Abstract
Gametophytic selection has potential to increase the efficiency of breeding for temperature tolerance. Here, we describe orchid seedlings after application of low and high temperatures during gametophytic development. In addition to phenotypic traits, amplified fragment length polymorphism (AFLP) markers were used to determine the genetic variability in seedlings. Two hybrid Phalaenopsis were cross-pollinated and exposed to 30°C day/25°C night for 3 days for a warm pollination or 15°C day/10°C night for 7 days as a cold pollination treatment. The plants were returned to the greenhouse after pollination and green capsules were collected after 150 days. Protocorms obtained from these treatments were evaluated 72 days after initial plating for germination and size on a thermogradient table ranging from 10 to 30°C. Seedlings were then evaluated 1 year after initial plating. The mean number of roots per seedling (4.2) was greater for plantlets that derived from the cold pollination treatment compared to those from warm pollination (3.6). Weight of the seedlings, number of roots and the average root length were significantly affected by the interaction between pollination treatment and germination temperature. The weight, number of leaves, and average root length were significantly affected by the interaction between pollination treatment and incubator/growth chamber. The results indicated that seedlings derived from warm pollination were more vigorous under warm growing conditions and those derived from cold pollination were more vigorous under cold growing conditions. Genetic variation among 16 F1 seedlings randomly selected from various temperature treatments was analyzed. A dendrogram based on 651 loci resulted in three major groups and one subgroup. The groups and subgroup revealed common selection pressure during the gametophytic stage. The AFLP data support genetic differentiation of Phalaenopsis hybrids pollinated under different temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orchids are exotic flowering house plants, second only to poinsettias in popularity in the U.S. (Griesbach 2002). According to the 2007 USDA floriculture crops survey, the potted orchid industry was valued at $126 million (USDA 2008). The genus Phalaenopsis, commonly known as moth orchids, comprises an estimated 50 to 90% of orchids marketed as cut flowers or potted plants in the world (Griesbach 2002; Laws 2004). This genus is native to tropical and subtropical climates (Christenson 2001). Greenhouses are required for commercial production of Phalaenopsis in temperate climates such as Virginia. For retail production, most Phalaenopsis species and hybrids require three phases; vegetative cultivation at high temperatures of 28–32°C, spike induction at low temperature of 17–25°C, and finishing at 17–26°C (Blanchard et al. 2005). Greenhouse facilities located in warm climates only fulfill one or two phases of Phalaenopsis growth and development. Therefore, growers need to heat or cool greenhouse facilities for Phalaenopsis production. The energy input in temperate climates contributes to the high price of orchids. If Phalaenopsis with greater tolerance for temperature fluctuations is developed, greenhouse costs involving temperature control can be reduced.
Gametophytic selection has been used as a tool for crop improvement (Hormaza and Herrero 1992; Sacher et al. 1983). Gametophytic selection plays an important role in angiosperms because pollen grains exposed to stressful environmental conditions can compete in a style for effecting fertilization. Gametophytic selection is expected to be more effective on male gametophytes than on female gametophytes (Pfahler 1975). Applying selective pressure at the gametophytic stage in the plant life cycle provides an opportunity to benefit from possible adaptive value of viable recombinants while avoiding the negative effects of poorly functioning recombinants in angiosperms (Mulcahy 1979). Direct selection on gametophytes avoids dominance issues of sporophytes. Selection pressure for temperature has been applied during the gametophytic generation in several studies (Chi et al. 1999; Clarke et al. 2004; Domínguez et al. 2005; Frova et al. 1995; Maisonneuve et al. 1986; Mandhu et al. 1992; Zamir et al. 1982) and proved successful for increasing the frequency of temperature tolerant progeny (Hormaza and Herrero 1996; Ravikumar and Patil 2002). Therefore, gametophytic selection may be useful for increasing thermotolerance in a temperature sensitive crop such as plants native to tropical and subtropical climates. In addition to gametophytic selection, selection pressure at the early development stage might also benefit plants that grow slowly and have a long life cycle from seed germination to first flowering, i.e., potted flowering orchids.
