Abstract
Natural triploids of Populus tomentosa (2n = 3x = 57) are presumably the result of sexual polyploidization through the union of normal n female gametes and numerically unreduced (2n) male gametes. In our microscopic study of microspore mother cells (MMCs) of diploid P. tomentosa (2n = 2x = 38), we observed that the first meiotic division was normal but that the second division was characterized by frequent abnormal spindle orientation (parallel, tripolar, and fused spindles) and premature cytokinesis. The parallel, fused spindles and premature cytokinesis were considered to be leading dyad formation, and tripolar spindles seemed to be leading triad formation at the tetrad stage. There was a higher frequency of parallel spindles than other spindle forms, but there were no significant correlations between parallel spindles and dyads. An indirect immunofluorescence examination of meiosis II revealed that four tetragonally arranged nuclei were formed in MMCs with parallel spindles and that there were radial microtubules systems (RMSs) among these four nuclei, leading to the tetragonal tetrad. In some MMCs, however, the parallel spindles led to the gathering of one or two non-sister groups of chromosomes, causing an incorporation of RMSs from two daughter nuclei. Thus, the incorporated RMSs established three or two nuclear cytoplasmic domains for the control of division plane, resulting in either triad or dyad formation. These results provide new insights on the mechanism of parallel spindles leading to numerically unreduced pollen formation and on the selection and utilization of this type of pollen in polyploid breeding of P. tomentosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of unreduced (2n) gametes that carry the chromosome number of the sporophyte rather than the gametophyte appears to be a common phenomenon in the plant kingdom and likely played a key role in the creation of polyploid series (Harlan and De Wet 1975; Den Nijs and Peloquin 1977; Veilleux 1985; Ramsey and Schemske 1998; Ramsey 2007; Rieseberg and Willis 2007). The induction of sexual polyploidization via controlled crosses between naturally occurring and artificially induced 2n gametes have also been widely used in the breeding of forest trees (Seitz 1954; Zhang et al. 2004), potato (Mendiburu et al. 1974; Ramanna 1979; Carputo and Barone 2005), grain crops (Veilleux 1985; Jauhar 2003), fruit-bearing trees (Sugiura et al. 2000), and ornamental plants (Van Tuyl and Lim 2003; Akutsu et al. 2007).
The mechanisms of 2n gamete formation have been researched in numerous plant species (see reviews by Veilleux 1985; Bretagnolle and Thompson 1995; Ramsey and Schemske 1998), and various possible routes leading to the formation of 2n gametes have been suggested: premeiotic disturbances, meiotic disturbances and restriction, abnormal cytokinesis, post-meiotic doubling, and apomeiotic cells of the ovule (apospory). However, the most accepted currently held view is that 2n gamete formation is the result of irregular orientations of spindles and abnormal cytokinesis at the second meiotic division (see reviews by Veilleux 1985; Bretagnolle and Thompson 1995; Ramsey and Schemske 1998). Disturbed spindles and cytokinesis related to 2n pollen formation have been reported to be associated with changes in the configurations of the microtubules (Genualdo et al. 1998; Conicella et al. 2003). In addition, d’Erfurth et al. (2008) recently isolated and characterized a gene (AtPS1) involved in the abnormal orientation of spindles at meiosis II that apparently controlled diploid (2n) gamete formation in Arabidopsis thaliana.
