Abstract
Gall midge is the third most destructive insect pests of rice after stem borers and planthoppers. Host plant resistance has been recognized as the most effective and economic, means for gall midge management. With the characterization of a new gall midge biotype (GMB) 4M, unique feature of gall midge resistance in the breeding line CR57-MR1523 was highlighted. Multi-location evaluation of F3 families derived from the cross TN1 × CR57-MR1523 against different gall midge biotypes helped to identify a new dominant gene conferring resistance against GMB4. This gene has been designated as Gm11t. Though CR57-MR1523 has been extensively used in breeding gall midge resistant rice varieties like Suraksha, neither the genetics of resistance nor chromosomal location of the resistance gene(s) is known. In the present study we have tagged and mapped the new gall midge resistance gene, Gm11t, on chromosome 12, using SSR markers. To map the gene locus, 466 F10 generation Recurrent Inbred Lines (RILs), from the cross of TN1 × CR57-MR1523 were used. Of the 471 SSR markers spread across the rice genome, 56 markers showed polymorphism and were used to screen a subset of the mapping population consisting of 10 resistant (R) and 10 susceptible (S) F10 RILs. Six SSR markers, RM28706, RM235, RM17, RM28784, RM28574 and RM28564 on chromosome 12 were initially found to be associated with resistance and susceptibility. Based on the linkage analysis in selected 158 RILs, we were able to map the locus between two flanking SSR markers, RM28574 and RM28706, on chromosome 12 within 4.4 and 3.8 cM, respectively. Further, two NILs with 99% genetic similarity, were identified from the RILs which differed in gall midge resistance. The tightly linked flanking SSR markers will facilitate marker-assisted gene pyramiding and map-based cloning of the resistant gene. NILs would be valuable materials for functional analysis of the identified candidate gene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Asian rice gall midge, Orseolia oryzae (Wood-Mason) (Diptera: Cecidomyiidae) is a serious pest of rice. The average annual yield loss due to gall midge in India is US$ 80 million (Widowsky and O’Toole 1996). Over 70 gall midge resistant rice varieties have been developed and released for commercial cultivation in the pest endemic areas in India since 1975 (Bentur et al. 2003). Wide spread cultivation of some of the resistant varieties carrying a single resistance gene has led to evolution of virulent populations of the pest, referred to as biotypes, that are capable of overcoming the resistance. Existence and emergence of new and virulent biotypes of the rice gall midge is a cause for concern, as it has resulted in the breakdown of resistance in many of the popular gall midge resistant varieties. Such a situation has called for new breeding strategies to meet the challenge of durable gall midge resistance.
So far, 10 gall midge resistance genes (Kumar et al. 2005), have been characterized in the plant and seven biotypes (Vijaya Lakshmi et al. 2006) of the pest have been reported. Among these resistance genes, Gm1, Gm2 and unknown gene(s) from a land race, Ptb21, have individually contributed resistance in 49 of the released varieties (Bentur et al. 2003). Improved rice varieties carrying Gm1 or Gm2, however, have lost their resistance against gall midge in most of the rice growing areas. Exceptionally, varieties deriving resistance from Ptb21 have displayed resistance against five of the seven biotypes (DRR 2006).
Interestingly, none of the identified genes confers resistance to all the gall midge biotypes, while none of the gall midge biotypes is virulent against all the resistance genes. One of the breeding options to develop effective varieties against the pest is to identify and utilize new resistance genes. Alternatively, gene pyramiding with Marker Assisted Selection (MAS) is being considered as a viable option for achieving durable gall midge resistance in rice. Molecular markers can support classical breeding in achieving such goals of gene pyramiding. So far, seven of the gall midge resistance genes have been tagged and mapped using molecular markers (Jain et al. 2004). Tightly linked (preferable <2 cM) and/or flanking co-dominant markers (<5 cM on either side of gene locus) help to effectively pyramid genes (Biradar et al. 2004). Availability of abundant and versatile microsatellite or Simple Sequence Repeat (SSR) markers in the rice genome (Mc Couch et al. 2002) and information on their physical position in the rice genome provide an opportunity to map any gene of interest. These markers could be used in fine mapping and pyramiding the gene through MAS.