Molecular marker analysis can be used to validate gametophytic selection (Chandler et al. 2000; Fedoroff et al. 1989; Jorgensen 1993). In plant species where little DNA sequence information is available, amplified fragment length polymorphism (AFLP) based markers have prominent advantages, such as reproducibility, high levels of polymorphism that can be detected in a single reaction and genome-wide distribution compared to other DNA based markers (Vos et al. 1995). AFLP has potential application for screening DNA markers linked to genetic traits (Blears et al. 1998). AFLP analysis uses selective amplification of a subset of restriction enzyme digested DNA fragments to generate a unique fingerprint of a particular genome (Mueller and Wolfenbarger 1999). Despite the advantages and potential applications of AFLP, published literature on its application for the analysis of Phalaenopsis genetic variation in an F1 population is scarce.
In this study, the effects of low temperature and heat stress were evaluated on gametophytic selection, seed germination and development, as well as seedling vigor in a hybrid Phalaenopsis population. AFLP analysis was then used to evaluate genetic variation in Phalaenopsis derived from male gametophytic selection under different temperature regimes.
Materials and methods
Plant material
Hybrid Phalaenopsis (Taisuco Windian × Sogo Yukidian) and a pink-flowered unknown hybrid (Bedford Orchids, Montreal, Canada) were used in this study. The tag of the unknown hybrid was lost during shipping; however, its AFLP fingerprint indicates it is closely related to Phalaenopsis Luchia Pink (Chang et al. 2009). All plants were maintained in a greenhouse at 15–20°C with 70% relative humidity under natural day length until just prior to flower anthesis.
Pollination and temperature treatment
A schematic presentation of the experimental design is given in Fig. 1. Briefly, reciprocal crosses were carried out by hand as inflorescences matured and flowers opened. A flower was pollinated and the plants were placed in a high temperature incubator (30°C day/25°C night) for 3 d. The plants were removed from the high temperature incubator and a second flower on the same plant was pollinated and then the plants were placed in a low temperature incubator (15°C day/10°C night) for 7 d. The length of time in each chamber was selected because 3 and 7 days were required under the warm and cold temperature treatments, respectively, for the pollinated flower petals to shrivel, the first sign that cross-pollination was effective. During the pollination and temperature treatments, 11 h photoperiod with a photosynthetic photon flux (PPF) of 180 μmol m−2 s−1 and 70% relative humidity were maintained in the growth chambers.
A schematic presentation of pollination and post pollination treatments. a Pollinations and crosses under two temperature treatments, warm (30/25°C) and cold (15/10°C) made between two hybrid Phalaenopsis. Each parent was cross-pollinated and then exposed to the warm temperature treatment for 3 days; a second flower on the same inflorescence was then cross-pollinated and the plants were exposed to the cold temperature treatment for 7 days in order to produce both capsules where initial pollen germination and tube growth occurred under warm and cold treatments. b After the pollination temperature treatments, plants were placed in the greenhouse at 15–20°C until pod maturation. c Protocorms germinated on the temperature gradient table (ranging from 30 to 8°C) were divided in half and transferred to fresh media and to either a warm incubator (30°C) or a cold incubator (25°C). Seedlings mature enough to be transferred to a greenhouse were removed from culture and placed on sphagnum moss. Seedlings were then transferred to a warm or cold growth chamber depending on whether grown in a warm or cold incubator. Growth chambers were set at 10/15°C and 25/30°C
Seed germination
After temperature treatments were completed, plants were returned to the greenhouse at 15–20°C. Seed pods were harvested after 150 d. Equal volumes of seeds were sterilized using a saturated solution of calcium hypochlorite (17 g l−1) containing Tween 20 for 10 min and plated onto sterile petri plates containing 35 ml Phytamax medium (Sigma Aldrich, St. Louis, MO) with 5% (v/v) coconut water. Plates were placed on a temperature gradient table with temperatures ranging from 10 to 30°C for seed germination. Multiple plates (4 to 5 per table position) were arranged from the 30°C at position 1 to the 10°C at position 12. The 12 positions on the temperature gradient table differed by approximately 2°C increments.
Protocorm and seedling evaluations
Protocorms were counted under a dissecting microscope 72 d after initial plating and plates were rated according to number and size of protocorms. Protocorms were then divided and transferred to fresh germination media. One of each of the new plates was placed in a warm incubator set at 30°C or a cooler incubator set at 25°C. Once the leaves and first roots developed, plates were evaluated for the number of protocorms (scored by dividing the plate into grids using a paper template), leaf number, root number, and spontaneous clump formation. The second evaluation was conducted 125 d following re-plating.
