Abstract
Pineapple is one of the most important tropical fruits and therefore intensive genetic improvement programs are being carried out in many countries, including Cuba. Our research team has previously introduced the bar gene, along with chitinase and AP24 genes, into the pineapple genome. Herein, we report on the biochemical side effects of the herbicide FINALE® on these transgenic plantlets during hardening. Levels of aldehydes and chlorophylls, and peroxidase activity were recorded. The transformed clone studied here, not sprayed with FINALE®, showed the following side effects because of transgenesis only. Levels of malondialdehyde, other aldehydes, chlorophyll b, and total chlorophyll pigments decreased. The most remarkable biochemical differences between transgenic and non-transgenic plantlets after application of FINALE® follow. Levels of malondialdehyde and other aldehydes in transgenic material were not decreased by FINALE®, perhaps because these levels were already low as a result of transformation. FINALE® increased peroxidase activity in transgenic plantlets but such increase was higher in non-transgenic material. The herbicide increased contents of chlorophyll pigments (a, b, total) in transformed plantlets. However, as expected, non-transgenic plantlets decreased levels of chlorophylls (a, b, total) after application of FINALE®. The genetic transformation of pineapple with the bar gene not only conferred resistance to the herbicide FINALE®, but also promoted other biochemical changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pineapple world production reached 18.2 million tons in 2006 (FAOSTAT 2008). Therefore, several research groups are developing basic and applied studies to create new varieties with better agronomic performance. We previously developed a protocol for pineapple genetic transformation introducing the chitinase, AP24 and bar genes into the pineapple genome under the control of the following promoters: OCS-35S CaMV-rice actin I, 35S CaMV and maize Ubi1, respectively (Espinosa et al. 2002). These promoters have been described as constitutive transcription promoters (Franck et al. 1980; Christensen et al. 1992).
Chitinase and AP24 have been described as antifungal genes. The chitinase gene (from Phaseolus vulgaris) product degrades chitin: an essential compound of most of the fungal cell walls (Broglie et al. 1986; Schlumbaum et al. 1986). The AP24 gene (from Nicotiana tabacum) codes for a protein that destabilizes the fungal membrane (Singh et al. 1989; Woloshuk et al. 1991). We introduced these two antifungal genes into the pineapple genome as an attempt to reduce the great losses caused by Phytophthora nicotianae var. parasitica (Kamoun 2001). These phytopathological studies are in progress in our field experimental station. Regarding the safety profile studies of these two genes, we have not found reports about AP24. However, class-I chitinases have been identified as the major panallergens in fruits associated with the latex-fruit syndrome, such as the uncooked-consumed avocado, banana and chestnut (Blanco et al. 1994; Brehler et al. 1997; Sanchez-Monge et al. 2000).
We used the bar gene as a selectable marker. It was cloned from Streptomyces hygroscopicus and encodes for phosphinotricin acetyltransferase (Thompson et al. 1987). This enzyme is capable of inactivating phosphinotricin that is the active compound of the non-selective herbicide FINALE® (Torres et al. 1999). After application of FINALE® onto non-transformed plants, phosphinotricin inhibits the enzyme glutamine synthetase (Mohapatra et al. 1999). Such an inhibition causes plant toxicity to ammonium provoking death (D'Halluin et al. 1992). Phosphinotricin acetyltransferase proteins have been deeply studied (Wehrmann et al. 1996) and have an excellent safety profile (Herouet et al. 2005).
The above mentioned references indicate that genes and promoters used for pineapple genetic transformation are well known, at least in their primary effect. However, biochemical side effects of the herbicide FINALE® on bar gene-containing transgenic pineapple plantlets have not been explored to date. Although several metabolic studies on transgenic plants have shown effects of transformation (Momma et al. 1999; Wilson and Latham 2006), investigation of the biochemical unexpected side effects could help to positively impact the public perception on genetically modified plant food (Kuiper et al. 2001).
