Abstract
The effect of the Embryo lethality mutant (Eml) of rye was studied in crosses between hexaploid wheat and corresponding inbred rye line (L2). Histological analysis of hybrid embryos revealed morphological differences 16 days after pollination. Eml was found to arrest the formation of shoot meristem but had no influence on root meristem formation. The effect of Eml cannot be overcome by in vitro embryo rescue via direct regeneration on Kruse medium. The possibility of complementary interactions between wheat and rye genes and of changes in gene expression through increased variation in dosage-regulated gene expression during hybrid formation is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The processes leading to postzygotic barriers in distant hybridizations are poorly known. The prevailing view is that postzygotic isolation is usually caused by extensive negative epistatic interactions of parental genomes in experimentally produced interspecific hybrids (Orr and Presgraves 2000; Burke and Arnold 2001). In flowering plants postzygotic barriers take effect after successful fertilization and have been revealed in different taxons both in intraspecific (Oka 1957; Hermsen 1963, 1967) and interspecific crosses (Hollingshead 1930; Gerstel 1954; Sawant 1956; Lee 1981). These barriers could arise from the first division of hybrid zygotes and during ontogenesis of F1 hybrids and later generations (Stebbins 1957; Rieseberg and Carney 1998). Besides hybrid embryo lethality, which results in seed abortion (Sears 1944; Lee 1981; Comai et al. 2000), distant hybridizations are often accompanied by seedling lethality, death at later developmental stages, and morphological abnormalities such as hybrid dwarfness or hybrid weakness (Hollingshead 1930; Oka 1957; Hermsen 1963, 1967; Takahashi et al. 1970; Shii et al. 1980; Worland and Law 1980). In these publications the description of embryo lethality during seed development relates only to seed viability and the stage at which embryo development was arrested. May and Appels (1984) reported that seedling lethality in Triticum aestivum × Secale cereale hybrids clearly results from the new combination of rye and wheat chromatin, so this example could be attributed to hybrid lethality. Wheat plants disomic for a 2RS/2BL translocation chromosome substituting for chromosome 2B show seedling lethality. However, this kind of seedling lethality can be overcome if 2RS/2BL is introduced into a different genetic background (May and Appels 1980, 1984).
Hybrid lethality at the seedling stage has been most thoroughly studied in the genus Nicotiana (Gerstel et al. 1979; Inoue et al. 1996; Kobori and Marubashi 2004). The process is characterised by an apoptosis-like programmed cell death (Marubashi et al. 1999; Yamada et al. 2000; Masuda et al. 2003). In the crosses Nicotiana suaveolens × N. tabacum and N. suaveolens × N. sylvestris hybrid lethality could be overcome by in vitro culture of embryos on a medium containing cytokinin (Inoue et al. 1994, 1997, 2000). Based on chromosome-specific DNA markers Tezuka and Marubashi (2006) demonstrated that the chromosome Q of N. tabacum encodes hybrid lethality in interspecific crosses with N. suaveolens.
For cereals three general causes which can limit the viability and fertility of wide hybrids have been postulated: (i) hybrid sterility, (ii) death of a potentially viable embryo due to failure endosperm development, or (iii) selective chromosome elimination of the parental sets of chromosomes during embryo development (Baum et al. 1992). (i) Crosses between relatively closely related species such as T. aestivum x S. cereale usually lead to sterile F1 hybrids. The sterility can be overcome successfully by chromosome doubling (Larter et al. 1968; Kaltsikes 1974). (ii) In crosses between tetraploid wheat (mainly T. durum) and rye (Raina 1984) hybrid lethality is characterized by abnormal development of embryo and endosperm. Most seeds obtained in such crosses fail to germinate. The failure in endosperm development can be overcome by culturing on an artificial medium (Taira and Larter 1978; Raina 1984; Sirkka and Immonen 1993). In contrast to this, seeds from crosses between hexaploid wheat and rye have a high germination capacity (Müntzing 1979). Depending on the rye genotype, the frequency of non-germinating grains does not exceed 42% (Oettler 1983; Tikhenko et al. 2003). (iii) Selective chromosome elimination has been shown in crosses between hexaploid wheat with Zea mays (Laurie and Bennett, 1986, 1988a), Pennisetum glaucum, Sorghum bicolor (Laurie and Bennett, 1988b; Matzk and Mahn 1994; Gernand et al. 2005) or Hordeum bulbosum (Snape et al. 1979; Sitch and Snape 1987). Until now, selective chromosome elimination has not been observed in crosses between hexaploid wheat with rye.