When the plantlets had at least one leaf and one root in culture, they were transferred to sphagnum moss medium in Phytatray II containers (P5929, Sigma, St. Louis, MO) and placed in growth chambers with one to ten seedlings per container depending upon the number of plantlets available. All seedlings from the warm incubator were placed in the corresponding warm growth chamber set at 30°C day/25°C night and seedlings from the cooler incubator were placed in the cold growth chamber set at 15°C day/10°C night. Each growth chamber was set at a 14 h photoperiod. Seedlings were fertilized every other week with an all-purpose plant food (24-8-16). Watering was done every other day in the warm growth chamber but only once a week in the cool growth chamber. One year after initial plating, seedlings were evaluated for fresh weight, number of leaves, leaf width, leaf length, leaf area, number of roots, and root length. Leaf area was estimated using a non-destructive method (Chen and Lin 2004).
DNA extraction and AFLP analysis
Phalaenopsis seedlings (n = 16) derived from four different treatments were selected randomly for AFLP analysis (Table 1). Genomic DNA was extracted from fresh leaves according to the method of Doyle and Doyle (1990) with some modifications. Fresh leaf samples (0.5 g) were pulverized in liquid nitrogen. CTAB extraction buffer [2% CTAB, 100 mM Tris (pH 8.0), 1.4 M NaCl, 20 mM EDTA, 0.2% (v/v) 2-mercaptoethanol and 4% (w/v) polyvinylpyrrolidine (PVP)] was added and incubated at 60°C for 1 h. The samples were extracted with 10 ml of chloroform: isoamyl alcohol (24:1) and centrifuged. DNA was precipitated and washed accordingly. The DNA was dissolved in 100 μl TE buffer containing 100 μg of RNase and incubated at 37°C for 1 h. The concentration of DNA was measured using a NanoDrop (Thermo Fisher Scientific, Waltham, MA) and quality was checked by electrophoresis on a 0.8% (w/v) agarose gel in TBE buffer.
AFLP analysis was performed according to the AFLP manual A-2015A (Beckman-Coulter, Fullerton, CA) described by Hayashi et al. (2005). EcoRI and MseI enzymes were used for DNA digestion. Adapter ligation, preselective and selective amplification were performed according to the above mentioned protocol. Selective amplification was carried out using six EcoRI and MseI primer combinations as described in Chang et al. (2009). The selective amplified PCR product was analyzed using a CEQ 8800 Genetic Analysis System (Beckman-Coulter, Fullerton, CA). The Frag-4 module of CEQ was used to size all the fragments using DNA size standard 600 (Beckman-Coulter, Fullerton, CA) as an internal DNA size standard.
Data analysis
Protocorm and seedling data were analyzed using SAS general linear models (SAS version 5.1.2600 for Windows, Cary, NC). Mean comparisons were done using Ryan-Einot-Gabriel-Welsch Multiple Range Test. Pollination treatment, germination temperature and incubator/growth chamber effects and their interactions were tested for significance at the P < 0.05 level. All AFLP fragments from CEQ were scored as present or absent. The binary scores were manually compared with the electropherograms to re-confirm presence or absence of peaks. Calculations for the genetic similarity/dissimilarity between all samples were performed with the NTSYSpc software version 2.20 (Rohlf 2005). A phylogenetic tree was constructed using the unweighted pair group method of arithmetic means (UPGMA) based on the Dice index (Nei and Li 1979).
Results
Pod set, seed germination and protocorm evaluation
Although we attempted nearly 200 cross-pollinations among different hybrid clones of Phalaenopsis in the different temperature treatments, we obtained only one set of capsules from hybridization between two clones where abundant seed was produced under both pollination conditions. Capsule set was high under the warm pollination conditions but low under the cold pollination conditions. In addition, capsules that were produced under cold pollination conditions frequently lacked seeds. Hence all seedlings used in the present study were derived from the cross between [‘Taisuco Windian’ × ‘Sogo Yukidian’] and unknown pink hybrid.
Protocorm development on the thermogradient table was first evaluated 72 d after initial plating. The seeds from capsules obtained under warm and cold pollination conditions had been divided into equal volumes and placed on medium in petri plates on the thermogradient table. A comparison of seed germination by two-way ANOVA (pollination treatment and germination temperature) indicated a significant (P < 0.05) effect of the pollination treatment, the germination temperature (P < 0.001) and the interaction between pollination treatment and germination temperature (P < 0.001). Cold pollinated seeds germinated better than warm pollinated seeds at almost all germination temperatures (results not shown).
