Abstract
Simple sequence repeat motifs are abundant in plant genomes and are commonly used molecular markers in plant breeding. In tomato, currently available genetic maps possess a limited number of simple sequence repeat (SSR) markers that are not evenly distributed in the genome. This situation warrants the need for more SSRs in genomic regions lacking adequate markers. The objective of the study was to develop SSR markers pertaining to chromosome 6 from bacterial artificial chromosome (BAC) sequences available at Solanaceae Genomics Network. A total of 54 SSR primer pairs from 17 BAC clones on chromosome 6 were designed and validated. Polymorphism of these loci was evaluated in a panel of 16 genotypes comprising of Solanum lycopersicum and its wild relatives. Genetic diversity analysis based on these markers could distinguish genotypes at species level. Twenty-one SSR markers derived from 13 BAC clones were polymorphic between two closely related tomato accessions, West Virginia 700 and Hawaii 7996 and were mapped using a recombinant inbred line population derived from a cross between these two accessions. The markers were distributed throughout the chromosome spanning a total length of 117.6 cM following the order of the original BAC clones. A major QTL associated with resistance to bacterial wilt was mapped on chromosome 6 at similar location of the reported Bwr-6 locus. These chromosome 6-specific SSR markers developed in this study are useful tools for cultivar identification, genetic diversity analysis and genetic mapping in tomato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is one of the most widely grown and economically important vegetables in the world. Breeding to enhance tomato with higher yield, tolerance to biotic and abiotic stresses, and better nutritional quality is a continuous process that aims to meet the demands of producers and consumers. Breeding efficiency in tomato has been improved by using molecular markers to tag and transfer useful alleles from germplasm to elite cultivars (reviewed by Foolad 2007). However, there is a lack of sufficient polymorphic markers between closely related tomato species and within cultivars of the same species because the majority of molecular markers were developed based on polymorphisms between domesticated tomato and its wild relatives (Tanksley et al. 1992; Fulton et al. 2002; Frary et al. 2005). This poses difficulty in developing and saturating several species-specific linkage maps. For instance, the mapping populations derived from a cross between West Virginia 700 and Hawaii 7996 have been used to map quantitative trait loci (QTLs) associated with bacterial wilt and late blight, but the low number of polymorphic markers between these two parents has been an obstacle towards fine-mapping and marker-assisted selection (MAS) of these QTLs (Thoquet et al. 1996; Moreau et al. 1998; Wang et al. 2000).
Simple sequence repeat (SSR) markers are often the preferred molecular markers for the purpose of marker-assisted plant breeding when they are available, because the SSR markers possess properties suitable for high-throughput genotyping, such as high reproducibility, co-dominance nature, multi-allelic variation, simplistic assay, low distributing cost and easy automation (Edwards and McCouch 2007). The conventional method for SSR marker development involves construction of genomic libraries and screening them for repeat motifs (Zane et al. 2002). Areshchenkova and Ganal (1999, 2002) used this approach to develop 32 SSR markers for tomato with longer repeats. However, this approach is cumbersome and intensive in terms of cost, time and labor. Alternatively, DNA sequences deposited in public databases provide an easy and economical source for development of SSR markers (Morgante and Olivieri 1993). The tomato genome sequencing project has generated sequences of many bacterial artificial chromosome (BAC) clones which augments the existing genomic resources (Mueller et al. 2009). The search for repeat motifs in genomic sequences and expressed sequence tags (ESTs) of Solanaceae family available in the European Molecular Biology Laboratory (EMBL), GenBank and Solanaceae Genomics Network (SGN) databases enabled to rapidly produce a few hundred SSR markers in tomato (Smulders et al. 1997; He et al. 2003; Frary et al. 2005). Currently, a total of 404 tomato SSR markers have been developed and mapped. Information for 256 and 148 SSR markers is available at SGN and Vegmarks, respectively (http://www.sgn.cornell.edu; http://vegmarks.nivot.affrc.go.jp/). Nevertheless, these SSR markers are not distributed evenly throughout the 12 chromosomes, and tend to cluster around the centromeres (Areshchenkova and Ganal 2002; Ohyama et al. 2009). This phenomenon may be attributed to the fact that a large proportion of SSR markers developed from BAC end sequences were located predominantly in the heterochromatin regions. Most of these SSR markers have not been evaluated for their ability to detect genetic variation within cultivated and closely related tomato species. The number of polymorphic markers is expected to be less for closely related cultivated tomatoes.