Parallel, fused, and tripolar spindles are the three main types of spindle configurations that have been observed in plants that produce 2n pollen (Mok and Peloquin 1975; Veilleux 1985; Bretagnolle and Thompson 1995; Ramsey and Schemske 1998). Some authors have pointed out that parallel, fused, and tripolar spindles are different phenotypic expressions of the same gene and that meiosis II spindle orientation is responsible for 2n pollen formation (Mok and Peloquin 1975; d’Erfurth et al. 2008). The parallel or fused spindles result in a dyad containing two numerically unreduced microspores, whereas the tripolar spindle produces a triad with one numerically unreduced microspore and two reduced microspores. However, in some Solanum species, plants that produce a high percentage of parallel spindles either at metaphase or anaphase II still produce only a low frequency of dyads and triads (Ramanna 1979). One hypothesis is that only fused, but not parallel spindles result in 2n pollen formation (Veilleux et al. 1982; Ramanna 1983). Carputo et al. (2000) have suggested that a parallel spindle is not the only condition for 2n pollen formation in potato and that a second mechanism acting at the end of cytokinesis may be involved. The irregular cytokinesis at the end of the second meiotic division results in microspore dyads or triads that have two or one unreduced microspores, respectively, instead of normal tetrads with four reduced microspores (Mok and Peloquin 1975; d’Erfurth et al. 2008). The microsporocytes of dicotyledons generally exhibit simultaneous cytokinesis; the failure of (or the incomplete) cytokinesis in dicotyledons leads to the incidence of 4n and 3n pollen grains (Pfeiffer and Bingham 1983; Tavoletti et al. 1991).
In the genus Populus, unreduced pollen has been documented in Populus canescens Moench. (2n = 2x = 38) (Seitz 1954), P. nigra L. (2n = 2x = 38) (Bradshaw and Stettler 1993), P. tomentosa Carr. (2n = 2x = 38) (Zhu et al. 1998), and P. tremula L. (2n = 2x = 38) (Muntzing 1936). This unreduced pollen is considered to be involved in the formation of these natural triploid Populus species, which have a rapid growth rate and improved pulp properties compared to their diploid counterparts (Yao and Pu 1998; Zhu et al. 1998). These are highly valuable economic attributes and has led to the search for successful triploid synthesis programs using natural and colchicine or temperature-induced 2n gametes in sexual polyploidization (Zhang et al. 2007). Investigations on the meiotic mechanisms of microspore mother cells (MMCs) have revealed that the abnormality of the final cytokinesis resulting from fused or parallel spindles at metaphase II is involved in the formation of 2n pollen in P. tomentosa (Kang 2002). However, how the abnormal cytokinesis occurred and in which ways the irregular spindles led to the formation of abnormal sporads at tetrad stage were not clearly determined.
The aim of this study was to elucidate the cytological mechanisms that lead to unreduced pollen formation in P. tomentosa. Spindle orientation and other cytological characteristics were examined during the meiosis of MMCs in squashed anthers by indirect immunofluorescence. Based on our results on the observed spindle configurations, sporad types, and diameters of pollen grains, we propose the cytological mechanisms involved in the formation of unreduced pollen and discuss the role of 2n pollen in triploid synthesis and polyploid formation.
Materials and methods
Plant material
Twelve clones of diploid P. tomentosa (2n = 2x = 38) were sampled from 224 clones maintained in our laboratory. Of these 12 clones, six produced a relatively higher frequency of larger pollen grains than the other six (Zhang et al. 2007). These clones were planted in the Guan County nursery garden of Shandong province. Floral branches were detached and forced, under greenhouse conditions, at Beijing Forestry University.
Cytological studies
After the floral branches had been forced, flower buds were collected every 2–4 h and fixed in Carnoy’s fluid (ethanol:acetic acid, 3:1) at 4°C until mature pollen grains appeared. For the meiosis analysis, anthers were dissected from the buds and squashed in a droplet of aceto-carmine solution (2%) onto a microscope slide using forceps. Developing microsporocytes were characterized in detail for their spindle orientations at metaphase II and anaphase II; dyads, triads, and tetrads were determined at the sporad stage. The frequency of cells with various types of spindle arrangements at metaphase and anaphase II was determined in 585–960 MMCs. More than 1000 sporads were observed to determine the proportion of tetrads, triads, and dyads at this stage.