At the second seedling evaluation conducted at 125 d after initial plating, seedling development varied both within plates and among treatments (Table 2). Some protocorms had leaves and roots whereas others had yet to develop either organ. The main effects of germination temperature (table position) and incubator were highly significant (P < 0.01) factors in protocorm mortality. The interactions between pollination treatment and incubation temperature, as well as germination temperature and incubation temperature, were significant (P < 0.05 for both interactions). Overall, the warm germination temperatures tended to have greater rates of mortality than cooler germination temperatures (more than 40% mortality for the three greatest germination temperatures compared to 14% for the five lowest germination temperatures).
Pollination treatment did not significantly affect the frequencies of protocorms at various developmental stages (protocorms without leaves or roots, seedlings with leaves, seedlings with leaves and roots, and clump development). However, germination temperature significantly affected frequency of protocorms without leaves or roots (P < 0.01), frequency of seedlings with leaves but no roots (P < 0.05) and percent of seedlings in clumps (P < 0.01). More than 40% of seedlings germinated at cooler temperatures (16–22°C) were scored as protocorms without leaves or roots whereas less than 18% of seedlings germinated at the warmer temperatures (24–30°C) fell into this category. Clump formation was greatest (67 and 42%) at germination temperatures of 26 and 28°C.
The incubation temperature significantly affected the frequencies of protocorms without leaves or roots, seedlings with both leaves and roots, and protocorms or seedlings in clumps (P < 0.01 for all three parameters). Development of protocorms was more advanced for seedlings incubated at 30°C compared to 25°C (Table 3). Only 3% of seedlings had both leaves and roots in the cooler incubator at 125 days after plating compared to 24% for the warm incubator. A greater percentage (45% compared to 16%) of protocorm forming “clumps” was produced in the warm incubator (30°C).
Final seedling evaluation
After the seedlings had grown for 1 year, six different measurements were taken as they were transferred from the growth chamber to the greenhouse. The only remaining significant main effect of pollination treatment at this stage was on the mean number of roots. Cold pollination derived seedlings had significantly more roots than warm pollination derived seedlings (4.2 and 3.6 roots per seedling, respectively). As might be expected, incubator was a significant source of variation for five of six traits (only leaf width was not significant) with greater growth in the warmer incubator (data not shown). In addition, five of six interactions between pollination temperature and incubation temperature were significant (mean weight of seedlings, mean number of leaves, mean leaf length, mean root number and mean root length). For nine of ten comparisons of these five traits, warm-pollination-derived seedlings outperformed cold-pollination seedlings in the warm incubator and conversely, cold-pollination-derived seedlings outperformed warm-pollination-derived seedlings in the cooler incubator (Table 4).
AFLP analysis
A total of 651 loci ranging in size from 100 to 350 bp was detected using six primer combinations, of which 387 loci (59.4%) were polymorphic. The number of polymorphic fragments for each primer combination ranged from 53 (E-CAG/M-CGT) to 81 (E-CAT/M-CCG). The average number of polymorphic loci detected was 64.5 per primer combination. Percentages of polymorphic loci among primer combinations ranged from 53.7% (E-CAT/M-CGC) to 64.6% (E-CAG/M-CGT). Seedlings derived from germination at 20°C, warm-pollination, and warm-incubation revealed 25.5% of polymorphism. The greatest polymorphism (35.9%) in different temperature treatments was found for seedlings derived from germination at 18°C, warm-pollination, and warm-incubation.
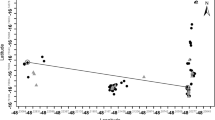
Genetic similarities among the 16 Phalaenopsis siblings derived from four combinations of germination, pollination, and incubation temperature conditions were estimated. Similarity values among individual samples ranged from 0.825 to 0.946 on the Dice index. Two Phalaenopsis seedlings (13 and 14) derived from the same condition (germination at 18°C and warm-pollination-derived seedlings in the warm incubator) were the most closely related, whereas two of Phalaenopsis seedlings (7 and 16) derived from different germination and incubation temperature treatments were the most distantly related. Relationships among 16 Phalaenopsis seedlings (Fig. 2) indicated three major groups (Group I, Group II, and Group III), representing three different temperature sets. Group I includes all seedlings derived from germination at 20°C, warm pollination and warm incubation treatments. Group II includes almost all seedlings derived from germination at 20°C, warm pollination and cold incubation treatments. All seedlings from germination at 18°C, cold pollination and warm incubation conditions were placed in Group III. One subgroup in Group III was distinguished for two individual seedlings (13 and 14) from germination at 18°C, warm pollination and warm incubation conditions.
