted to a bird-net-equipped field in the experimental farm of Huazhong Agricultural University in Wuhan, China, in the 2004 and 2006 rice growing seasons. Field experiments were carried out following a randomized complete block design with two replicates. Fourteen seedlings (approximately 25 days old) for each line were transplanted into a two-row plot, with a between-plant distance of 16.5 cm within a row and 26.4 cm between rows. The ten plants in the middle of the two rows of each plot were harvested individually to score the following traits: (1) the number of SPP, calculated as the total number of spikelets from the entire plant divided by the number of tillers; (2) the TGW (g), calculated as the yield per plant divided by the number of spikelets multiplied by 1,000. Trait measurements averaged over the two replications within each year were used as the raw data in the analyses.
Two sets of near-isogenic line (NIL) F2 populations of SPP7a were obtained by consecutively backcrossing Zhenshan 97, with Minghui 63 and Teqing as donor parents, respectively (Xing et al. 2008; Xue et al. 2008). For easy description, the two NIL-F2 populations, hereafter, were named after NIL-F2 (ZM) and NIL F2 (ZT), respectively. Minghui 63 homozygotes in NIL-F2 (ZM) and Teqing homozygotes in NIL-F2 (ZT) were screened to cross each other and followed one selfing for producing NIL-F2 (TM). In total, 190 plants of each NIL-F2 population were grown in the same field as the RIL populations in the 2007 rice growing season. The number of SPP was measured individually.
DNA markers and assays
A total of 694 simple sequence repeat (SSR) markers that were well distributed in the whole genome were chosen to screen polymorphism between Zhenshan 97 and Teqing, according to the map developed by Temnykh et al. (2000, 2001) and the rice genome sequences of the Monsanto Company (McCouch et al. 2002). Polymorphic SSR markers between Zhenshan 97 and Teqing that evenly covered all 12 chromosomes were chosen for genotyping the PZT population. The SSR assay was carried out essentially as described by Wu and Tanksley (1993).
Data analyses
The genetic linkage map was constructed with the use of the computer software program MAPMAKER/EXP 3.0 (Lincoln et al. 1993). The Kosambi function was used to calculate genetic distance. Composite interval mapping was performed with the use of Windows QTL CARTOGRAPHER 2.0 (Wang et al. 2001–2003). Window size was set at 10 cM. Forward stepwise regression was used to find significant markers as cofactors. The significance level of the experiment-wise limit of detection (LOD) threshold was determined by computing 1,000 permutations (P < 0.05) as implemented by Windows QTL CARTOGRAPHER. These permutations can account for non-normality in marker distribution and trait values. The threshold of LOD values ranged from 2.4 to 2.7.
Heritability for the traits SPP and TGW was estimated in RILs using the formula:
where σ 2g , σ 2ge , and σ 2e were the estimates of genetic, G × E, and error variances derived from the mean square expectations of the analysis of variance, with n = 2 being the number of environments and r = 2 being the number of replicates. Expected genotypic variance and expected G × E interaction variance were estimated as the model in Table 1.
Comparative QTL analysis
Combining the QTL results of PZM for TGW (Xing et al. 2002) and PMT for SPP and TGW (Liu et al. 2010) with the QTLs identified in this study, we then compared the QTLs. The comparative method is as follows: (1) the physical locations of flank markers of QTLs were ascertained from the database (www.gramene.org); (2) the QTLs identified in different populations for the same trait were considered to be the same if their 1 – LOD confidential intervals overlapped each other; (3) the allele with the largest increasing effects at a given QTL was considered to be the beneficial allele among three parents.
Results
Variation of the traits
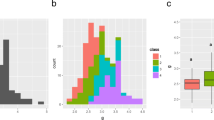
Teqing had an average of approximately 200 SPP in three environments, which had the largest panicle among the three parents. Zhenshan 97 had the smallest panicle, with an average of fewer than 115 spikelets under four environments. Minghui 63 had an intermediate panicle, with an average of approximately 140 spikelets. With respect to TGW, Minghui 63 had the largest TGW, about 25–28 g, whereas Teqing and Zhenshan 97 had equivalent TGW values, with an average weight of about 22 g (Table 2). Although the PZT population was derived from the two parents with the largest difference in SPP and also exhibited the largest SPP variation, this population has the smallest variation in TGW (Fig. 1). The mean TGW of the three connected populations is approximately equal to their mid-parent values. Transgressive segregation for SPP was observed in PZM, but not in PZT and PMT. However, TGW in three populations expressed transgressive segregation in both directions in all investigated environments. Among the three populations, significant negative correlations were observed between SPP and TGW (Table 3); TGW and SPP showed a high heritability of more than 80% in all three populations (Table 4).