The rice breeding line CR57-MR1523 derives its gall midge resistance from unknown gene(s) of Ptb21 parent. Though a complex pattern of inheritance of resistance has been suspected in Ptb21 (Kalode and Bentur 1989), genetics of resistance in this breeding line has not been studied well. In the present study we report a new gene in the rice breeding line CR57-MR1523 conferring resistance against GMB4 and we have mapped the gene with closely linked flanking SSR markers that can be used in MAS. Preliminary report of the study has been published earlier (Himabindu et al. 2009).
Materials and methods
Plant material used and development of mapping population
The gall midge resistant rice breeding line CR57-MR1523 and a gall midge susceptible variety TN1 were used as parental lines for developing the mapping population. The breeding line CR57-MR1523 was developed at the Central Rice Research Institute, Cuttack from the cross IR8 × Ptb21. F1s of the cross TN1 × CR57-MR1523 were advanced to F2 generation and later individual F3 families were developed from each F2 plant. Simultaneously, a mapping population consisting of Recurrent Inbred Lines (RILs) was developed by pooling five seeds each from plant (~200 plants) in each of the generations beginning with F3 and advancing them to next generation in the field. Final mapping population consisted of 466 RILs in F10 generation. All the lines were evaluated against GMB4 under greenhouse conditions. Of these, 158 (34 resistant and 124 susceptible) lines with clear ‘R’ (<10% damage) and ‘S’ (>80% damage) were used for genotyping. From the ‘R’ and ‘S’ groups 10 lines each were selected as subset mapping population for initial mapping studies.
Studies on inheritance of resistance
CR57-MR1523 was crossed with the rice varieties with the known resistance genes viz., Kavya (with Gm1), Phalguna (Gm2), RP2068-18-3-5 (gm3), Abhaya (Gm4) and ARC5984 (Gm5), to study inheritance of gall midge resistance and to test for allelism. Along with the parents, genotypes Kavya, Phalguna and Abhaya carrying gall midge resistance gene Gm1, Gm2 and Gm4, respectively, were selected for allelism test with flanking markers.
Greenhouse evaluation for gall midge resistance
Parents and F3 families were evaluated under greenhouse conditions for resistance against GMB1, GMB3, GMB4 and GMB4M following the standardized screening procedure (Bentur and Kalode 1996). F10 lines along with the genotypes Kavya, Phalguna and Abhaya were evaluated against GMB4. F3 families from individual F2 plants of the crosses involving CR57-MR1523 and the rice varieties with known genes were evaluated against GMB1 and GMB4. Individual families were scored for resistance.
Insect damage was recorded 20 days after adult release, when the susceptible checks showed fully exerted galls. A test was considered valid only when >90% of the susceptible check TN1 plants showed damage. Test genotypes recording nil or <10% plant damage were rated as resistant while those with >80% level of damage were rated as susceptible. Plant damage in F10 RILs was recorded on a line basis. Lines with galls were rated as susceptible while the rest were dissected to confirm the presence of dead maggots and/or expression of hypersensitive reaction (HR) for rating as resistant. Lines which segregated with >10 but <80% damage were scored as heterozygous. Lines that did not show these features were labeled as escapes and were not considered under the total of test lines. Escape lines did not exceed 10% of the total in any of the experiments.
DNA extraction and PCR
Total genomic DNA was isolated from leaf tissue of selected genotypes and RILs through the modified method of Zheng et al. (1995) and used for PCR amplification following the protocol of Chen et al. (1997). In order to map the new gene in CR57-MR1523 on the rice genome and to identify linked SSR markers, we used a set of 471 SSR markers uniformly spread across the 12 chromosomes of rice (RM series, Research Genetics, USA). The map locations, primer sequences and other details of these markers are available online at http://www.gramene.org. The PCR products were resolved on 3.5% agarose (US Biochemicals, USA) in 0.5× TBE buffer, stained with ethidium bromide (0.5 μg/ml) and photographed under UV light. The size of the amplified fragments was calculated using Alphaease software (Alpha Innotech, USA) with 50-bp ladder (MBI Fermentas, Lithuania) as size reference standards. The exact physical positions of the linked markers were determined through BLAST search using bioedit software against the indica sequence database (http://rice.genomics.org.cn/rice/index2.jsp). Polymorphic SSR markers were used for co-segregation analysis with the trait phenotype, initially, in a subset of 20 F10 RILs (consisting of 10 resistant and 10 susceptible lines). This was done in order to quickly identify SSR marker(s) co-segregating with the trait phenotype and the tentative chromosomal location. Once the tentative chromosome location was known, all the SSR markers on that particular chromosome were further analyzed in the selected 158 F10 population showing clear ‘R’ and ‘S’ phenotype. The linked SSR markers for the genes Gm1, Gm2 and Gm4 were used for allelism test with the markers (Himabindu et al. 2007) to predict the corresponding allele in CR57-MR1523.