Genetic similarity analysis of 16 Phalaenopsis seedlings derived from the same cross but pollinated and cultivated at different temperature conditions. Dendrogram was generated from the AFLP data based on the Dice coefficient (Nei and Li 1979) of genetic similarities using UPGMA analysis. The seedling numbers as described in Table 1. Pollination, germination and incubation treatments occur beside each seedling
Discussion
The significant interaction effects in the ANOVA of growth traits of Phalaenopsis seedlings measured 1 year after initial plating indicated that exposure of male gametophytes of Phalaenopsis to different temperature regimes during pollination influenced seedling thermotolerance. It is known that in Phalaenopsis, ovules do not develop prior to pollination but rather their differentiation is induced by pollination; within 2 days after pollination, cell proliferation is initiated along the placental ridges marking the first visible stage of the future female gametophytes (Zhang and O’Neill 1993). Within 14 d, the placental protuberances further enlarge and vascular bundles only begin to differentiate from a single epidermal layer of the placenta (Zhang and O’Neill 1993). In our study, although these cells may have been affected by the temperature treatments the timing and short duration of the treatments (warm temperature treatment was applied for 3 d and the cold treatment for 7 d), would be expected to influence male rather than female gametophyte development (Hormaza and Herrero 1996).
Hypothetically, selective pressures during pollen germination and tube growth should have been applied through the entire 85 d between pollination and fertilization in Phalaenopsis (Zhang and O’Neill 1993). However, treatment for this period was not realistically feasible. The temperature treatments selected in our study were too extreme and would have caused the reproductive tissues to fail. Thus, the treatment durations were applied for a 3 to 7 d period in 198 crosses that we attempted. Only two pollinations were done at a time on a single plant and care was taken emulating natural pollination progression so that spontaneous abortion would be less likely to occur. Less extreme temperatures might have been used, but selective pressures may not have been strong enough to ensure the progeny would demonstrate any alternation in allele frequency favoring thermotolerance (Hormaza and Herrero 1996). Due to the lag between pollination and the maturation of the ovules, pollen tube growth is arrested and the sperm and vegetative cell are not active. Gametophytic selection for thermotolerance is thought to be most effective while pollen is active during microspore development or pollen germination and tube growth (Frova et al. 1995; Hormaza and Herrero 1996). Male gametophytic selection in our study most likely occurred through the death of pollen that were not resistant to the temperatures applied during the pollination treatments. Although the temperature treatments were applied only during the initial stages of pollen germination, due to the extreme nature of the selective pressure, male gametophytic selection may have been successful in producing progeny with the ability to outperform in a selected environment.
Seedling development at 125 d after initial plating indicated that germination temperature and incubation temperatures influenced leaf development, root development and mortality. However, seedling characteristics measured at this time may not necessarily be indicative of the future capability of the progeny. Phalaenopsis species and hybrids are categorized by the American Orchid Society as warm growing orchids, so it is not surprising that colder germination temperatures produced fewer seedlings. Growth was also slower in the cool incubator for both warm and cold pollination derived seedlings. However, the two incubators where protocorms were transferred after initial germination only differed by 5°C, i.e., 25°C for cooler incubation and 30°C for warmer incubation. This temperature difference was enough to significantly improve leaf and root production of protocorms at the higher temperature (greater seedling weight, more and longer leaves and roots).
Warm-pollination-derived seedlings at the two coldest germination temperatures, 16 and 18°C, germinated poorly and developed slowly. However, they exhibited a low rate of mortality whereas cold-pollination-derived seeds had greater germination. The improved germination of cold-pollination-derived seeds is supported by Johannsson and Stephenson (1998a). They found that Cucurbita pollen developed at 20°C (low temperature) produced more seeds than pollen developed at 30°C (high temperature) indicating that temperature affected pollen performance. In addition, sporophytic provisioning of pollen and seeds has been a favored explanation in another study (Johannsson and Stephenson 1998b) where progeny derived from one pollination treatment consistently outgrew another in post pollination environmental conditions. Sporophytes grown under cooler conditions may provide more resources to developing pollen, thus allowing for paternal provisioning to affect the future progeny (Delph et al. 1997). However, pollen used in our study was produced under the same conditions. Any effect of paternal provisioning during pollen development would be irrelevant, because pollen used in both the high and low temperature treatments would have had the same resources allocated from the sporophyte.