Based on these prospects, we studied some of the biochemical side effects of pineapple genetic transformation in interaction with the herbicide FINALE®. The present report is focused on the evaluation of the early stage of greenhouse hardening of transformed and non-transformed pineapple plantlets. Levels of aldehydes and chlorophyll, and the activity of peroxidases were recorded. We selected these compounds because they are related to a wide range of important biochemical and physiological pathways such as plant response to stress and photosynthesis (Porras 1991; Gross et al. 2000; Moller 2001; Yaginuma et al. 2002). We explored other biomarkers different from those recommended by the OECD (1993) because we evaluated very young pineapple plantlets far for fruiting and marketing. Plant survival was also evaluated.
Materials and methods
Pineapple c.v. Serrana Smooth Cayenne was used. Transgenic plantlets were obtained according to Espinosa et al. (2002). Embryogenic calli were gently passed through polypropylene meshes (2000 μm pore diameter, Spectrum) and dried for 15 min in the laminar flow cabinet. Agrobacterium tumefaciens suspension was then added (strain AT2260, pHCA58, containing a bar gene controlled by maize Ubi 1 promoter, a class-I bean chitinase gene controlled by a hybrid OCS-35S CaMV-rice actin I promoter, and a tobacco AP24 gene controlled by 35S CaMV promoter). After 10 min, infected calli were washed with distilled water and dried with filter paper. Co-culture was allowed for 24 h (darkness, 25°C). Calli were then transferred to the callus proliferation medium supplemented with 0.2 g l−1 cefotaxime (4 weeks, 25°C, 16 h light photoperiod, 2000 lux). Calli were transferred to temporary immersion bioreactors for plant regeneration in a selective medium (2.5 mg l−1 phosphinotricin). After 45 days, phosphinotricin-resistant plantlets were recovered. Non-transformed plantlets (control treatment) were obtained following the protocol described above but avoiding contact with Agrobacterium tumefaciens and phosphinotricin.
About 120 non-transformed and 120 transformed (one clone) plantlets were transferred to a greenhouse for hardening according to Yanes et al. (2000). Plantlets were placed in plastic trays containing 82 cm3 of a mixture zeolite ± filter cake (1:1). Microject automated irrigations for 25 s every 30 min were applied. Plantlets were kept under a photosynthetic photon flux density of 458 μmol m−2 s−1. Standard phytosanitary controls were applied. About 60 non-transformed and 60 transformed plantlets were sprayed with the herbicide FINALE® at 12 l ha−1 as recommended by Bayer CropScience (2005). Evaluations started immediately after application of the herbicide (day 0: less than 1 min after herbicide application) and after 15 or 30 days. The experimental design was completely randomized. In a previous experiment (data not shown) we compared resistance to herbicide FINALE® of 100 transformed clones. In the present study we used the transgenic clone that previously showed the lowest foliar damage after application of FINALE®.
Plant survival was recorded. Leaf samples were stored in liquid nitrogen at 15 days after application (or not) of the herbicide. Each biochemical determination started from three independent pooled samples (100 mg each). They were finely grounded in liquid nitrogen. Contents of malondialdehyde and other aldehydes (Heath and Packer 1968), chlorophyll (a, b, total; (Porras 1991)), and peroxidase activity (Hammerschmidt et al. 1982) were measured. The experiment was repeated 3 times.
Results and discussion
Genetic transformation with the bar gene protected the plantlets from the effect of herbicide FINALE®. Most of non-transformed plantlets had died at 15 days after the herbicide application (Fig. 1a).
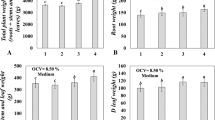
Survival, levels of aldehydes and chlorophylls, and peroxidase activity during hardening of transformed or non-transformed pineapple plantlets, sprayed or not with herbicide FINALE®. For each indicator, results with the same letter are not statistically different (ANOVA, Tukey, P > 0.05, Statistical Package for Social Sciences, version 8.0 for Windows, SPSS Inc.). Plant survival percentages were transformed, for statistical analysis only, according to y′ = 2 × (arcsine (y/100)0.5)
Biochemical analyses of surviving plant leaves (15 days), showed a detrimental effect of FINALE® on malondialdehyde level in non-transformed plantlets. However, this effect of the herbicide was not observed in transgenic plantlets. Level of malondialdehyde in non-transformed plantlets, not spayed with FINALE®, was relatively high (Fig. 1b). Regarding content of other aldehydes, results were similar to malondialdehyde evaluations (Fig. 1c). FINALE® increased peroxidase activity in both non-transformed and transformed plantlets but its effect on non-transgenic plantlets was more remarkable (Fig. 1d).