Linkage analysis
Linkage analysis and map construction were performed using MAPMAKER/EXP, version 3 (Lander et al. 1987). Initially, linkage groups were obtained using two-point analysis with a log-likelihood of odds (LOD) score of 4.0 and maximum recombination level of 0.3. This step was implemented by using the ‘group’ command. Linked markers within the linkage groups were ordered using multipoint analysis with ‘compare’, ‘suggest subset’ and ‘try’ commands. Best order of marker was then confirmed with the ‘ripple’ command, using a minimum LOD of 4.0. Finally, the map distances were calculated using ‘map’ command. The map distances were converted into centiMorgans, using the Kosambi (1944) function.
Candidate-gene analysis
In silico analysis of putative candidate genes in the target region was done with available sequence annotation database http://rapdb.dna.affrc.go.jp. The location and function of these genes were noted to identify the putative candidate for gall midge resistance.
Identification of NILs
Ten lines each from ‘R’ (lines with nil damage) and ‘S’ (lines with 100% damage) groups of the F10 mapping population were used for the study. A total of 471 SSR markers, spread across the rice genome were used. The pairs with maximum similarity were identified for phenotypic study.
Results
Unique gall midge resistance gene in CR57-MR1523
With the characterization of a new gall midge biotype GMB4M, unique feature of gall midge resistance in the breeding line CR57-MR1523 was highlighted (Vijaya Lakshmi et al. 2006). This feature was again tested for confirmation by screening all the gene differential rice genotypes against gall midge biotypes GMB1, GMB4 and GMB4M.
Results confirmed that, against GMB1, all the ten genes were found to confer resistance. Against GMB4 only three genes viz., gm3 (RP2068-18-3-5), Gm4 (Abhaya) and Gm8 (Jhitpiti) along with CR57-MR1523 provided resistance. However, CR57-MR1523 was susceptible against GMB4M while the other three genes viz., gm3, Gm4 and Gm8 conferred resistance (data not shown). This suggested that the gene(s) in CR57-MR1523 conferring resistance to GMB4 is likely to be a new gene(s).
Genetic analysis of gall midge resistance in CR57-MR1523 and test for allelism
Inheritance of resistance in the cross TN1 × CR57-MR1523 revealed a complex segregation pattern of resistance depending upon the biotype used for evaluation. F3 families showed segregation ratio of 13 resistant: 3 susceptible families when evaluated against GMB1, 15:1 ratio against GMB3 and 3:1 ratio against GMB4, while none was resistant to GMB4M (Table 1). It is obvious from these results that gene(s) controlling gall midge resistance in CR57-MR1523 interacted differently against different biotypes tested. For GMB1 two gene, one dominant and one recessive while for GMB3 two independent dominant gene interaction was evident. For GMB4, a single dominant gene segregation ratio was observed. Against GMB4M, none of the genes was effective.
Results of allelism tests involving crosses between CR57-MR1523 and rice varieties with other known gall midge resistance genes did not show any consistent pattern of segregation (data not shown). This suggested varying and higher order of interactions and reaffirmed presence of a single gene in the breeding line CR57-MR1523 conferring resistance against GMB4. We set to identify this dominant gene conferring resistance against GMB4.
Allelism test using gene linked markers
Since classical allelism tests through the crosses involving CR57-MR1523 with the other known gene sources did not clearly reveal identity of the gene in CR57-MR1523, we used the reported linked SSR markers for different gall midge resistance genes to identify the gene. The markers linked to Gm1, Gm2 and Gm4 genes were used to amplify genomic DNA of CR57-MR1523 along with the gene specific genotypes Kavya, Phalguna, Abhaya and the susceptible parent TN1 for allelism test with the markers. Results suggested lack of any of the three gene specific amplification with CR57-MR1523 (Table 2). Thus the gene conferring resistance to gall midge biotype GMB4 in CR57-MR1523 is, most likely, non allelic to the three genes Gm1, Gm2 and Gm4.