Another explanation for the improved germination of cold-pollination-derived seeds is a possible link between cold tolerance and germination. Thermotolerance is a complex trait and is regulated at all levels of plant organization including components on the cellular and subcellular level that are difficult to detect (Ottaviano and Sari Gorla 1993). Many genes that are active in pollen mediate basic metabolic activities such as those involved with energy production and starch synthesis (Ottaviano and Mulcahy 1989). Due to the overlap between sporophytic and gametophytic transcriptomes, it is likely that genes conferring adaptability to low temperatures could also improve germination through enhanced cellular or sub-cellular activity.
Spontaneous proliferation of plantlets from a single protocorm or clump was observed in seedlings 125 d after initial plating. The number of plantlets from a single seed ranged from 2 to 15. This phenomenon has been reported in orchid biology (Arditti 1992; Batygina 1998; Batygina and Andronova 2000; Batygina and Shevtsova 1985; Shevtsova et al. 1986; Singh and Thimmappaiah 1982). Protocorms are rarely observed in nature (Tatarenko and Vakhrameeva 1998), whereas many protocorms may be obtained under in vitro culture conditions (Batygina and Shevtsova 1985; Shevtsova et al. 1986). Seeds in this study were germinated asymbiotically on a nutrient medium containing coconut water. Coconut water is liquid endosperm obtained from immature coconuts (Hartmann et al. 1997). Nine cytokinins present in coconut water, including isoprenoid and aromatic cytokinins, play an important role to promote cell division in callus tissue (Ge et al. 2004, 2006). The polyol myo-inositol, a constituent of coconut water has been reported to increase callus induction in Phalaenopsis PLB (Ishii et al. 1998). Therefore, we suspect that coconut water in the medium may have affected the proliferation of protocorms. However, this hypothesis needs to be confirmed.
The present study represents the first known use of AFLP markers to define genetic differentiation in gametophytic selection in Phalaenopsis. We initially attempted to identify markers associated with the temperature tolerance trait using pools of individuals from each pollination treatment; however, this was unsuccessful (results not shown). Therefore, we randomly sampled individual plants representing each treatment. The AFLP data revealed relatively low polymorphism among different germination, pollination, and incubation temperature treatments. The similarity coefficient obtained from the AFLP analysis indicated that the amount of genetic diversity was low among the siblings within our gametically selected population. Chen et al. (1999) observed low genetic differences (11.6%) between intergenic hybrid clones of Vandaceous orchids. Our Phalaenopsis F1 hybrids under various temperature treatments exhibited 13.9 to 24.3% polymorphism, much higher than Vandaceous hybrid clones. The difference in polymorphism may be due to innate differences between the genera. Commercial Phalaenopsis have been developed through extensive interspecific hybridization that would generate polymorphism in segregating progenies (Chang et al. 2009).
The dendrogram shows clustering of Phalaenopsis hybrids pollinated under different temperature treatments. Only a few studies have been done on temperature-based selection of populations using AFLP analysis. Kelly et al. (2003) found clear genetic difference between Betula pendula samples acclimated under different climatic conditions. In Lolium, Skøt et al. (2002) identified markers that were associated with low temperature tolerance. Their cluster analyses showed that populations from cold regions distinguished clearly from the other populations. The results of this study indicate that genetic differentiation may have occurred within populations in response to selection pressure. However, the clustering of individuals would be better served by a greater population size.
In conclusion, the use of gametophytic selection as a tool in breeding Phalaenopsis for thermotolerance was evaluated. Poor pod set of Phalaenopsis at cool temperatures limited our comparisons to only a single family. Despite this limitation, there were indications that gametophytic selection for thermotolerance affected subsequent seedling performance. It may be possible to exploit this selection to develop hybrids more tolerant of temperature extremes. However, the study would need to be continued through subsequent development of the seedlings through flowering while growing under different temperature regimes. Further research on marker analysis for seedlings derived from gametophytic selection would improve the ability to select for thermotolerance in Phalaenopsis. Functional genomics tools such as expressed sequence tag (EST) analysis, gene expression analysis using microarray or proteome comparisons would help identify thermotolerance genes and understand their function under stress conditions.