Indirect immunofluorescence
The protocol for microtubule localization during microsporogenesis was adapted from Brown and Lemmon (1992). Accordingly, the anthers were fixed for 45 min in a 4% solution of paraformaldehyde, freshly prepared in a PEM buffer (50 mM PIPES, 5 mM EGTA, 1 mM MgSO4; pH 6.8), then permeabilized for 30 min in the buffer with 10% (v/v) dimethyl sulfoxide (DMSO), and finally extracted in 1% (v/v) Triton X-100 for 15 min. After three washings in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4; pH 7.4), the anthers were dissected with forceps to release the meiocytes, tetrads, or young microspores. Cells were then affixed to a slide coated with 0.1% (w/v) poly-l-lysine (P1274; Sigma, St. Louis, MO) and incubated in a moist chamber with an anti-α-tubulin antibody (T9026; Sigma) diluted 1:200 in PBS for 1 h at 37°C. Following further washing in PBS, the samples were labeled with a 1:50 (v/v) dilution of fluorescein isothiocyanate (FITC) conjugated anti-mouse immunoglobulin (Ig)G antibody (F0219; Sigma) for at least 1 h at 37°C in a moist chamber. For correlation with nuclear stages, samples were finally washed in PBS, counterstained with 1 μg ml−1 DAPI (4′,6-diamidino-2-phenylindole) for 5 min and mounted in a Prolong Gold antifade reagent (P36934; Invitrogen, Carlsbad, CA). The preparations were observed using a fluorescence microscope (model BX51; Olympus, Japan) and photographed with a CCD camera (model DP70; Olympus).
Measurement of 2n pollen grain diameter
Separate pollen samples were collected from mature catkins of each clone and stored in glass bottles containing allochronic silica gel. Pollen grains were taken from the glass bottles with a dissecting needle, then mounted on a microscope slide, and stained with a 2% aceto-carmine solution. The diameter of each pollen grain was assessed using an eyepiece micrometer. A total of 840 pollen grains were measured per clone.
Results
Cytological characteristics of male meiosis
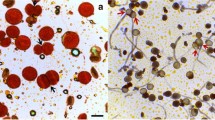
The first meiotic division of all 12 clones was observed to progress normally, but a number of cytological events occurred during the second meiotic division that resulted in unreduced pollen formation. At metaphase and anaphase II, the orientations of the spindles of homologous chromosomes were perpendicular, parallel, tripolar, and fused, as observed in pollen specimens that had been stained in the aceto-carmine solution (Fig. 1a–d). There was a predominance of parallel spindles in all 12 clones of P. tomentosa, but the frequency of perpendicular spindles ranged only from 5.0 to 18.9%. Triploid and fused spindles were observed most frequently in clones 347 and 314, respectively, whereas clone 346 was the highest total producer of these two abnormal spindles (see Table 1). The angle of the meiotic spindles at anaphase II was also arranged from parallel to tripolar, which was illustrated by the position of the four-sister chromosome complement (Fig. 1f–i). Indirect immunofluorescence microscopy of microtubule cytoskeleton patterns during meiosis revealed that the poles of the triploid and fused spindles in some MMCs were joined at one or two axes of the cell (Fig. 1m, n) compared to perpendicular and parallel spindles (Fig. 1k, l). Moreover, a single narrow fissure between the mutually parallel spindles, with no microtubules around them, was observed in some MMCs at second meiosis (Fig. 1e, j, o).
Aspects of meiotic spindles at the second meiosis in microspore mother cells (MMCs) of diploid Populus tomentosa. Bar: 10 μm. a Perpendicular spindle, b parallel spindle, c tripolar spindle, d fused spindle, e furrow between parallel spindle at metaphase II, f–j spindles at anaphase II, which form a–e. k–o Indirect immunofluorescence study of microtubule cytoskeleton patterns at metaphase II corresponding to a–e
Cytokinesis and abnormal sporads formation
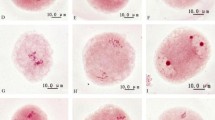
Phragmoplasts were not observed at telophase II, but radial microtubule systems (RMSs), which expanded from the sister nuclei and non-sister nuclei, were observed (Fig. 2a–c). The formation of cleavage furrows pinpointed the beginning of the simultaneous cytokinesis that occurred among the RMSs, and this cleavage formation progressed from the center to the periphery of the dividing cells (Fig. 2d–f). Even a small nucleus had RMSs formed around it, leading to cytoplast division (Fig. 2g–i). Meiosis and cytokinesis resulted in the production of generally tetragonal tetrads with four reduced microspore (Fig. 3a), but different types of dyads (Fig. 3b–f) and triads (Fig. 3g–i) with one or two unreduced microspores were observed at the tetrad stage. These unreduced microspores had a restitution nucleus with one (Fig. 3b, d, g, i), or two (Fig. 3c, f, h) and three nucleoli (Fig. 3e). The triads presented asymmetric cytokinesis (Fig. 3g–i), but an unbalanced division of cytoplasm also occurred in the dyads (Fig. 3d, e). The frequency of triads, dyads, and tetrads was calculated for 12 clones of P. tomentosa with staminate buds at the end of telophase II (Table 1). Of the more than 1000 spores counted in randomly selected anthers from each clone, the frequency of triads, dyads, and tetrads ranged from 0.24 to 18.91, 0.08 to 65.70, and 34.04 to 99.68%, respectively. The correlation analysis showed a significant association (r = 0.64, P < 0.05) only between the fused spindles and dyads (data not show), but the association of parallel and tripolar spindles between dyads and triads were not significant.