Tagging and mapping of the new gene in CR57-MR1523
Of the 471 SSR markers used for parental polymorphism survey, 56 markers showed polymorphism between the parental lines TN1 and CR57-MR1523 (data not shown). The markers RM28706, RM235, RM17, RM28784, RM28574 and RM28564 located on chromosome 12, co-segregated with the trait phenotype in the subset of mapping population consisting of 10 ‘R’ and 10 ‘S’ lines (Fig. 1). However, other polymorphic markers on chromosome 12 did not show strong linkage with the trait. The analysis was extended to rest of the selected RIL lines. Of the 466 RILs, 158 (34 resistant and 124 susceptible) RILs with clear phenotypic data were selected for genotyping. Remaining 308 lines were not included in the analysis to avoid phenotypic error. Genomic DNA from these 158 lines was used for PCR amplification with the six linked markers to check the marker-trait co-segregation.
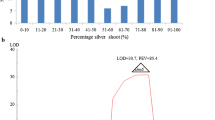
MAPMAKER analysis indicated that SSR markers RM28706, RM235, RM17 and RM28784 are located at a genetic distance of ~3.8 cM, ~7.6 cM, ~8.9 cM and ~11.1 cM, respectively, from the gene. Whereas, the markers RM28574 and RM28564 were found to be located at a genetic distance of ~4.4 cM and ~6.5 cM, respectively, on the other side of the gene (Fig. 2). The recombination frequency among the markers RM28706, RM235, RM17 and RM28784 was always lesser than the recombination frequency between the marker and the gene. This suggested that all four markers lie on one side of the gene. However, the recombination frequency between the markers RM28706, RM235, RM17, RM28784 and RM28574, RM28564 was high than the recombination frequency between these markers and the trait (gene), suggesting that the markers RM28574 and RM28564 flank the gene locus on chromosome 12. Thus, the new gall midge resistance gene in CR57-MR1523 is located on chromosome 12.
Analysis of candidate genes for Gm11t
Based on the linkage map, about 1.7 MB genomic region of rice encompassing the closest markers, starting from RM28574 (located at 24.2 MB) and RM28706 (located at 25.9 MB) and the target genome was analyzed for the gene content. A total of 139 genes are reported in this genomic region. Of these, 43 genes encoded for hypothetical proteins whose functions are unknown while 6 genes encode for cyclin-like F-box domain containing protein which recruit particular substrates for ubiquitin–proteosome pathway. Two genes encoding tetratricopeptide-like helical domain containing protein widely implicated in defense mechanism and three genes coding for transcriptional factor B3 family protein involved in auxin-regulated gene expression of primary response genes were also detected. Further, genes encoding uridine kinase family protein, CXC domain containing protein etc. were also noted in the region. Of the enumerated genes in the Gm11t encompassing region, two classes of genes encoding for cyclin-like F-box domain containing protein and tetratricopeptide-like helical domain containing protein, qualify to be the candidates for Gm11t.
Development of NILs through RILs using SSR markers
NILs were developed from the ten selected resistant (designated R1 through R10) and ten susceptible (S1–S10) RILs based on clear phenotype when exposed to GMB4. These selected RILs were again individually subjected to genotyping using all 56 parental polymorphic SSR markers. Two paired lines were identified from R and S RILs which shared the same allele at most of the loci examined. The maximum similarity observed between the pair R5 and S1 was 88% while 86% similarity was seen between the pair R5 and S7 with reference to the 56 polymorphic SSR markers. These included four of the six linked markers RM235, RM28706, RM28784 and RM28574. But two of the flanking markers RM17 and RM28564 showed polymorphism within the pairs. Considering the data of all the 471 SSR loci examined earlier, similarity between these pairs was 99% and 98%, respectively (Table 3). Hence R5 and S1, R5 and S7 can be considered as NILs developed through RILs.