Genetic linkage map
A total of 694 SSR markers were polymorphic between Zhenshan 97 and Teqing, and the polymorphism rates between the parents were 32.8%. We chose 176 genome-wide evenly distributed SSR markers to develop the genetic map. The genetic map spans 1432.1 cM, and the average genetic distance between neighboring markers is 8.1 cM.
QTL analysis
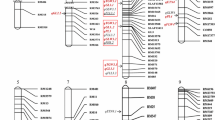
In PZT, five SPP QTLs (SPP1, SPP6a, SPP6b, SPP7a, and SPP7b) were detected on chromosomes 1, 6 (two QTLs), and 7 (two QTLs) (Fig. 2); the Teqing alleles of the five QTLs increased SPP. SPP6a and SPP6b were detected in only 1 year. Two TGW QTLs (TGW1a and TGW1b) were detected on chromosome 1. The alleles with improvement effects came from Zhenshan 97 and Teqing, respectively (Table 5). Four of these seven QTLs were detected in both environments (Table 5).
Utilizing the same data of PZM used in the report by Xing et al. (2002), six SPP QTLs (SPP1, SPP2, SPP3a, SPP6a, SPP6b, and SPP7a) were detected on chromosomes 1, 2, 3, 6 (two QTLs), and 7, respectively. With the except of SPP2 and SPP3a, Minghui 63 alleles increased the SPP at all QTLs (Table 6). Only SPP1 and SPP7a were identified in both years (Table 6).
QTL comparison across three connected populations
For the convenience of comparisons, QTLs detected in this study, together with QTLs detected in PZM (Xing et al. 2002) and in PMT (Liu et al. 2010) were commonly incorporated into Table 6. A total of nine SPP QTLs and nine TGW QTLs were detected in several environments. Remarkably, SPP1 was commonly detected among the three populations in five different environments. This QTL explained about 25% of the SPP variation in PZT, and its additive effects in 2 years were the largest, namely, double the estimates in PMT and PZM. SPP3a, TGW3a, TGW3b, TGW5, and TGW9 were commonly detected in PMT and PZM; SPP6a, SPP6b, SPP7a, TGW1a, and TGW1b were commonly detected in PZT and PZM. SPP7b was commonly detected in PZT and PMT. For all the QTLs commonly detected in two populations, their estimated additive effects in the two populations were not very different. SPP3b and SPP8 were repeatedly detected in only PMT and showed considerable effects. SPP2, TGW1c, TGW6, and TGW11 were detected only once in PZM, with small additive effects. Interestingly, all nine QTLs for TGW were detected in PZM.
Genetic effects of SPP7a estimated in three NIL-F2 populations
In the NIL-F2 (ZM) and NIL-F2 (ZT) populations, SPP7a exhibited a major QTL with a large LOD score of more than 48 and explained a large part of the trait variation. In the NIL-F2 (ZM) and NIL-F2 (ZT) populations, the additive effect was 43.6 and 49.1, respectively (Table 7). However, the in NIL-F2(TM) population, no QTL was detected at the level of the LOD threshold of 2.4. If the LOD threshold was decreased to 2.0, one QTL in the region was mapped with very small additive effect (3.5) and a small contribution (3.7%) to SPP variation.
The most beneficial allele in the three parents
SPP1 was commonly detected in three populations. The Teqing allele at SPP1 had positive effects in PZT and PMT, whereas the Minghui 63 allele increased SPP in PZM. Hence, the Teqing allele of SPP1 was the most beneficial, the Zhenshan 97 allele was the most inferior, and the Minghui 63 allele was intermediate. SPP3a, TGW3a, TGW3b, TGW5, and TGW9 were commonly detected in PMT and PZM. At TGW3a and TGW3b, Minghui 63 alleles expressed positive effects in both populations, indicating that Minghui 63 alleles are beneficial. With regard to SPP3a, TGW5, and TGW9, both Zhenshan 97 and Teqing alleles had positive effects against the Minghui 63 allele. Therefore, it is likely that the Zhenshan 97 allele of TGW5 and the Teqing allele of TGW9 were the most beneficial alleles according to a comparison of the additive effects detected in the two populations. The most beneficial allele at SPP3a was difficult to determine due to an equivalent effect estimated in the two populations. SPP6a, SPP6b, SPP7a, TGW1a, and TGW1b were commonly detected in PZT and PZM, but not in PMT. Similarly, Zhenshan 97 carried the most beneficial TGW1a allele because it exhibited positive effects in the two populations. With respect to SPP6a, SPP6b, SPP7a, and TGW1b, Teqing alleles increased trait values in PZT, whereas the Minghui 63 alleles increased trait values in PZM. Comparison of the size of additive effects estimated in the two populations showed that the Teqing SPP6a allele and Minghui 63 SPP6b and TGW1b alleles are likely to be the most beneficial. However, for SPP7a it was still difficult to decide which allele was the most beneficial. SPP7b were commonly detected in PZT and PMT. The Teqing allele of SPP7b expressed positive effects in both populations, indicating that Teqing alleles are beneficial in this locus.