Microtubules arrays, chromatin location, and cytokinesis at second meiotic division of MMCs in diploid P. tomentosa. a, d, g Microtubules, b, e, h DAPI staining of chromosome, c, f, I aceto-carmine staining of chromosomes. Bar: 10 μm. a–c Cytokinesis after meiosis, with radial microtubule systems (RMSs) surrounding each nucleus. d–f The beginning of the furrow (arrow), showing the occurrence of simultaneous cytokinesis. g–I RMSs of a small nucleus resulting in a mini-spore (arrows)
Different types of sporads at tetrad stage of diploid P. tomentosa. Bar: 10 μm. a A tetragonal tetrad with four reduced microspores. b A dyad with two nucleoli of nearly the same size being included in each of the two restitution nuclei. c A dyad with two restitution nuclei, but each of them containing two nucleoli. d A dyad with one larger microspore and one smaller microspore, each of the two dyad microspores containing one restitution nucleus. e A dyad with one larger microspore containing three nucleoli and one smaller microspore containing one nucleolus. f A dyad with two microspores of nearly the same size, but with each of them containing two nucleoli. g A triad with the large microspore containing one restitution nucleus and two smaller microspores each containing one nucleolus. h The large microspore of the triad with one restitution nucleus containing two nucleoli. I A linear triad with one restitution nucleus
Pollen grain size distribution
The fertile pollen grains were stained deeply by the 2% aceto-carmine solution, while the aborted ones could either not be stained or could only be stained lightly. Consequently, it was very easy to measure the pollen grains under a light microscope. The size of the pollen grains ranged from 17.50 to 63.75 μm among the 12 clones of diploid P. tomentosa. However, the pollen grain-size distribution of the 12 clones was different. The diameters of the pollen grains of clone 518 ranged from 23.75 to 42.50 μm (Fig. 4a, b), but the largest range was found for clone 539, from 20.00 to 63.75 μm (Fig. 4c, d).
Discussion
Pollen grain size and ploidy level
Pollen size in many plants positively correlates with their ploidy level (Jacob and Pierret 2000). Because the diameter of pollen grains may become larger due to the doubling of chromosome numbers, pollen size has been widely used as an indicator of 2n male gametes (Veilleux et al. 1982). Orjeda et al. (1990) found that the 2n pollen of the sweet potato (Ipomoea trifida) was about 30% larger than normal pollen.
Seitz (1954) observed the presence of giant pollen grains in the anomalous androgynous flowers of the grey poplar (P. canescens) and, subsequently the formation of triploids after self-pollination. The pollen grain of the triploid aspen (P. tremuloides) was found to be between 37 and 44 μm in diameter, and tetraploid hybrids were obtained by crossing pollen of the triploid tree with a diploid female (Winton and Einspahr 1970). The induced unreduced pollen of P. tomentosa was distinctly larger than normal pollen, which can reach >37 μm in diameter (Zhu et al. 1998). Based on these studies, the diameter of unreduced pollen grains in the poplar genus is at least 37 μm in diameter, but in clone 539, the number of triads and dyads (0 and 0.1%, respectively; Table 1) was far too low when compared with the high percentage (more than 50%; Fig. 4c) of pollen grains with a diameter >37 μm. Whether this discrepancy resulted from pollen grains expanding in aceto-carmine or from the genetic background of the clone, more research is needed to elucidate the underlying process. Pollen grains with diameters >37 μm were frequently observed at different frequencies among all 12 clones, and giant pollen grains were also observed in some clones. These giant pollen grains are considered to be either 3n or 4n pollen grains (Tavoletti et al. 2000; Camadro et al. 2008), which would indicate that over-diploid pollen grains do occur in these clones of P. tomentosa.