The purpose of the present study was (1) to develop substantial SSR markers evenly distributed on chromosome 6; (2) to characterize their utility for genetic diversity assessment in tomato; and (3) to map resistance QTLs associated with bacterial wilt using a recombinant inbred population derived from a cross between two closely related tomatoes West Virginia 700 and Hawaii 7996. Chromosome 6 was chosen because only 15 unevenly distributed SSR markers are currently available on this chromosome. Moreover, a major QTL associated with bacterial wilt caused by Ralstonia solanacearum in Hawaii 7996 has been mapped on chromosome 6 (Thoquet et al. 1996; Wang et al. 2000; Carmeille et al. 2006). And several genes and QTLs conferring resistance to various biotic stresses such as bacterial canker (Sandbrink et al. 1995; van Heusden et al. 1999), powdery mildew (Bai et al. 2003), leaf mold (Jones et al. 1993), tomato yellow leaf curl virus (Zamir et al. 1994; Hanson et al. 2000) and root knot nematode (Ammiraju et al. 2003) were mapped to chromosome 6. Additional useful SSR markers on chromosome 6 would facilitate the transfer of these useful alleles in breeding processes.
Materials and methods
Plant materials
A panel of 16 accessions comprising of cultivated tomato and its wild relatives were used for genetic diversity analysis (Table 1). These accessions represented diverse origins and possessed useful horticultural traits such as resistance to biotic and abiotic stresses. Seeds of these accessions were obtained from Genetic Resources and Seed Unit, AVRDC—The World Vegetable Center (AVRDC), Taiwan. A mapping population comprising of 188 recombinant inbred lines (RILs) (F9) derived from a cross between West Virginia 700 (S. pimpinellifolium) and Hawaii 7996 (S. lycopersicum) (Carmeille et al. 2006) was used for genotyping and linkage map construction with SSR markers. Genomic DNA was isolated from fresh young leaves of RILs using GenElute™ plant genomic DNA miniprep kit (Sigma, USA) following the user’s instruction manual.
Database search, primer designing, and nomenclature
Eighteen BAC clones on chromosome 6, starting from zero to 101 cM and placed at a distance of approximately 5–10 cM from each other according to SGN were selected for the study. The selected BACs were searched for perfect di-, tri- and tetra nucleotide SSR motifs using simple sequence repeat identification tool downloadable from http://www.gramene.org/db/markers/ssrtool. A minimum number of eight repeat motifs was used as the search criterion. Sixty-one primer pairs were designed from sequences flanking the repeat motifs using PRIMER3.0 (http://www.-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). The parameters for primer designing included a product size of 100–300 bp, primer length of 20–25 bp, melting temperature of 54–64°C and a GC content of 40–60%. The primer nomenclature included a serial number with the prefix SLM6 (S. lycopersicum microsatellites of chromosome 6). The details of BAC clones, SSR motifs and the designed primers are listed in Table 2.
PCR amplification
PCR amplification of SSRs was performed in a PTC 200 DNA engine thermal cycler (MJ Research, USA). Each 15 μl reaction mixture consisted of 20 ng DNA, 0.3 μM of each forward and reverse primer (Invitrogen, USA), 200 μM of deoxyribonucleotides, 50 mM KCl, 10 mM Tris HCl (pH 8.3), 1.5 mM MgCl2, and 0.5 unit of hot start Taq DNA polymerase. The temperature profile used for PCR amplification included initial denaturation at 94°C for 10 min, 30 cycles of 94°C for 30 s, 50–55°C for 45 s, 72°C for 45 s, followed by a final extension at 72°C for 7 min. Annealing temperature was adjusted based on the specific requirement of each primer combination. PCR products (3 μl) were analyzed on 6% non-denaturing polyacrylamide gel in 0.5× TBE buffer. After electrophoresis, the gels were stained with 5 μg/ml ethidium bromide and the bands were visualized under UV light using the Alpha Imager 3300 system.