The herbicide dramatically decreased content of chlorophyll a in non-transformed plantlets. This may be the result of the deleterious effect of FINALE®. Contrastingly, the herbicide increased levels of chlorophyll a in transgenic plantlets. This experimental treatment reached the highest chlorophyll a level observed (Fig. 1e).
Levels of chlorophyll b and total chlorophyll contents were modified by FINALE® in the same way (Fig. 1f, g). Transgenic plantlets sprayed with the herbicide showed the highest total chlorophyll content recorded (Fig. 1g).
The transformed clone studied here, not sprayed with FINALE®, showed the following side effects because of transgenesis with chitinase, AP24 and bar genes. Levels of malondialdehyde, other aldehydes, chlorophyll b, and total chlorophyll pigments decreased. For plants generated by recombinant technology, side effects (such as those observed in our experiment) may arise from the process of introducing foreign genes or as a result of the interaction among the transgene, the genetic background of the plant and the environment (Meyer 1999). Moreover, random insertion of DNA sequences can cause modification, interruption or silencing of existing genes as well as activation of silent genes (Codex 2003).
The most remarkable biochemical differences between transgenic and non-transgenic plantlets after application of FINALE® follow. Levels of malondialdehyde and other aldehydes in transgenic material were not decreased by FINALE®, perhaps because these levels were already low as a result of transformation. FINALE® increased peroxidase activity in transgenic plantlets but such increase was higher in non-transgenic material. The herbicide increased contents of chlorophyll pigments (a, b, total) in transformed plantlets. However, as expected, non-transgenic plantlets decreased levels of chlorophylls (a, b, total) after application of FINALE®.
Levels of malondialdehyde and other aldehydes, and peroxidase activity, have been described to be closely connected. Malondialdehyde is one of the primary metabolite of plant response to stress (e.g. herbicides, (Dumet and Benson 2000). It results from peroxidation of cell membrane lipids, and promotes formation of other aldehydes (Moller 2001). It is also well documented that as a result of reactive oxygen species action, besides aldehydes, hydrogen peroxide is formed. Then peroxidase activity is increased (Gross et al. 2000; Breusegem et al. 2001; Moller 2001; Arora et al. 2002; De Jong et al. 2002; Kuo and Kao 2004). According to these references, levels of malondialdehyde and other aldehydes, and peroxidase activity were expected to increase after FINALE® application. However, results shown in Fig. 1 (b, c) do not support these statements. Levels of malondialdehyde and other aldehydes were not increased by the herbicide. Perhaps, biochemical evaluations were performed too late (15 days) after application of the herbicide and therefore, the initial changes were not recorded. Only measurements of peroxidase activity agreed these previous reports (Fig. 1d).
Regarding measurements of chlorophyll pigments, it is convenient to take into consideration that reaction of glutamine synthetase 2 takes place in chloroplasts (Maldonado 1993) and this is the target enzyme inhibited by phosphinotricin (Metz et al. 1998). Therefore, it was expected that chlorophyll structures were affected in non-transformed pineapple plantlets after herbicide application (Fig. 1e, f, g). Contrastingly, the positive effect of FINALE® on formation of chlorophyll pigments (a, b, total) in transgenic plantlets was not previewed. It might be that stress caused by the herbicide on transgenic material, somehow destabilized temporarily the chloroplast structure. Then, transformed plantlets synthesized more chlorophyll to keep photosynthesis efficiency at the same level as under non-stress conditions. This kind of general physiological response to compensate for damages has been previously described (Scarpari et al. 2005). At present, pineapple transgenic plants are being studied at the Bioplant Center’s Field Experimental Station.