Cytological aspects of unreduced pollen production
There are three main types of spindle anomalies: parallel spindles, fused spindles, and tripolar spindles at the second meiotic division. All three types have been well documented in many plant species (see reviews in Veilleux 1985; Bretagnolle and Thompson 1995). However, there is some evidence that parallel, tripolar, or fused spindles may be different phenotypic expressions of the same gene (d’Erfurth et al. 2008). An abnormal spindle can cause abnormal cytokinesis and lead to the formation of dyads and triads with 2n pollen (Mok and Peloquin 1975; d’Erfurth et al. 2008). However, the occurrence of parallel spindles at the second division does not necessarily mean that 2n pollen has been formed (Ramanna 1979; Tavoletti et al. 1991). The ‘fused spindle’ at metaphase II has been associated with the formation of dyads (Veilleux et al. 1982; Ramanna 1983).
Using squashed anthers as experimental material and an aceto-carmine solution as the stain, we also observed 2n pollen clones of P. tomentosa. However, no correlation has been found between parallel spindles and dyads (Kang 2002). In our study, we only found a significant association correlation (r = 0.64, P < 0.05) between fused spindles and dyads; the data on the higher occurrence of triads and dyads were conflicting. The formation of predominantly tetrads in clones with a higher frequency of parallel spindles (e.g., Clone 539 in Table 1) as well as our observations that dyads and triads occurred in clones with no fused or no tripolar spindles (e.g., Clones 311 and 539 in Table 1, respectively) demonstrate that parallel spindles not only lead to the formation of dyads, but also to the formation of tetrads and triads. Owing to simultaneous cytokinesis, the RMSs emanating from daughter nuclei define the spore domains and determine the placement of intersporal walls (Brown and Lemmon 1992). The establishment of spore domains via RMSs can account for both the regular and irregular patterns of tetrad formation (Brown and Lemmon 1991).
In P. tomentosa, the position of the sister nuclei confined the RMSs as well as the plane of division. The statistical analysis of abnormal spindles and sporads showed that the frequent parallel spindles may lead to triads and dyads formation at the tetrad stage. This could be due to the gathering of one or two non-sister sets of chromosomes at telophase II, which results in three or two nuclear cytoplasmic domains in late-telophase cells and the division of MMCs into either triads or dyads. The results of our study indicate that the occurrence of numerically unreduced pollen in diploid P. tomentosa was mainly due to the formation of abnormal spindles and premature cytokinesis at the second meiotic division.
Genetic consequences and sexual polyploidization
Depending on the cytological mechanisms involved in the production of unreduced gametes, such gametes are generally categorized as being equivalent to either first-division restitution (FDR) or second-division restitution (SDR) according to their genetic consequences. FDR gametes without crossover will transmit the parental genotype virtually intact to their progenies and preserve a much larger amount of parental heterozygosity and epistasis compared to SDR gametes. Consequently, FDR gametes are particularly valuable in plant sexual polyploidization breeding programs (Ramanna 1983; Ortiz and Peloquin 1994; Bretagnolle and Thompson 1995). The unreduced pollen resulting from abnormal spindles is genetically equivalent to the FDR mechanism, while that from premature cytokinesis is genetically equivalent to SDR (Ramanna 1979; Mok and Peloquin 1975). The unreduced pollen of P. tomentosa caused by abnormal spindles is genetically equivalent to the FDR mechanism, while that from premature cytokinesis is genetically equivalent to SDR. However, there were meiotic chromosome synapsis and recombination was involved in meiosis. As our cytological analysis was performed only to determine the expected heterozygosity of unreduced gametes, more research is needed to reveal the actual level of heterozygosity of the unreduced gametes and their contributions to the progeny.