Estimation of polymorphism information content and genetic diversity analysis
Individual SSR alleles observed for the 16 genotypes were scored as present (1) or absent (0) to generate a binary data matrix. Using this data matrix, the polymorphism information content (PIC) of SSR markers and genetic similarity coefficients for genotypes were computed. The PIC value (Hn) for each SSR marker was calculated based on the formula, Hn = 1 − Σpi 2, where pi is the allele frequency for the ith allele (Nei 1973). A data matrix was assembled and analyzed using Populations v.1.2.30 (Langella, 1999; http://bioinformatics.org/~tryphon/populations/) and a pairwise distance matrix was generated based on total character difference. The genetic relatedness was analyzed using Unweighted Pair Group Method with Arithmetic Averages (UPGMA). Bootstrap analysis with 1,000 replicates was performed to obtain the confidence of branches of the UPGMA tree. The output dendrogram file was viewed and plotted by TreeView (Page 1996).
Linkage map construction
All the polymorphic SSR markers between West Virginia 700 and Hawaii 7996 were used to genotype the 188 RILs, and an approximate 1:1 ratio with 0.39% expected heterozygosity was tested using chi-square (χ2) goodness-of-fit analysis. Two publicly available SSR markers SSR47 and SSR350 which showed polymorphism in the mapping population were genotyped and used as anchors on chromosome 6. Linkage analysis was performed using Mapmaker/Exp ver. 3.0b with Kosambi mapping function (Kosambi 1944; Lander et al. 1987). The GROUP command was used to define the tightly linked markers at LOD ≥ 6.0 with genetic distance less than 2 cM. In each group of tightly linked markers, the marker with the smallest chi-square statistics for segregation distortion was selected to construct the framework of the linkage map. The ORDER command was used to define linear order of markers. The RIPPLE command was used to confirm marker order at LOD ≥ 3.0. The NEAR command was used to mark the most likely mapping position on the framework of the linkage map for the remaining unmapped SSR markers. The heterozygous genotypes in the mapping population were treated as missing value for the linkage analysis. The linkage map was drawn using the Adobe Illustrator CS2 (version 12.0).
Mapping resistance associated with bacterial wilt
The 188 RILs and the two parents were evaluated for their reactions against the R. solanacearum strain Pss4 (Phylotype I, race 1, and biovar 3) at seedling stage. The inoculation was conducted following methods described by Wang et al. (2000). Percentages of wilted plants were recorded 28 days after inoculation (dai). For QTL analysis, the percentage data were transformed using arcsin squareroot. QTL detection was conducted using composite interval mapping method with the Windows QTL Cartographer Version 2.5 (Wang et al. 2007). The empirical LOD threshold obtained was 2.7 by computing with permutation test (1,000 permutations; P = 0.01).
Results and discussions
Strategy to develop new SSR markers
Previously developed SSR markers mapped on tomato chromosome 6 were unevenly distributed (http://sgn.cornell.edu/) and showed low polymorphism between closely related tomato species (Frary et al. 2005). To circumvent the issue of uneven distribution, a set of fully sequenced BAC clones uniformly distributed over chromosome 6 were selected for developing new SSR markers. According to SGN, the International Tomato Genome Sequencing Project has released genomic sequences of 157 BAC clones which was equivalent to 56 percentage of euchromatic sequences on chromosome 6 (http://sgn.cornell.edu/). Eighteen BAC clones 5–10 cM apart on the genetic map of chromosome 6 were selected for marker development (Table 2). Among them, 15 BAC clones have been anchored based on RFLP and COS markers. In addition, from each BAC clone, all repeat sequence features with flanking regions amenable for primer designing, were used to develop new SSR markers, in order to ensure that at least one SSR marker from each chosen BAC clone detects polymorphism across closely related genotypes for the use of genetic mapping.
Occurrence of microsatellites in BAC clones
A total of 124 perfect di- and tri-nucleotide SSR motifs were identified from 18 BAC clones belonging to chromosome 6. The number of repeat motifs per BAC clone ranged from 1 to 16. Dinucleotide repeats were predominant followed by trinucleotide repeats. No perfect tetranucleotide repeats were identified. Among the dinucleotide repeats, AT repeat motifs were abundant, depicting a common feature of plant genome (Morgante and Olivieri 1993). Length criterion, represented by number of repeat motifs or nucleotides, is an important factor in the identification of SSRs. Earlier studies showed that adopting a longer length criterion greater than 10 repeat motifs or 20 nucleotides could result in higher level of polymorphism in human genome (Weber 1990). However, recent studies show that the level of polymorphism cannot be reliably predicted from length, emphasizing the utility of short SSRs (Sanwen et al. 2000). In the present study, a length criterion of a minimum of eight repeat motifs was applied to identify SSRs. This represents a lower level of stringency, especially for dinucleotide motifs; a lesser level of stringency has been adopted in other studies as well to minimize the loss of potential markers being identified. Smulders et al. (1997) used six repeat units to identify SSR markers in tomato. Kumpatla and Mukhopadhyay (2005) used a minimum of five repeat units to identify SSR markers in 55 dicotylednous species including tomato, and Portis et al. (2007) included mononucleotide motifs and seven dinucleotide repeats for SSR identification in pepper.