After crossing 101 different self-fertile rye inbred lines from the Peterhof genetic collection (Smirnov and Sosnikhina 1984) with hexaploid wheat cultivar ‘Chinese Spring’ (CS) we found that the germination rate of seeds from most hybrid combinations ranged between 60% and 100%. However, four rye lines yielded only hybrid seeds that failed to germinate. Three of these four rye lines (L2, L3 and L564) are closely related, and one line (L535) has an independent origin. Seeds from crosses of eight common wheat accessions with rye line L2 had an abnormal embryo development. This trait was further analysed by segregation analysis using inter-line F1 rye hybrids and two wheat accessions (Voylokov and Tikhenko 2002). Rye line L2 carries the mutation Eml (Embryo lethality), which terminates the development of the hybrid embryos in amphihaploids. Monogenic control of development of the hybrid embryo by the rye parent was confirmed by crossing CS with a set of rye recombinant inbred lines (Tikhenko et al. 2005). Embryos of non-germinating swelling hybrid seeds from these amphihaploids were not normally differentiated, whereas the endosperms from these seeds looked similar to those from control crosses. The degree of embryo development in hybrid seeds obtained by crossing hexaploid wheat varieties with rye inbred lines L2 and L535 showed that the proportions of undifferentiated embryos of various sizes was affected by parental genotypes (Tikhenko et al. 2005). After crossing CS with rye line L2 44.7% of the hybrid seeds contain a normal sized embryo without visible signs of tissue differentiation. This result suggested in vitro embryo rescue as the method to produce wheat-rye hybrids in this combination.
The aim of this study was to the test whether in vitro embryo rescue could be used to overcome hybrid lethality caused by the rye gene Eml. Histological analysis provides clear evidence that rye gene Eml arrests the development of shoot apical meristem (SAM) in hybrid embryos.
Materials and methods
Plant material
The wheat cultivar ‘Chinese Spring’ (CS) (Triticum aestivum, 2n = 6× = 42) was used as female parent. The following self-fertile rye (Secale cereale, 2n = 2× = 14) lines were used as pollen donors: (1) line L2, carrying the mutant Eml gene; (2) control line L6 , with the wild type eml allele allowing the development of viable hybrid embryos; (3) inter-line F1 hybrid L6 × L2. Rye lines L2 and L6 were supplied by the Peterhof genetic collection of the Plant Genetics Laboratory of the Biological Institute of St. Petersburg State University. Wheat spikes were emasculated 1–2 days before anthesis, and pollinated with fresh rye pollen.
In vitro embryo rescue
Embryos were excised 16 and 20 days after pollination (DAP) under a dissecting microscope. Caryopses were surface sterilized by washing in 70% ethanol for 1 min, and soaking for 15 min in 3.0% (v/v) sodium hypochlorite with two drops of Tween 80, followed by three rinses in sterile water for 15 min. Isolated embryos were classified according to the scale of Clark and Sheridan (1988), which includes six developmental stages: stage 1—proembryo, stage 2—lemon-like stage, stage 3—transition stage, stage 4—with first step of the differentiation (coleoptile stage), stage 5—embryonic axis and scutellum are present, stage 6—fully developed embryo with shoot and root meristems. Isolated embryos 16 and 20 DAP were classified visually and ranged from stage 2 to stage 6. Embryos at each stage were cultured scutellum side down for regeneration on Kruse medium (Kruse 1974) and incubated at 24°C in the dark. Regenerative capacity was determined after 20 days.
Histological analysis
Embryos of wheat × rye crosses were fixed with 2% glutaraldehyde and 2% formaldehyde in cacodylate buffer (50 mM, pH 7.0) for 16 h. After three 15 min washes with the same buffer, the embryos were postfixed with 1% OsO4 for 6 h. The embryos were washed with buffer and distilled water and subsequently dehydrated in a graded ethanol series followed by embedding in Spurr’s low viscosity resin. Longitudinal median sections of 1 μm were cut on a Reichert-Jung Ultracut S (Leica, Vienna, Austria) and stained with methylene blue. Digital images were made on a Zeiss Axiovert equipped with an Axiocam (Carl Zeiss, Jena, Germany).