References
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Bayer (2005) Technical information. In: Bayer (ed) Glufosinate-ammonium. Bayer CropScience, 38 pp
Blanco C, Carrillo T, Castillo R et al (1994) Latex allergy: clinical features and cross-reactivity with fruits. Ann Allergy 73:309–314
Brehler R, Thiessen U, Mohr C et al (1997) “Latex-fruit syndrome”: frequency of cross-reacting IgE antibodies. Allergy 52:404–410. doi:10.1111/j.1398-9995.1997.tb01019.x
Breusegem FV, Vranová E, Dat JF et al (2001) The role of oxygen species in plant signal transduction. Plant Sci 161:405–414. doi:10.1016/S0168-9452(01)00452-6
Broglie KE, Gaynor JJ, Broglie RM (1986) Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci USA 83:6820–6824. doi:10.1073/pnas.83.18.6820
Codex (2003) Codex Alimentarius Commission. In: Joint FAO/WHO Food Standard Programme Codex ad hoc intergovernmental task force on foods derived from biotechnology. Available via: FAO/WHO. http://www.who.int/fsf/GMfood/codex_index.htm, http://www.codexalimentarius.net/ccfbt4/bt03_01e.htm. Accessed 27 May 2008
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689. doi:10.1007/BF00020010
D’Halluin K, De Block M, Denecke J et al (1992) The bar gene as a selectable and screenable marker in plant genetic engineering. Methods Enzymol 216:415–426. doi:10.1016/0076-6879(92)16038-L
De Jong AJ, Yakimova ET, Kapchina VM et al (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214:537–545. doi:10.1007/s004250100654
Dumet D, Benson EE (2000) The use of physical and biochemical studies to elucidate and reduce cryopreservation-induced damage in hydrated/desiccated plant germplasm. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm: current research progress and application. JIRCAS/IPGRI, Tsukuba/Rome, pp 43–56
Espinosa P, Lorenzo JC, Iglesias A et al (2002) Production of pineapple transgenic plants assisted by temporary immersion bioreactors. Plant Cell Rep 21:136–140. doi:10.1007/s00299-002-0481-9
FAOSTAT (2008) Available via: FAO STATISTIC DIVISION. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567. Accessed 23 May 2008
Franck A, Guilley H, Jonard G et al (1980) Nucleotide sequence of cauliflower mosaic virus DNA. Cell 21:285–294. doi:10.1016/0092-8674(80)90136-1
Gross NT, Hultenby K, Mengarelli S et al (2000) Lipid peroxidation by alveolar macrophages challenged with Cryptococcus neoformans, Candida albicans or Aspergillus fumigatus. Med Mycol 38:443–449. doi:10.1080/714030972
Hammerschmidt R, Nuckleus EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:61–71
Heath RL, Packer I (1968) Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Herouet C, Esdaile DJ, Mallyon BA et al (2005) Safety evaluation of the phosphinothricin acetyltransferase proteins encoded by the pat and bar sequences that confer tolerance to glufosinate-ammonium herbicide in transgenic plants. Regul Toxicol Pharmacol 41:134–149. doi:10.1016/j.yrtph.2004.11.002
Kamoun S (2001) Non-host resistance to Phytophthora: novel prospects for a classical problem. Plant Biol 4:295–300
Kuiper HA, Kleter GA, Noteborn HP et al (2001) Assessment of the food safety issues related to genetically modified foods. Plant J 27:503–528. doi:10.1046/j.1365-313X.2001.01119.x
Kuo MC, Kao CH (2004) Antioxidant enzyme activities are up-regulated in response to cadmium in sensitive, but not in tolerant, rice (Oryza sativa L.) seedlings. Bot Bull Acad Sin 45:291–299
Maldonado JM (1993) Assimilation of nitrogen and sulfur. In: Azcon-Bieto J, Talon M (eds) Plant physiology and biochemistry. EDIGRAFOS, Madrid, pp 215–237
Metz PLJ, Stiekema WJ, Nap JP (1998) A transgene-centered approach to the biosafety of transgenic phosphinothricin-tolerant plants. Mol Breed 4:335–341. doi:10.1023/A:1009695124173