Out of the 124 SSRs identified using the length criterion of eight repeat motifs, 61 SSRs were selected for marker development. The rest were excluded because flanking sequences around these repeat motifs were not amenable for primer designing. The number of SSR primer pairs designed for each BAC clone ranged from 1 to 7. Out of 61 primer pairs screened, seven failed to produce any amplification product, which could be due to unsuitable primer sequences and/or improper PCR conditions resulting in undetectable amounts of amplified products. The remaining 54 primer pairs derived from 17 BAC clone sequences, produced clear banding patterns of DNA fragments with expected sizes. The only exception was SLM6-14, for which the genotype Hawaii 7996 produced a larger fragment size than expected, which could be due to insertions (Fig. 1). These 54 primer pairs were used for diversity estimation and genetic mapping.
Allelic distribution of five microsatellite loci across 16 tomato genotypes. M, 25-bp ladder markers; Lane 1, LA1579 (S. pimpinellifolium); 2, LA1940 (S. pennellii); 3, CLN2498E (S. lycopersicum); 4, Arka Meghali (S. lycopersicum); 5, CA4 (S. lycopersicum); 6, CLN1621L (S. lycopersicum); 7, FLA456 (S. lycopersicum); 8, CL5915-93-D4 (S. lycopersicum); 9, Hawaii 7996 (S. lycopersicum); 10, LA716 (S. pennellii); 11, West Virginia700 (S. pimpinellifolium); 12, M82 (S. lycopersicum); 13, LA1033 (S. habrochaites); 14, LA3708 (S. pimpinellifolium); 15, LA407 (S. habrochaites); 16, T4065 (S. lycopersicum)
Polymorphism of SSR markers
For each of the 54 SSR markers, number of alleles across 16 accessions ranged from 1 (SLM6-8, SLM6-9, SLM6-44, SLM6-60) to 10 (SLM6-5) with an average of 4.35 alleles per loci (Table 2). Examples for the allelic polymorphism are given in Fig. 1. When the estimation of allelic polymorphism was restricted within nine cultivated accessions, the average number of alleles for 54 SSR markers was 2.15 (Table 2). This number was close to the value reported previously (Frary et al. 2005).
Null allele was assigned whenever an amplification product could not be detected for a particular genotype-marker combination. Thirty SSR markers showed null alleles. The frequency of genotypes showing null allele ranged from a minimum of 1 (SLM6-4, SLM6-6, SLM6-23, SLM6-31 and SLM6-38) to a maximum of 7 (SLM6-35 and SLM6-40). Null alleles were mostly observed in S. pennelli and S. habrochaites accessions. Occurrence of null alleles could represent sequence diversity at the primer annealing sites. A higher frequency of null alleles in S. pennelli has been reported by Areshchenkova and Ganal (1999, 2002). Frequencies of null alleles were excluded in the calculation of PIC values for each SSR locus.
The PIC values reflected allele diversity and frequency among different accessions. A large variation in PIC values was observed for all the SSR loci tested. The average PIC value was 0.5 and it ranged from zero (SLM6-8, SLM6-9, SLM6-44, SLM6-60) to 0.83 (SLM6-14). Within the nine cultivated accessions, the average PIC value was 0.25 and it ranged from zero to 0.69. No clear relationship could be established between PIC values and the number of repeat units. For example, some shorter SSRs such as SLM6-57 with (TC)9 and SLM6-58 with (GA)8 had PIC values of 0.68 and 0.61 respectively, while some longer SSRs such as SLM6-32 with (AT)30 and SLM6-53 with (TA)25 had lower PIC values of 0.44 and 0.31 respectively. In a similar study by Smulders et al. (1997), involving seven S. lycopersicum cultivars and three wild species S. pennellii, S. peruvianum and S. habrochaites, no clear association of total repeat length with the degree of polymorphism could be deduced between species. This absence of correspondence between PIC and repeat length has been reported in arabidopsis (Bell and Ecker 1994), potato (Milbourne et al. 1998), pepper (Nagy et al. 1998; Sanwen et al. 2000) and common bean (Yu et al. 2000). In contrast, positive association between the number of repeat units and polymorphism has been observed among the genotypes of S. lycopersicum (Smulders et al. 1997; He et al. 2003, Frary et al. 2005). This may be due to a naturally higher frequency of polymorphism exhibited by longer microsatellites within a genetically homogenous group (Smulders et al. 1997).