Discussion
Comparison of QTLs across connected populations
Although an additive effect is heritable across generations (Garant et al. 2003), very few studies have reported on QTLs across connected populations (Joseph and Gebisa 2008). One of the more important results of this study is that additive effects could also be accumulative across connected populations. Three populations in this study were not grown simultaneously in the same environments because of the lack of space for this exceedingly large-scale field experiment and arduous management control. As compensation, all experiments were conducted at the same spot and on similar dates, with the same field management. Great precautions were taken to eliminate, as much as possible, the potential confounding effects of the environment. The additive effects of the QTLs estimated in the different populations were also additively expressed. For example, the Teqing SPP1 allele was the best among the three parents. Additive effects for SPP in PZT were 16.5 and 15.4, which are almost equal to the sum of 8.6 and 8.3 in PMT, and 8.7 and 10.3 in PZM, regardless of environment effects. If the additive effects estimated in the connected populations were expressed additively, the QTL (existence and effect) in the third population could be predicted according to the situations in the two previous populations. If SPP3a is taken as an example, the Teqing allele increased SPP by 8.5/6.6 compared with the Minghui 63 allele in PMT; similarly, the Zhenshan 97 allele increased SPP by 8.4 compared with the Minghui 63 allele. Compared with the Zhenshan 97 SPP3a allele, the Teqing allele could possibly increase or decrease the number of spikelets by no more than two, which is too small a difference to be significantly detected in PZT. We were able to prove that no SPP QTL was detected in PZT. With respect to all 11 QTLs detected in two populations, potential additive effects were too small to be detected in the third population without any exception. However, if the statistically significant level was decreased, QTLs with an LOD value of <1.5 would occur at some loci in the third population (data not shown). Alternatively, phenotypic data of the three populations collected from the same environment would provide direct comparison of QTL effects and provide us with more reliable results.
The genetic effects of SPP7a estimated in three NIL-F2 populations confirmed the finding that additive effects of QTLs estimated in different populations were also expressed additively (Table 7). The three NIL-F2 populations shared the genetic background of Zhenshan 97 with different allele segregations of SPP7a, thus the genetic background noise was minimized; meanwhile, measurements were made at the same location and same year, thereby blocking the environmental effect. In this case, SPP7a clearly expressed additive accumulative effects across the three NIL-F2 populations, namely, 49.1, 43.6, and 3.5, respectively. The Teqing allele showed the largest effect; in contrast, the Zhenshan 97 allele showed the least effect. These results are in agreement with the results from the three RIL populations. The additive effect of 49.1 in NIL-F2 (ZT) was approximate to the sum of those (43.6 and 3.5) in the other two populations. SPP7a was not a major QTL in the two RIL populations of PZM and PZT, but its additive effects was greatly enhanced in the corresponding NIL-F2 populations. Even in this case, statistically, SPP7a was not easily detected in NIL-F2(TM). Hence, the finding in the RIL populations was convincible.
Ascertainment of beneficial allele
Hundreds of rice QTLs for SPP and TGW have been reported during the last 20 years (www.gramene.org). Among these, Yoon et al. (2006) identified five SPP QTLs on chromosomes 2, 3, 4, 6, and 11 and two TGW QTL on chromosomes 2 and 11, Kwon et al. (2008) identified three SPP QTLs on chromosomes 3, 10, and 11, Yoshida et al. (2002) detected six TGW QTLs on chromosomes 2, 3, 4, 4, 5, and 11, and Redoña and Mackill (1998) detected two TGW QTLs on chromosome 4 and 8. Some of the QTLs identified in our study share a similar or same interval with those identified in these previous studies. However, because of the low mapping resolution in primary populations, such as F2, RILs, and doubled haploids, it is still far from ideal to claim a relationship even the QTLs are located in similar or the same regions in different populations. It is difficult to distinguish the most beneficial allele among multiple alleles using the QTL mapping method, which takes advantage of only bi-allele variations. Alternative association studies based on linkage disequilibrium (LD) have been applied in plant genetics to tackle complex traits (Buckler and Thornsberry 2002; Nordborg and Tavare 2002). Analysis based on LD has been suggested to be more powerful than the traditional QTL method based on linkage analysis in terms of identifying the most beneficial alleles (Flint-Garcia et al. 2003).