Natural triploids occur frequently in the genus Populus, and have been widely used in forestation and forestry production (Zhang et al. 2004). These natural triploid hybrids are presumably due to the production of numerically unreduced pollen (Zhang et al. 2004; Zhu et al. 1998). Research on the genetic control of unreduced pollen and the techniques for discovering producers of a higher frequency of unreduced pollen will provide a platform for the genetic improvement and triploid breeding of P. tomentosa.
References
Akutsu M, Kitamura S, Toda R, Miyajima I, Okazaki K (2007) Production of 2n pollen of Asiatic hybrid lilies by nitrous oxide treatment. Euphytica 155:143–152. doi:10.1007/s10681-006-9317-y
Bradshaw HD, Stettler RF (1993) Molecular genetics of growth and development in Populus. I. Triploidy in hybrid poplars. Theor Appl Genet 86:301–307. doi:10.1007/BF00222092
Bretagnolle F, Thompson JD (1995) Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol 129:1–22
Brown RC, Lemmon BE (1991) Pollen development in orchids 2. The cytokinetic apparatus in simultaneous cytokinesis. Protoplasma 163:9–18. doi:10.1007/BF01322286
Brown RC, Lemmon BE (1992) Control of division plane in normal and griseofulvin-treated microsporocytes of Magnolia. J Cell Sci 103:1031–1038
Camadro EL, Saffarano SK, Espinillo JC, Castro M, Simon PW (2008) Cytological mechanisms of 2n pollen formation in the wild potato Solanum okadae and pollen-pistil relations with the cultivated potato, Solanum tuberosum. Genet Resour Crop Evol 55:471–477. doi:10.1007/s10722-007-9254-1
Carputo D, Barone A (2005) Ploidy level manipulations in potato through sexual hybridisation. Ann Appl Biol 146:71–79. doi:10.1111/j.1744-7348.2005.04070.x
Carputo D, Barone A, Frusciante L (2000) 2n gametes in potato: essential ingredients for breeding and germplasm transfer. Theor Appl Genet 101:805–813. doi:10.1007/s001220051547
Conicella C, Capo A, Cammareri M, Errico A, Shamina N, Monti LM (2003) Elucidation of meiotic nuclear restitution mechanisms in potato through analysis of microtubular cytoskeleton. Euphytica 133:107–115. doi:10.1023/A:1025636321757
d’Erfurth I, Jolivet S, Froger N, Catrice O, Novatchkova M, Simon M et al (2008) Mutations in AtPS1 (Arabidopsis thaliana Parallel Spindle 1) lead to the production of diploid pollen grains. PLoS Genet 4(11):1000274. doi:10.1371/journal.pgen.1000274
Den Nijs TPM, Peloquin SJ (1977) 2n gametes in potato species and their function in sexual polyploidization. Euphytica 26:585–600. doi:10.1007/BF00021684
Genualdo G, Errico A, Tiezzi A, Conicella C (1998) α-Tubulin and F-action distribution during microsporogenesis in a 2n pollen producer of Solanum. Genome 41:636–641
Harlan JR, De Wet JMJ (1975) On Ö. Winge and a prayer: the origins of polyploidy. Bot Rev 41:361–390
Jacob Y, Pierret V (2000) Pollen size and ploidy level in the genus Rosa. Acta Hortic 508:289–292
Jauhar PP (2003) Formation of 2n gametes in durum wheat haploids: sexual polyploidization. Euphytica 133:81–94. doi:10.1023/A:1025692422665
Kang XY (2002) Mechanism of 2n pollen occurring in Chinese white poplar. J Beijing For Univ 24(5, 6):67–70
Mendiburu AO, Peloquin SJ, Mok DWS (1974) Potato breeding with haploids and 2n gametes. In: Kasha K (ed) Haploids in higher plants. University of Guelph, Guelph
Mok DWS, Peloquin SJ (1975) Three mechanisms of 2n pollen formation in diploid potatoes. Can J Genet Cytol 17:217–225
Muntzing A (1936) The chromosomes of a giant Populus tremula. Hereditas 21:383–393