Genetic diversity analysis
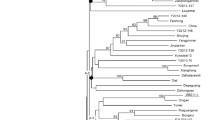
Similarity matrices constructed based on shared allele analysis revealed that the average genetic similarity between genotypes was 0.08 using chromosome 6 specific SSR markers. The dendrogram produced four distinct clusters, one cluster each for S. lycopersicum, S. pimpinellifolium, S. habrochaites and S. pennellii accessions (Fig. 2). However, West Virginia 700, despite being an accession of S. pimpinellifolium showed relatively lower polymorphism with S. lycopersicum compared to other S. pimpinellifolium accessions and was separated from the S. lycopersicum as well as S. pimpinellifolium genotypes in this study. This agrees with previous studies indicating the low polymorphism between West Virginia 700 and Hawaii 7996 (Thoquet et al. 1996; Wang et al. 2000). Our results suggest that SSR markers derived from a single chromosome were sufficient to discriminate the 16 genotypes at the species level. This is due to the rich genetic variability present in wild relatives that render SSR markers to be highly polymorphic at species level. However, within S. lycopersicum gene pool, the total genetic diversity is low and only 10% of the tested marker were polymorphic. Among the S. lycopersicum genotypes, the lines developed at AVRDC, e.g. CLN1621L, CLN2498E and CLN5915-93D4, shared high similarity and CLN1621L and CL5915-93D4 were closely related. This is anticipated as CL5915-93D4 is one of the parents of CLN1621L and also present in the genetic background of CLN2498E. And the introgression of Ty-2 (a gene derived from S. habrochaites) in CLN2498E could make the line genetically more distanced from the other two AVRDC lines. Because the pedigree details were not known for other cultivars, it was not possible to infer their genetic relationship. It was found that as few as 1–2 SSR markers developed in this study were enough to differentiate T4065, CA4, FLA456 and Hawaii 7996 from other S. lycopersicum genotypes. Bredemeijer et al. (1998) reported that as few as four SSR markers could differentiate 16 tomato cultivars. In another study by He et al. (2003), use of five SSR loci could effectively differentiate 19 tomato cultivars. Considering the low levels of polymorphism within cultivated tomato, these SSRs are useful in differentiating cultivars and in development of cultivar specific markers.
Linkage map of chromosome 6
Survey of polymorphism between West Virginia 700 and Hawaii 7996 resulted in the identification of 21 polymorphic markers from 54 newly developed SSR markers on chromosome 6. These 21 markers belonged to 13 BAC clones with one or more polymorphic markers representing each BAC clone. Including SSR47 and SSR350, only four out of 23 polymorphic SSR loci on chromosome 6, i.e. SSR47, SLM6-07, SLM6-14, SLM6-48, showed no significant segregation distortion in the F9 RILs derived from the cross between West Virginia 700 and Hawaii 7996. All the distorted markers showed skewness towards the Hawaii 7996 allele. Such a high level of skewness could be due to the accumulation of distorted alleles in the population with progressive cycles of selfing during the development of RIL. Paran et al. (1995) reported that 73% markers in a RIL population derived from S. lycopersicum × S. cheesmanii cross showed significant segregation distortion in favor of S. lycopersicum alleles. Furthermore, high level of skewed segregation has been observed for markers on chromosome 6 even in other mapping populations involving various interspecific crosses of tomato such as S. lycopersicum × S. pimpinellifolium (Grandillo and Tanksley 1996; Chen and Foolad 1999; Sharma et al. 2008) and S. lycopersicum × S. hirsutum (Bernacchi and Tanksley 1997; Zhang et al. 2002). This was mainly attributed to the presence of the self-pruning (sp) locus on chromosome 6, near the RFLP marker TG279 (Fulton et al. 1997). According to tomato-EXPEN2000 map (http://www.sgn.cornell.edu), TG279 is close to the BAC clone C06HBa0055E14 (anchored based on T0405) from which the highly distorted markers SLM6-29 and SLM6-30 were derived.