In our study, we derived three sets of connected RIL populations (PZM, PZT, and PMT) from crosses between Zhenshan 97 and Minghui 63, Zhenshan 97 and Teqing, and Minghui 63 and Teqing, respectively, which provided us with the opportunity to directly compare additive effects among three alleles. A total of 18 QTLs were detected in these three populations in five environments. The most beneficial alleles at all 18 QTLs were identified among these three parents by comparing QTL effects and the directions of their action (Table 6). Pyramiding of the most beneficial alleles could theoretically result in the development of a high-yield variety. For example, Minghui 63, an elite restorer for the three-line hybrid system in China, can increase the SPP to 80–100 spikelets if its alleles are substituted with positive TQ alleles at SPP1, SPP3a, SPP3b, SPP6a, SPP7b, and SPP8. Again, if we use Zhenshan 97 alleles of TGW1a, TGW1c, TGW5, and TGW9 to substitute the Minghui 63 alleles, theoretically the TGW of the modified Minghui 63 variety will be increased by 5 g, resulting in a new Minghui 63 variety with a TGW of up to 31–33 g.
The characteristic of transgressive segregation in three populations
An important finding in this study is that the results of our analysis of transgressive segregation are in agreement with the pattern of positive QTL distribution in parents. The accumulation of positive additive effects and an overdominant effect are regarded as the genetic bases of transgressive segregation (deVicente and Tanksley 1993). In this study, transgressive segregation of SPP and TGW in PZM and of TGW in PZT and PMT was observed. The RILs in these three populations are genome-wide homozygous; thus, overdominance does not underlie the transgressive segregation phenomena. However, positive alleles for SPP and TGW were identified in both inferior and superior parents in PZM, and for TGW in PZT and PMT. Recombination of these positive or negative alleles can produce better or worse genotypes, which can explain why some lines exhibit better or worse performance than their parents. In contrast, no transgressive segregation was observed for SPP in PZT and PMT. This finding is in agreement with the result that all positive alleles of the detected SPP QTLs originated from the superior parent Teqing.
References
Blanc G, Charcosset A, Mangin B, Gallais A, Moreau L (2006) Connected populations for detecting quantitative trait loci and testing for epistasis: an application in maize. Theor Appl Genet 113:206–224
Buckler ES, Thornsberry JM (2002) Plant molecular diversity and applications to genomics. Curr Opin Plant Biol 5:107–111
deVicente MC, Tanksley SD (1993) QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics 134:585–596
Flint-Garcia SA, Thornsberry JM, Buckler ES IV (2003) Structure of linkage disequilibrium in plants. Annu Rev Plant Biol 54:357–374
Garant D, Dodson JJ, Bernatchez L (2003) Differential reproductive success and heritability of alternative reproductive tactics in wild Atlantic salmon (Salmo salar L.). Evol Int J Org Evol 57:1133–1141
Jannink JL, Jansen R (2001) Mapping epistatic quantitative trait loci with one-dimensional genome searches. Genetics 157:445–454
Joseph K, Gebisa E (2008) Marker-assisted selection for early-season cold tolerance in sorghum: QTL validation across populations and environments. Theor Appl Genet 116:541–553
Kwon SJ, Cho YC, Kwon SW, Oh CS, Shu JP, Shin YS, Kim YG, Holligan D, Wessler SR, Hwang HG, Ahn SN (2008) QTL mapping of agronomic traits using RIL population derived from a cross between temperate japonica cultivars in rice (Oryza sativa L.). Breed Sci 58:271–279
Lander ES, Botstein D (1989) Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Li ZK, Pinson SRM, Park WD, Paterson AH, Stansel JW (1997) Epistasis for three grain yield components in rice Oryza sativa L. Genetics 145:453–465
Lincoln SE, Daly MJ, Lander ES (1993) Mapping genes controlling quantitative traits with MAPMAKER/QTL1.1: a tutorial and reference manual, 2nd edn. Whitehead Institute Technical Report. Whitehead Institute, Cambridge
Liu T, Shao D, Kovi M, Xing Y (2010) Mapping and validation of QTL for spikelets per panicle and 1,000-grain weight in rice (Oryza sativa L.). Theor Appl Genet 120:933–942
Liu T, Mao D, Zhang S, Xu C, Xing Y (2009) Fine mapping SPP1, a QTL controlling the number of spikelets per panicle, to a BAC clone in rice (Oryza sativa). Theor Appl Genet 118:1509–1517