References
Arditti J (1992) Fundamentals of orchid biology. Wiley, New York, NY
Batygina TB (1998) Certain aspects of the reproductive system in orchids. Byulleten Botanicheskogo Sada IS Kosenko 7:155–157
Batygina TB, Andronova EV (2000) The orchid protocorm: an opinion. In: Mukerji K (ed) Glimpses in botany. APH Publishing, New Delhi, India, pp 60–77
Batygina TB, Shevtsova GG (1985) Metamorphosis in orchid ontogenesis (on the example of Cymbidium hybridum, Orchidaceae). Botanicheskii Zhurnal 70:1614–1621
Blanchard M, Lopez R, Runkle E, Wang YT (2005) The orchid grower. Greenhouse Grower 10:86–89
Blears MJ, De Grandis SA, Lee H, Trevors JT (1998) Amplified fragment length polymorphism (AFLP): a review of the procedure and its applications. J Ind Microbiol Biotechnol 21:99–114
Chandler VL, Eggleston WB, Dorweiler JE (2000) Paramutation in maize. Plant Mol Biol 43:121–145
Chang YK, Iqbal MJ, Veilleux RE (2009) Analysis of genetic variability among Phalaenopsis species and hybrids using amplified fragment length polymorphism. J Am Soc Hortic Sci 134:58–66
Chen C, Lin RS (2004) Nondestructive estimation of dry weight and leaf area of Phalaenopsis leaves. Appl Eng Agric 20:467–472
Chen XL, Lim SH, Wong SM, Lee YH, Kuo J, Yam TW, Lin JJ (1999) Amplified fragment length polymorphism analysis of vandaceous orchids. Plant Sci 141:183–189
Chi HS, Straathof TP, Löffler HJM, Van Tuyl JM (1999) In vitro selection for heat tolerance in lilies. In: Clement C, Pacini E, Audran JC (eds) Anther and pollen from biology to biotechnology. Springer-Verlag, Heidelberg/Berlin, Germany, pp 175–182
Christenson EA (2001) Phalaenopsis. Timber Press, Portland, OR
Clarke HJ, Khan TN, Siddique KHM (2004) Pollen selection for chilling tolerance at hybridisation leads to improved chickpea cultivars. Euphytica 139:65–74
Delph LF, Johannsson MH, Stephenson AG (1997) How environmental factors affect pollen performance: ecological and evolutionary perspectives. Ecology 78:1632–1639
Domínguez E, Cuartero J, Fernández-Muñoz R (2005) Breeding tomato for pollen tolerance to low temperatures by gametophytic selection. Euphytica 142:253–263
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fedoroff N, Masson P, Banks JA (1989) Mutations, epimutations, and the developmental programming of the maize suppressor-mutator transposable element. Bioessays 10:139–144
Frova C, Portaluppi P, Villa M, Gorla MS (1995) Sporophytic and gametophytic components of thermotolerance affected by pollen selection. J Hered 86:50–54
Ge LY, Yong JWH, Tan SN, Yang XH, Ong ES (2004) Analysis of some cytokinins in coconut (Cocos nucifera L.) water by micellar electrokinetic capillary chromatography after solid-phase extraction. J Chromatogr A 1048:119–126
Ge LY, Yong JWH, Tan SN, Ong ES (2006) Determination of cytokinins in coconut (Cocos nucifera L.) water using capillary zone electrophoresis-tandem mass spectrometry. Electrophoresis 27:2171–2181
Griesbach RJ (2002) Development of Phalaenopsis orchids for the mass-market. In: Janick J, Whipkey A (eds) Trends in new crops and new uses. ASHS Press, Alexandria, VA, pp 458–465
Hartmann HT, Kester D, Davies F, Geneve R (1997) Plant propagation: principles and practices. Prentice Hall, Upper Saddle River, NJ
Hayashi E, Chi HC, Boyer SK, Still DW (2005) Amplified fragment length polymorphism protocol for plant science on CEQ series genetic analysis system. Beckman Coulter, Fullerton, CA
Hormaza JI, Herrero M (1992) Pollen selection. Theor Appl Genet 83:663–672
Hormaza JI, Herrero M (1996) Male gametophytic selection as a plant breeding tool. Hort Sci 65:321–333
Ishii Y, Takamura T, Goi M, Tanaka M (1998) Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rpt 17:446–450
Johannsson MH, Stephenson AG (1998a) Effects of temperature during microsporogenesis on pollen performance in Cucurbita pepo L. (Cucurbitaceae). Int J Plant Sci 159:616–626
Johannsson MH, Stephenson AG (1998b) Variation in sporophytic and gametophytic vigor in wild and cultivated varieties of Cucurbita pepo and their F1 and F2 generations. Sex Plant Reprod 11:265–271