Twenty-one polymorphic SSR markers were placed along with two anchor markers SSR47 and SSR350 in a single linkage group. The genetic map spanned a total length of 117.6 cM (Fig. 3). The order of these markers on the genetic map was in the same order of BAC clones on the high density linkage map of tomato (Table 2). The map distance between markers derived from adjacent BAC clones in the West Virginia 700 × Hawaii 7996 RIL population were comparable to distances between the actual BAC clones anchored on the tomato-EXPEN2000 map except for one marker interval (SLM6-17 to SLM6-53) where an expansion of 27 cM was observed. Such interval specific expansions have been observed in the other S. lycopersicum × S. pimpinellifolium map (Sharma et al. 2008). The present map with SSR markers derived from BAC clones could provide a good representation of chromosome 6, when integrated with other published SSR markers.
SSR based linkage map of chromosome 6 based on a RIL population derived from a cross between West Virginia 700 and Hawaii 7996. Markers shown in the parenthesis were tightly linked with the marker indicated on the framework. Map location of the QTL associated with resistance to R. solanacearum strain Pss4 was highlighted
A major QTL associated with resistance to bacterial wilt
Maximum percentage of wilted plants was observed at 28 dai, after inoculating R. solanacearum strain Pss4. And distinct reactions were observed between the resistant Hawaii 7996 (19.8% of wilted plants) and susceptible WVa700 (96.9% of wilted plants). The mean percentage of wilted plants among the 188 RILs was 70.4%. Using the linkage map constructed in this study, a major QTL was identified associated with the marker interval SLM6-17–SLM6-53. The estimation of the percentage of phenotypic variation explained by this QTL was 11.2% and the allele from the resistant parent contributed to the resistance phenotype. According to EXPEN2000 map, the location of this QTL is similar with Bwr-6 reported by Carmeille et al. (2006), which was detected by Wang et al. (2000) as well. Bwr-6 is linked with TG73 located at 43.3 cM on chromosome 6, and SLM6-17 is located at 47.9 cM. More markers are needed to saturate this interval in order to find a closely linked maker for MAS.
In conclusion, 54 SSR markers specific to chromosome 6 were developed from anchored BAC clone sequences available in the Solanaceae Genomics Network. These SSR markers add to the present repository of molecular markers available for chromosome 6 in tomato and their utility in genetic diversity analysis and mapping studies have been demonstrated in this study.
References
Ammiraju JSS, Veremis JC, Huang X, Roberts PA, Kaloshian I (2003) The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor Appl Genet 106(3):478–484
Areshchenkova T, Ganal MW (1999) Long tomato microsatellites are predominantly associated with centromeric regions. Genome 42:536–544
Areshchenkova T, Ganal MW (2002) Comparative analysis of polymorphism and chromosomal location of tomato microsatellite markers isolated from different sources. Theor Appl Genet 104:229–235
AVRDC (2007) Tomato trials for the APSA workshop. In: AVRDC report 2004. AVRDC—The World Vegetable Center, Shanhua, p 32
Bai Y, Huang CC, van der Hulst R, Meijer-Dekens F, Bonnema G, Lindhout P (2003) QTLs for tomato powdery mildew resistance (Oidium lycopersici) in Lycopersicon parviflorum G1.1601 co-localize with two qualitative powdery mildew resistance genes. Mol Plant Microbe Interact 16(2):169–176
Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19:137–144
Bernacchi D, Tanksley SD (1997) An interspecific backcross of Lycopersicon esculentum × L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147:861–877
Bredemeijer GMM, Arens P, Wouters D (1998) The use of semiautomated fluorescent microsatellite analysis for tomato cultivar identification. Theor Appl Genet 97:584–590
Carmeille A, Caranta C, Dintinger J, Prior P, Luisetti J, Besse P (2006) Identification of QTLs for Ralstonia solanacearum race 3-phylotype II resistance in tomato. Theor Appl Genet 113:110–121