Meyer P (1999) The role of chromatin remodeling in transgene silencing and plant development. In vitro Cell Dev Biol Plant 35:29–35. doi:10.1007/s11627-999-0006-0
Mohapatra U, McCab M, Power J et al (1999) Expression of the bar gene confers herbicide resistance in transgenic lettuce. Transgenic Res 8:33–44. doi:10.1023/A:1008891216134
Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Plant Mol Biol 52:561–591. doi:10.1146/annurev.arplant.52.1.561
Momma K, Hashimoto W, Ozawa S et al (1999) Quality and safety evaluation of genetically engineered rice with soybean glycinin: analyses of the grain composition and digestibility of glycinin in transgenic rice. Biosci Biotechnol Biochem 63:314–318. doi:10.1271/bbb.63.314
OECD (1993) Safety evaluation of foods derived by modern biotechnology. Available via: OECD. www.oecd.org/dsti/sti/s_t/biotech/prod/modern.htm. Accessed 27 May 2008
Porras RJ (1991) Recent advances and re-assessments in chlorophyll extraction and assay procedures for terrestrial, aquatic and marine organisms including recalcitrant algae. In: Scheer H (ed) Chemistry of chlorophyll. CRC Press, Boca Raton, p 320
Sanchez-Monge R, Blanco C, Díaz Perales A et al (2000) Class I chitinases, the panallergens responsible for the latex-fruit syndrome, are induced by ethylene treatment and inactivated by heating. J Allergy Clin Immunol 106:190–195. doi:10.1067/mai.2000.107599
Scarpari LM, Meinhardt LW, Mazzafera P et al (2005) Biochemical changes during the development of witches′ broom: the most important disease of cocoa in Brazil caused by Crinipellis perniciosa. J Exp Bot 56:865–877. doi:10.1093/jxb/eri079
Schlumbaum A, Match F, Vogeli U et al (1986) Plant chitinases differ in antifungal activity. Nature 324:325–367. doi:10.1038/324365a0
Singh NK, Nelson DE, Kuhn D et al (1989) Molecular cloning of osmotin and regulation of its expression by ABA and adaptation to low water potential. Plant Physiol 90:1096–1101
Thompson CJ, Movva NR, Tizard R et al (1987) Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J 6:2523–2527
Torres AC, Nagata RT, Ferl RJ et al (1999) In vitro assay selection of glyphosate resistance in lettuce. J Am Soc Hortic Sci 1:86–89
Wehrmann A, Van Vliet A, Opsomer C et al (1996) The similarities of bar and pat gene products make them equally applicable for plant engineers. Nat Biotechnol 14:1274–1278. doi:10.1038/nbt1096-1274
Wilson AK, Latham JR (2006) Transformation-induced mutations in transgenic plants: analysis and biosafety implications. Biotechnol Genet Eng Rev 23:209–234
Woloshuk CP, Meulenhoff JS, Sela-Buurlage M et al (1991) Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell 3:619–628
Yaginuma S, Shiraishi T, Ohya H et al (2002) Polyphenol increases and cucumber seedlings exposed to strong visible light limited water. Biosci Biotechnol Biochem 66:65–72. doi:10.1271/bbb.66.65
Yanes PE, González OJ, Rodríguez R (2000) A technology of acclimatization of pineapple vitroplants. Pineap News 7:24
Acknowledgements
This research was supported by the Cuban Ministry for Science, Technology and the Environment through a grant to Mrs. Lourdes Yabor Cabrera. The authors are grateful to Dr. Lazaro Hernandez (CIGB, Havana, Cuba) for providing gene constructs; to Mr. Conroy Cassan Huggins (Saint Vincent and the Grenadines) for his critical reading of the manuscript; and to Ms. Mayda Arzola, Mrs. Julia Martínez and Mrs. Alitza Iglesias for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yabor, L., Aragón, C., Hernández, M. et al. Biochemical side effects of the herbicide FINALE® on bar gene-containing transgenic pineapple plantlets. Euphytica 164, 515–520 (2008). https://doi.org/10.1007/s10681-008-9743-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9743-0