Discussion
Discovery of new gene/gene sources would provide breeders the choice to improve rice varieties with broad range and durable gall midge resistance. To facilitate this objective, additional DNA markers for novel genes are necessary. The breeding line CR57-MR1523 was selected in the present study for tagging and mapping of a new resistance gene conferring resistance against GMB4. This line has multiple pest resistance against planthoppers (Kalode et al. 1977) and broad spectrum of gall midge resistance against gall midge biotypes GMB1, GMB2, GMB3, GMB4 and GMB6 (DRR 2006) and exhibits hypersensitive (HR) + type of resistance (Bentur et al. 2003).
Complex inheritance of gall midge resistance in CR57-MR1523 was evident in the earlier studies. Sastry and Prakasa Rao (1976) and Sastry et al. (1984) were the first to note genetics of gall midge resistance in CR57-MR1523. Their study suggested involvement of three complementary genes in CR57-MR1523 based on the segregation ratio from the crosses with ARC7328 and Ptb33. They further suggested that Ptb21, progenitor of CR57-MR1523, also had a recessive allele of the dominant inhibitory gene present in Leb Muey Nahng (Sastry et al. 1984). Presence of more than one gene and their possible complex interactions among themselves and with the biotypes could be the possible cause for such variations in CR57-MR1523.
In the present study, we further confirmed the complex inheritance pattern of resistance in the breeding line CR57-MR1523 based on the reaction of F3 families of the cross TN1 × CR57-MR1523. Identity of the genes in the breeding line CR57-MR1523 was attempted through classical allelism tests and also through marker alleliesm test. Classical allelism tests could not establish the identity of resistance genes in this breeding line. However, the novel molecular allelism test (Himabindu et al. 2007) using linked markers revealed that the gene(s) in CR57-MR1523 is(are) nonallelic to the Gm1, Gm2 and Gm4. Further the single dominant gene in CR57-MR1523 conferring resistance to GMB4 may not be allelic to gall midge recessive gene gm3 and it may not be allelic to Gm8, which exhibits HR- type of reaction, conferring resistance to both GMB4 and GMB4M.
Thus the present study confirmed presence of a new single dominant gene in CR57-MR1523 conferring resistance against GMB4. In the process of tagging and mapping of the new single dominant gene in CR57-MR1523, 56 polymorphic SSR markers, out of the 471 tested, were identified. However, results of phenotyping 466 F10 RILs posed a problem of selecting R and S pools for linking the gene with markers. Our data on 466 RILs showed a normal distribution of frequency under different levels of pest damage which is unique and exceptional for gall midge resistance trait. Such a deviation could have resulted either due to complex genetics of resistance in CR57-MR1523 or due to unintended bias in selection during the development of RILs. To circumvent the complexity, 158 RILs (34 resistant and 124 susceptible) at the extreme range of the phenotypic scale were selected for tagging and mapping the new gene. Selective genotyping approach has been adopted to identify major genes in complexly inherited traits involving both major genes and QTLs (Nandi et al. 1997). Six SSR markers located on chromosome 12 co-segregated with the trait phenotype. The order of these SSR loci agreed with the published SSR genetic linkage maps (Temnykh et al. 2000, 2001; Mc Couch et al. 2002; http://www.gramene.org) and the physical map of IRGSP (2005) with minor variations in the linkage distances. Thus flanking SSR markers for the new major gall midge resistance gene were identified. Flanking markers are of great value in introgression of the target gene through MAS. Based on product rule of probability, flanking SSR markers help in accurate prediction of the gene with less than 1% error (Biradar et al. 2004).
Of the 139 annotated genes identified, in the genomic region encompassing the target gene, two class of genes encoding for tetratricopeptide-like helical domain containing protein and cyclin-like F-box domain containing protein widely implicated in defense mechanism were observed. Sebastian et al. (2006) reported tetratricopeptide (TPR) repeat-like structure for AvrBs3-homologous proteins. The AvrBs3 proteins are involved in gene-for-gene mediated recognition of nuclear-targeted bacterial effector proteins which play a vital role in plant defense mechanism against pathogen (Lamb et al. 1995; Jacobsen et al. 1996).
Another class of genes encoding for cyclin-like F-box domain containing protein which interacts with SKP-1 protein and playing a major role in defense mechanism (Hirofumi et al. 2002) was also observed. Thus, two classes of genes have been identified through the present study, which could be potential candidates for the new gall midge resistance gene. It could be possible that one or more of these genes may be directly or indirectly associated with gall midge resistance.