Chen FQ, Foolad MR (1999) A molecular linkage map of tomato based on a cross between Lycopersicon esculentum and L. pimpinellifolium and its comparison with other molecular maps of tomato. Genome 42:94–103
Edwards JD, McCouch SR (2007) Molecular markers for use in plant molecular breeding and germplasm evaluation. In: Guimarães EP, Ruane J, Scherf BD, Sonnino A, Dargie JD (eds) Marker-assisted selection. Current status and future perspectives in crops, livestock, forestry, and fish. Food and Agriculture Organization of the United Nations, Rome, pp 29–49
Foolad MR (2007) Genome mapping and molecular breeding of tomato. Int J Plant Genomics. doi:10.1155/2007/64358
Frary A, Xu Y, Liu J, Mitchell S, Tedeschi E, Tanksley SD (2005) Development of a set of PCR-based anchor markers encompassing the tomato genome and evaluation of their usefulness for genetics and breeding experiments. Theor Appl Genet 111(2):291–312
Fulton TM, Nelson JC, Tanksley SD (1997) Introgression and DNA marker analysis of Lycopersicon peruvianum, a wild relative of the cultivated tomato, L. esculentum, followed through three successive backcross generations. Theor Appl Genet 95:895–902
Fulton TM, Van der Hoeven R, Eannetta NT, Tanksley SD (2002) Identification, analysis, and utilization of conserved ortholog set markers for comparative genomics in higher plants. Plant Cell 14:1457–1467
Grandillo S, Tanksley SD (1996) Genetic analysis of RFLPs, GATA microsatelllites and RAPDs in a cross between L. esculentum and L. pimpinelliflium. Theor Appl Genet 92:957–965
Hanson PM, Bernacchi D, Green S (2000) Mapping a wild tomato introgression associated with tomato yellow leaf curl virus resistance in a cultivated tomato line. J Am Soc Hortic Sci 125(1):15–20
Hanson PM, Chen J, Kuo G (2002) Gene action and heritability of high temperature fruit set in tomato line CL5915. HortScience 37(1):172–175
He C, Poysa V, Yu K (2003) Development and characterization of simple sequence repeat (SSR) markers and their use in determining relationships among Lycopersicon esculentum cultivars. Theor Appl Genet 106:363–373
Jones DA, Dickinson MJ, Balint-Kurti PJ, Dixon MS, Jones JDG (1993) Two complex resistance loci revealed in tomato by classical and RFLP mapping of Cf-2, Cf-4, Cf-5, and Cf-9 genes for resistance to Cladosporium fulvum. Mol Plant Microbe Interact 6:348–357
Kosambi D (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kumpatla SP, Mukhopadhyay S (2005) Mining and survey of simple sequence repeats in expressed sequence tags of dicotyledonous species. Genome 48:985–998
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural population. Genomics 1:174–181
Langella O (1999) Populations 1.2.30. http://bioinformatics.org/~tryphon/populations/
Milbourne D, Meyer RC, Collins AJ, Ramsay LD, Gebhardt C, Waugh R (1998) Isolation, characterization and mapping of simple sequence repeat loci in potato. Mol Gen Genet 259:233–245
Moreau P, Thoquet P, Olivier J, Laterrot H, Grimsley N (1998) Genetic Mapping of Ph-2, a single locus controlling partial resistance to Phytophthora infestans in tomato. Mol Plant Microbe Interact 11(4):259–269
Morgante M, Olivieri AM (1993) PCR-amplified microsatellites as markers in plant genetics. Plant J 3:175–182
Mueller LA, Lankhorst RK, Tanksley SD, Giovannoni JJ et al (2009) A snapshot of the emerging tomato genome sequence. Plant Genome 2:78–92. doi:10.3835/plantgenome2008.08.0005
Nagy I, Polley A, Ganal M (1998) Development and characterization of microsatellite markers in pepper. In: Xth EUCARPIA meeting on genetics and breeding on Capsicum & Eggplant, Avignon, France
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Ohyama A, Asamizu E, Negoro S, Miyatake K, Yamaguchi H, Tabata S, Fukuoka H (2009) Characterization of tomato SSR markers developed using BAC-end and cDNA sequences from genome database. Mol Breed 23:685–691
Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computer. Bioinformatics 12:357–358