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2,240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Muranty H (1996) Power of tests for quantitative trait loci detection using full-sib families in different schemes. Heredity 76:156–165
Nordborg M, Tavare S (2002) Linkage disequilibrium: what history has to tell us. Trends Genet 18:83–90
Paterson AH, Lander ES, Had JD, Patrson S, Lincoln SE, Tanksley SD (1988) Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 335:721–726
Paterson AH, Damon S, Hewitt JD, Zamir D, Rabinowitch HD, Lincoln SE, Lander ES, Tanksley SD (1991) Mendelian factors underlying quantitative traits in tomato: comparison across species, generations, and environments. Genetics 127:181–197
Rebai A, Goffinet B (1993) Power of tests for QTL detection using replicated progenies derived from a diallel cross. Theor Appl Genet 86:1014–1022
Redoña ED, Mackill DJ (1998) Quantitative trait locus analysis for rice panicle and grain characteristics. Theor Appl Genet 96:957–963
Septiningsih EM, Prasetiyono J, Lubis E, Tai TH, Tjubaryat T, Moeljopawiro S, McCouch SR (2003) Identification of quantitative trait loci for yield and yield components in an advanced backcross population derived from the Oryza sativa variety IR64 and the wild relative O. rufipogon. Theor Appl Genet 107:1419–1432
Temnykh S, Park WD, Ayres N, Cartihour S, Hauck N, Lipovich L, Cho YG, Ishii T, McCouch SR (2000) Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor Appl Genet 100:697–712
Temnykh S, Declerck G, Luashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Tian F, Li DJ, Fu Q, Zhu ZF, Fu YC, Wang XK, Sun CQ (2006) Construction of introgression lines carrying wild rice (Oryza rufipogon GriV.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor Appl Genet 112:570–580
Wang S, Basten CJ, Zeng ZB (2001–2003) Windows QTL CARTOGRAPHER 2.0. Department of Statistics, North Carolina State University, Raleigh. Available at: http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Wu KS, Tanksley SD (1993) Abundance, polymorphism and genetic mapping of microsatellites in rice. Mol Gen Genet 241:225–235
Xing YZ, Tan YF, Hua JP, Sun XL, Xu CG, Zhang Q (2002) Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor Appl Genet 105:248–257
Xing YZ, Tang WJ, Xue WY, Xu CG, Zhang Q (2008) Fine mapping of a major quantitative trait loci, qSSP7, controlling the number of spikelets per panicle as a single Mendelian factor in rice. Theor Appl Genet 116:789–796
Xiong L, Liu K, Dai X, Xu C, Zhang Q (1999) Identification of genetic factors controlling domestication-related traits of rice using an F2 population of a cross between Oryza sativa and O. rufipogon. Theor Appl Genet 98:243–251
Xu SZ (1998) Mapping quantitative trait loci using multiple families of line crosses. Genetics 148:517–524
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40:761–767
Yoon DB, Kang KH, Kim HJ, Ju HG, Kwon SJ, Suh JP, Jeong OY, Ahn SN (2006) Mapping quantitative trait loci for yield components and morphological traits in an advanced backcross population between Oryza grandiglumis and the O. sativa japonica cultivar Hwaseongbyeo. Theor Appl Genet 112:1052–1062
Yoshida S, Ikegami M, Kuze J, Sawada K, Hashimoto Z, Ishii T, Nakamura C, Kamijima O (2002) QTL analysis for plant and grain characters of sake-brewing rice using a doubled haploid population. Breed Sci 52:309–317
Yu SB, Li JX, Xu CG, Tan YF, Gao YJ, Li XH, Zhang QF (1997) Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 94:9226–9231
Yu SB, Li JX, Xu CG, Tan YF, Li XH, Zhang QF (2002) Identification of quantitative trait loci and epistatic interactions for plant height and heading date in rice. Theor Appl Genet 104:619–625
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zhuang JY, Lin HX, Lu J, Qian HR, Hittalmani S, Huang N, Zheng KL (1997) Analysis of QTL × environment interaction for yield components and plant height in rice. Theor Appl Genet 95:799–808
Acknowledgments
This work is partly supported by grants from the National Key Program on the Development of Basic Research, Program for New Century Excellent Talents in University (NCET-07-0348) and the National Natural Science Foundation of China (30830064, 30921091).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Zhang, Y., Xue, W. et al. Comparison of quantitative trait loci for 1,000-grain weight and spikelets per panicle across three connected rice populations. Euphytica 175, 383–394 (2010). https://doi.org/10.1007/s10681-010-0186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0186-z