RIL and NIL populations have been extensively used in genetic studies (Eshed and Zamir 1995; Rae et al. 1999; Von Korff et al. 2004) due to the advantages derived from their homozygosity at majority of the background genome. During development of NILs back cross breeding approach is generally adopted to ensure introgression of almost entire genome of the recurrent parent. But development of NILs through classical backcross breeding is tedious and will take longer time since it requires six to eight generations (Allard 1960). Many attempts were made to address these problems using molecular markers. Advanced backcross QTL analysis for the development of QTL–NILs was proposed by Tanksley and Nelson (1996). Prabuddha et al. (2008) proposed the use of molecular markers to identify NILs for root and shoot morphological characters in a mapping population of rice. Identifying NILs using molecular marker data of a mapping population and subsequent phenotypic evaluation enables us to identify NILs directly, without repeated back crossing. Two lines each, significant for gall midge resistance and susceptibility are considered as NILs identified through RILs for their respective traits, and would be the valuable material for functional dissection of the gall midge resistance involving gene expression studies.
Conclusion
Both genetic and molecular analysis enabled us identify a new dominant gall midge resistance gene conferring resistance against GMB4 in CR57-MR1523. We propose to designate this as Gm11t. None of the previously mapped gall midge resistance genes is located on chromosome 12. Despite the complex inheritance pattern in CR57-MR1523, flanking and linked SSR markers identified in the present study can be used in marker assisted breeding program to introgress this new gene. In addition, NILs developed in the present study form important genetic resource for gene discovery and gene expression studies.
Abbreviations
- GMB:
-
Gall midge biotype
- HR:
-
Hypersensitive reaction
- MAS:
-
Marker assisted selection
- NILs:
-
Near isogenic lines
- RILs:
-
Recurrent inbred lines
- SSR:
-
Simple sequence repeat
References
Allard RW (1960) Principles of plant breeding. John Wiley, New York
Bentur JS, Kalode MB (1996) Hypersensitive reaction and induced resistance in rice against Asian rice gall midge Orseolia oryzae. Entomol Exp Appl 78:77–81
Bentur JS, Pasalu IC, Sarma NP, Prasada Rao U, Mishra B (2003) Gall midge resistance in rice. DRR Research paper series 01/2003. Directorate of Rice Research, Hyderabad, India, p 20
Biradar SK, Sundaram RM, Thirumurugan T, Bentur JS, Amudhan S, Shenoy VV, Mishra B, Bennet J, Sarma NP (2004) Identification of flanking SSR markers for a major rice gall midge resistance gene Gm1 and their validation. Theor Appl Genet 10:1468–1472
Chen X, Temnykh S, Xu Y, Cho YG, Mc Couch SR (1997) Development of a microsatellite frame work map providing genome wide coverage in rice (Oryza sativa L.). Theor Appl Genet 95:553–567
DRR (2006) Progress report. All India coordinated rice improvement programme (ICAR), vol 2. Directorate of Rice Research, Rajendranagar, Hyderabad, 27 pp
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
Himabindu K, Vijaya Lakshmi P, Sundaram RM, Neeraja CN, Mishra B, Bentur JS (2007) Flanking SSR markers for allelism test for the Asian rice gall midge (Orseolia oryzae) resistance genes. Euphytica 157:267–279
Himabindu K, Suneetha K, Sama VSAK, Cheralu C, Rao PRM, Bentur JS (2009) A new gene for gall midge resistance in rice variety MR1523. Rice Genet Newsl 25 (in press)
Hirofumi K, Naoki T, Hiroaki S, Motoaki, Kazuo S, Minami M (2002) Classification and expression analysis of arabidopsis F-box-containing protein genes. Plant Cell Physiol 43(10):1073–1085
Jacobsen SE, Binkowski KA, Olszewski NE (1996) SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Nat Acad Sci USA 93:9292–9296
Jain A, Ariyadasa R, Kumar A, Srivastava MN, Mohan M, Nair S (2004) Tagging and mapping of a rice gall midge resistance gene, Gm8, and development of SCARs for use in marker-aided selection and gene pyramiding. Theor Appl Genet 109:1377–1384