Paran I, Goldman I, Tanksley S, Zamir D (1995) Recombinant inbred lines for genetic mapping in tomato. Theor Appl Genet 90:542–548
Portis E, Nagy I, Sasvari Z, Stagel A, Barchi L, Lanteri S (2007) The design of Capsicum spp. SSR assays via analysis of in silico DNA sequence, and their potential utility for genetic mapping. Plant Sci 172:640–648
Sandbrink JM, van Ooijen JW, Purimahua CC, Vrielink M, Verkerk R, Zabel P, Lindhout P (1995) Localization of genes for bacterial canker resistance in Lycopersicon peruvianum using RFLPs. Theor Appl Genet 90:444–450
Sanwen H, Baoxi Z, Milbourne D, Cardle L, Guimei Y, Jiazhen G (2000) Development of pepper SSR markers from sequence databases. Euphytica 117:163–167
Sharma A, Zhang L, Nino-Liu D, Ashrafi H, Foolad MR (2008) A Solanum lycopersicum × Solanum pimpinellifolium linkage map of tomato displaying genomic locations of R-Genes, RGAs, and candidate resistance/defense-response ESTs. Int J Plant Genomics. doi:10.1155/2008/926090
Smulders MJ, Bredemeijer G, Rus-Kortekaas W, Arens P, Vosman B (1997) Use of short microsatellites from database sequences to generate polymorphisms among Lycopersicon esculentum cultivars and accessions of other Lycopersicon species. Theor Appl Genet 97:264–272
Srinivasa Rao NK, Bhatt RM, Sadashiva AT (2000) Tolerance to water stress in tomato cultivars. Photosynthetica 38(3):465–467
Tanksley SD, Ganal MW, Prince JP, De Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O, Roder MS, Wing RA, Wu W, Young ND (1992) High-density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Thoquet P, Olivier J, Sperisen C, Rogowsky P, Laterrot H, Grimsley N (1996) Quantitative trait loci determining resistance to bacterial wilt in tomato cultivar Hawaii7996. Mol Plant Microbe Interact 9(9):826–836
Van Heusden AW, Koornneef M, Voorrips RE, Brüggemann W, Pet P, Van Vrielink-Ginkel RV, Chen X, Lindhout P (1999) Three QTLs from Lycopersicon peruvianum confer a high level of resistance to Clavibacter michiganensis ssp. michiganensis. Theor Appl Genet 99:1068–1074
Wang JF, Olivier J, Thoquet P, Mangin B, Sauviac L, Grimsley NH (2000) Resistance of tomato line Hawaii7996 to Ralstonia solanacearum Pss4 in Taiwan is controlled mainly by a major strain-specific locus. Mol Plant Microbe Interact 13:6–13
Wang S, Basten CJ, Zeng ZB (2007) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Wann EV (1997) Tomato germplasm lines T4065, T4099, T5019, and T5020 with unique genotypes that enhance fruit quality. HortScience 32(4):747–748
Weber JL (1990) Informativeness of human (dC-dA)n, (dG-dT)n polymorphisms. Genomics 7:524–530
Yu K, Park SJ, Poysa V, Gebts P (2000) Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J Hered 91(6):429–434
Zamir D, Ekstein-Michelson I, Zakay Y (1994) Mapping and introgression of a tomato yellow leaf curl virus tolerance gene, Ty-1. Theor Appl Genet 88(2):141–146
Zane L, Bargelloni L, Patarnello T (2002) Strategies for microsatellite isolation: a review. Mol Ecol 11:1–16
Zhang LP, Khan A, Niño-Liu D, Foolad MR (2002) A molecular linkage map of tomato displaying chromosomal locations of resistance gene analogs based on a Lycopersicon esculentum × Lycopersicon hirsutum cross. Genome 45(1):133–146
Acknowledgements
The authors would like to thank Drs. Kadirvel Palchamy and Robert de la Peña for providing DNA samples of the tomato genotypes used in the study, Dr. Peter Hanson for providing information on tomato accessions, and Ms. Chiou-fen Hsu for technical assistance. This study is supported by funding provided by GTZ 81070160: Deutsche Gesellshaft fur Technishe Zusammenarbeit GmbH of Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Subramaniam Geethanjali and Kai-Yi Chen contributed equally for this manuscript.
Rights and permissions
About this article
Cite this article
Geethanjali, S., Chen, KY., Pastrana, D.V. et al. Development and characterization of tomato SSR markers from genomic sequences of anchored BAC clones on chromosome 6. Euphytica 173, 85–97 (2010). https://doi.org/10.1007/s10681-010-0125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-010-0125-z