Orjeda G, Freyre R, Iwanaga M (1990) Production of 2n pollen in diploid Ipomoea trifida, a putative wild ancestor of sweet potato. J Hered 81:462–467
Ortiz R, Peloquin SJ (1994) Effect of sporophytic heterozygosity on the male gametophyte of the tetraploid potato (Solanum tuberosum L.). Ann Bot 73:61–64
Pfeiffer TW, Bingham ET (1983) Abnormal meiosis in alfalfa, Medicago sativa: cytology of 2n eggs and 4n pollen formation. Can J Genet Cytol 25:107–112
Ramanna MS (1979) A re-examination of the mechanisms of 2n gamete formation in potato and its implications for breeding. Euphytica 28:537–561. doi:10.1007/BF00038921
Ramanna MS (1983) First division restitution gametes through fertile desynaptic mutants of potato. Euphytica 32:337–350. doi:10.1007/BF00021442
Ramsey J (2007) Unreduced gametes and neopolyploids in natural populations of Achillea borealis (Asteraceae). Heredity 98:143–150. doi:10.1038/sj.hdy.6800912
Ramsey J, Schemske DW (1998) Pathway, mechanisms, and rates on polyploid formation in flowering plant. Annu Rev Ecol Syst 29:467–501
Rieseberg LH, Willis JH (2007) Plant speciation. Science 317:910–914. doi:10.1126/science.1137729
Seitz FW (1954) The occurrence of triploids after self-pollination of anomalous androgynous flowers of a grey poplar. Z Forstgenet 3(1):1–6
Sugiura A, Ohkuma T, Choi YA, Tao R, Tamura M (2000) Production of nonaploid (2n = 9x) Japanese persimmons (Diospyros khaki) by pollination with unreduced (2n = 6x) pollen and embryo rescue culture. J Am Soc Hortic Sci 125(5):609–614
Tavoletti S, Mariani A, Veronesi F (1991) Cytological analysis of macro- and microsporogenesis of a diploid alfalfa clone producing male and female 2n gamete. Crop Sci 31:1258–1263
Tavoletti S, Pesaresi P, Barcaccia G, Albertini E, Veronesi F (2000) Mapping the jp (jumbo pollen) gene and QTLs involved in multinucleate microspore formation in diploid alfalfa. Theor Appl Genet 101:372–378. doi:10.1007/s001220051493
Van Tuyl JM, Lim K-B (2003) Interspecific hybridization and polyploidisation as tools in ornamental plant breeding. Acta Hortic 612:13–22
Veilleux R (1985) Diploid and polyploid gametes in crop plants: Mechanisms of formation and utilization in plant breeding. Plant Breed Rev 3:253–288
Veilleux R, McHale NA, Lauer FI (1982) 2n gametes in diploid Solanum: frequency and type of spindle abnormalities. Can J Genet Cytol 24:301–314
Winton L, Einspahr DW (1970) Tetraploid aspen production using unreduced triploid pollen. For Sci 16:180–182
Yao CL, Pu JW (1998) Timber characteristics and pulp properties of the triploid of Populus tomentosa. J Beijing For Univ 20:18–21
Zhang S, Qi L, Chen C, Li X, Song W, Chen R et al (2004) A report of triploid Populus of the section Aigeiros. Silvae Genet 53:69–75
Zhang Z, Kang X, Zhang P, Li Y, Wang J (2007) Incidence and molecular markers of 2n pollen in Populus tomentosa Carr. Euphytica 154:145–152. doi:10.1007/s10681-006-9280-7
Zhu ZT, Kang XY, Zhang ZY (1998) Studies on selection of natural triploid of Populus tomentosa. Sci Silvae Sin 34:22–31
Acknowledgements
We thank the Guan County nursery garden of Shandong province for providing the plant materials. The research was supported by the National Natural Science Foundation of China (Grant No. 30471407).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Kang, X. Cytological characteristics of numerically unreduced pollen production in Populus tomentosa Carr.. Euphytica 173, 151–159 (2010). https://doi.org/10.1007/s10681-009-0051-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0051-0