Jorgensen R (1993) The germinal inheritance of epigenetic information in plants. Phil Trans R Soc Lond B 339:173–181
Kelly CK, Chase MW, de Bruijn A, Fay MF, Woodward FI (2003) Temperature-based population segregation in birch. Ecol Lett 6:87–89
Laws N (2004) The World’s fascination with potted orchids. Floraculture Intl 14:26–27
Maisonneuve B, Hogenboom NG, Dennijs APM (1986) Pollen selection in breeding tomato (Lycopersicon-Esculentum Mill) for adaptation to low temperature. Euphytica 35:983–992
Mandhu B, Cresti M, Shivanna KR (1992) Effects of high temperature and humidity stresses on tobacco pollen and their progeny. In: Ottaviano E, Mulcahy DL, Sari Gorla M, Mulcahy GB (eds) Angiosperm pollen and ovules. Springer-Verlag, Heidelberg/Berlin, Germany, pp 349–354
Mueller UG, Wolfenbarger LL (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Mulcahy DL (1979) Rise of the angiosperms: a genecological factor. Science 206:20–23
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Ottaviano E, Mulcahy DL (1989) Genetics of angiosperm pollen. Adv Genet 26:1–64
Ottaviano E, Sari Gorla M (1993) Gametophytic and sporophytic selection. In: Hayward MD, Bosemark NO, Romagosa I (eds) Plant breeding: principles and prospects. Chapman, New York, NY, pp 332–352
Pfahler PL (1975) Factors affecting male transmission in maize (Zea mays L.). In: Mulcahy DL (ed) Gamete competition in plants and animals. North Holland, Amsterdam, Netherlands, pp 115–124
Ravikumar RL, Patil BS (2002) Pollen response as markers (PRM) and pollen selection: Novel tools in crop improvement. In: Dris R, Bary-Ryan C (eds) Plant physiology: characteristics, breeding and genetics. Science Publishers, Enfield, NH, p 212
Rohlf FJ (2005) NTSYS-pc numerical taxonomy and multivariate analysis system. Version 2.2. Exeter software, Setauket, NY
Sacher RF, Mulcahy DL, Staples RC (1983) Developmental selection during self-pollination of Lycopersicon × Solanum F1 for salt tolerance of F2. In: Mulcahy DL, Ottaviano E (eds) Pollen: biology and implication for plant breeding. Elsevier Press, New York, NY, pp 329–334
Shevtsova GG, Batygina TB, Lavrenteva AN (1986) Certain aspects of renewal system in orchids in Cymbidium hybridum (Orchidaceae) as an example. Botanicheskii Zhurnal 71:1457–1467
Singh F, Thimmappaiah M (1982) Polyembryony in orchid seeds. Seed Sci Technol 10:29–33
Skøt L, Hamilton NRS, Mizen S, Chorlton KH, Thomas ID (2002) Molecular genecology of temperature response in Lolium perenne: II. Association of AFLP markers with ecogeography. Mol Ecol 11:1865–1876
Tatarenko IV, Vakhrameeva MG (1998) On vegetative propagation in orchids. Byulleten Botanicheskogo Sada IS. Kosenko 7:155–157
US Department of Agriculture (2008) Floriculture crops 2007 summary. Agr Stat Board, Washington, DC
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Zamir D, Tanksley SD, Jones RA (1982) Haploid selection for low temperature tolerance of tomato pollen. Genetics 101:129–137
Zhang XS, O’Neill SD (1993) Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell 5:403–418
Acknowledgements
The authors thank Chadwick and Son Orchids, Floradise Orchids, Bedford Orchids, and Carmel Orchids for the plant material used in this project and orchid information, and Rubina Ahsan for technical advice with AFLP. We would also like to thank the Institute for Advanced Learning and Research (IALR) for supplying materials. This project was supported by a grant from the United States Department of Agriculture (USDA 2003-38891-02112), USDA HATCH funds (135816), as well as through operating funds provided by the Commonwealth of Virginia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yeun-Kyung Chang and Leslie A. Blischak contributed equally.
Rights and permissions
About this article
Cite this article
Chang, YK., Blischak, L.A., Veilleux, R.E. et al. Effect of temperature on gametophytic selection in a Phalaenopsis F1 population. Euphytica 171, 251–261 (2010). https://doi.org/10.1007/s10681-009-0040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0040-3