Kalode MB, Pophaly DJ, Kasi Viswanathan PR, Sreeramulu M (1977) Studies on resistance and mass rearing of rice gall midge, Orseolia oryzae (Wood-Mason). Madras Agric J 64:733–739
Kalode MB, Bentur JS (1989) Characterization of Indian biotypes of the rice gall midge Orseolia oryzae (Wood- Mason) (Diptera: Cecidomyiidae). Insect Sci Applic 10:219–224
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kumar A, Jain A, Sahu RK, Shrivastava MN, Nair S, Mohan M (2005) Genetic analysis of resistance genes for the rice gall midge in two rice genotypes. Crop Sci 45:1631–1635
Lamb JR, Tuguenreich S, Hieter P (1995) Tetratrico peptide repeat interactions: to TPR or not to TPR. Trends Biochem Sci 20:257–259
Lander ES, Green P, Abrahamson J, Barlow A, Daly MG, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic maps of experimental and natural populations. Genomics 1:174–181
Mc Couch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kano I, Yano M, Fjellstrom R, De CG, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Nandi S, Subudhi PK, Senadhira D, Manigbas NL, Sen-Mandi S, Huang N (1997) Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Mol Gen Genet 255(1):1–8
Prabuddha HR, Manjunatha K, Venuprasad R, Vinod MS, Jureifa JH, Shashidhar HE (2008) Identification of near-isogenic lines: an innovative approach, validated for root and shoot morphological characters in a mapping population of rice (Oryza sativa L.). Euphytica 160:357–368
Rae AM, Howell EC, Kearsey MJ (1999) More QTL for flowering time revealed by substitution lines in Brassica oleracea. Heredity 83:586–596
Sastry MVS, Prakasa Rao PS (1976) Promising new multiple insect resistant rice varieties. Curr Sci 45:424–425
Sastry MVS, Rao UP, Kalode MB, Sain M (1984) Inheritance of resistance to gall midge (Orseolia oryzae) in rice. Indian J Genet 44:325–328
Sebastian S, Annett M, Patrick R, Tina J, Thomas L (2006) Gene-for-gene-mediated recognition of nuclear-targeted AvrBs3-like bacterial effector proteins. J Plant Physiol 163(3):256–272
Tanksley SD, Nelson JC (1996) Advanced backcross QTL analysis: a method for the simultaneous discovery and transfer of valuable QTLs from un-adapted germplasm into elite breeding lines. Theor Appl Genet 92:191–203
Temnykh S, Cartinhour S, Park W, Ayres N, Hauck N, Lipovich L, Cho YG, Mc Couch SR (2000) Mapping and genome organization of microsatellite sequence in rice (Oryza sativa L.). Theor Appl Genet 100:697–712
Temnykh SG, Declerck A, Lukashova L, Lipovich L, Cartinhour S, Mc Couch SR (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations and genetic marker potential. Genome Res 11:1441–1452
Vijaya Lakshmi P, Amudhan S, Bindu KH, Cheralu C, Bentur JS (2006) A new biotype of the Asian rice gall midge Orseolia oryzae (Diptera: Cecidomyiidae) characterized from the Warangal population in Andhra Pradesh, India. Int J Trop Insect Sci 26:207–211
Von Korff, Wang MH, Leon J, Pillen (2004) Development of candidate introgression lines using an exotic barley accession (Hordeum vulgare ssp. Spontaneum) as donor. Theor Appl Genet 109:1736–1745
Widowsky DA, O’Toole JC (1996) Prioritizing rice research agenda for eastern India. In: Evanson RE, Herdt RW, Hussain M (eds) Rice research in Asia: progress and priorities. International Rice Research Institute, Manila, Philippines, pp 109–129
Zheng K, Huang N, Bennett J, Khush GS (1995) PCR-based marker assisted selection in rice breeding. IRRI Discussion paper series no. 12. International Rice Research Institute, Manila, Philippines
Acknowledgements
We thank the Project Director, DRR, Hyderabad for the facilities and encouragement. This work was supported by a grant from the Department of Biotechnology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Himabindu, K., Suneetha, K., Sama, V.S.A.K. et al. A new rice gall midge resistance gene in the breeding line CR57-MR1523, mapping with flanking markers and development of NILs. Euphytica 174, 179–187 (2010). https://doi.org/10.1007/s10681-009-0106-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0106-2