Results
Regenerative capacity of immature embryos was analyzed in four crosses: CS × CS, CS × L6 (Table 1), CS × L2 and CS × F1 (L6 × L2) (Table 2). Up to 98% of the 16 and 20 DAP old CS × CS embryos were in developmental stage 6. Regenerative capacity of these embryos after 20 days in vitro culture was almost 100% (Table 1). Embryos obtained from the control cross CS × L6 were morphologically similar, but had their regenerative capacity reduced to only 75% after 20 days in vitro culture (Table 1). CS × L2 hybrid embryos showed a delayed development with 34.4% and 64.8% of the 16 DAP old embryos showing features characteristic for developmental stages 2 and 3, respectively. At 20 DAP the embryos were at stages 2 (12.1%), 3 (50.7%) and 4 (37.2%). Even after cultivation on Kruse medium root formation was only sometimes observed and shoot development was completely absent (Table 2). Only hybrid embryos in stages 3 and 4 formed roots when placed on rescue medium. Of the embryos cultured 16 DAP 17.6% of those in stage 3 and 100% of those in stage 4 formed small roots (1–3 mm) before dying without shoot formation. For embryos cultured 20 DAP root formation was observed in 73.2% of the stage 3 and 80.0% of the stage 4. Embryos 16 DAP and 20 DAP occasionally developed primary roots 2.5–3 cm long without lateral roots (data not shown). Amphihaploid embryos 16 and 20 DAP derived from the cross CS × inter-line F1 rye hybrid (L6 × L2) showed the expected 1:1 segregation behaviour. Half of the embryos ceased development at stages 2 and 3. The remainder continued to stages 5 and 6 and showed a high regenerative capacity from embryos cultured at 16 DAP (84%) and 20 DAP (72.7%) (Table 2). In control crosses, only a few embryos excised at 16 and 20 DAP were at stage 4. However, the comparative analysis of the regenerative capacity of stage 4 hybrid embryos, showed that direct regeneration was possible for embryos from cross CS × CS 16 DAP (33.3%), as well as from cross CS × L6 20 DAP (66.7%) but not for embryos from cross CS × L2 (Table 1, 2). Thus, the effect of rye gene Eml on the development of the hybrid embryos becomes evident 16 DAP and cannot be overcome by direct regeneration on Kruse medium.
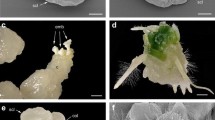
Histological studies of median longitudinal sections of hybrid and control embryos revealed no obvious morphological differences at 10 DAP (Fig. 1a–c). At this developmental stage first signs of differentiation were observed. The epidermis is formed and an internal cone-shaped group of cells marks the position of the apical meristem (Fig. 1a–c). The shoot apical meristem is visible as a dimple on the flattened face of the embryo (arrows in Fig. 1b, c). This primary meristematic region later forms the shoot-root axis of the mature embryo. Throughout stage 4 and 5 the scutellum greatly increases in size through cell division and cell enlargement. The vascular system differentiates simultaneously and links the embryonic axis with the scutellum. In the control, embryo morphogenesis ends 16 DAP. At this time the embryonic axis constitutes a well-differentiated miniature plant with leaf primordia covering the face of the shoot apex and a strand of vascular procambium extending into the scutellum (Fig. 1d). The location of the root apex is evident (Fig. 1d–f). Significant differences between control and CS × L2 hybrid embryos become obvious 16 DAP. At the location where the apical shoot meristem should be, an area with degenerating cells can be discerned in CS × L2 hybrid embryos. The scutellum is also much smaller and without a clear vascular system (Fig. 1e). Only the root meristem is similar to that of the control line embryos (Fig. 1e,f).
Histological analysis of embryos 10 DAP (a-c) and 16 DAP (e-f). (a) wheat CS × L6, (b) wheat CS × wheat CS and (c) wheat CS × rye L2 embryos. The shoot apical meristem is visible as a dimple on the flattened face of the embryo (arrowed). (d) normal embryo of wheat CS × wheat CS. Embryonic axis constitutes a well-differentiated miniature plant with leaf primordia covering the face of the shoot apex (am) and a strand of vascular procambium extending into the scutellum. (e, f) abnormal wheat CS × rye L2 embryo. At the site of the apical shoot meristem (am) an area with degenerating cells can be seen. The scutellum is much smaller and without a clear vascular system. Note that the root meristems (rm) appear similar to those in the control lines. In 1a, b, c, f bars = 100 μm and in 1d, e bars = 200 μm
At 20 DAP the abnormal embryos had not significantly changed in size when compared to 16 DAP. They showed no signs of further morphogenesis. The root meristematic region was reduced and the suspensor and scutellum regions were less distinct than in earlier stages (data not shown).
Discussion
At the start of the present study, we proposed that the nature of the hybrid lethality in cross common wheat with inbred rye line L2 may resemble the abortion of the embryos in crosses Triticum durum with Secale cereale (Krowlow 1970). The major problem with these crosses is poor endosperm development: embryos abort early in their development. Recently effective methods for in vitro culture for immature hybrid embryos were developed (Taira and Larter 1978; Raina 1984; Sirkka and Immonen 1993) We applied the embryo rescue method which had shown good results for overcoming the lethality of abnormal embryos for many wide crosses in the Poaceae (Matzk and Mahn 1994). Using Kruse medium we tried to prevent callus formation, which results in a chromosome number mosaicism in both shoot and root apical meristem (Shao and Taira 1990). One of the main factors influencing development of wheat-rye hybrid embryos in vitro is the time for rescuing. Taira and Larter (1978) shown that wheat-rye hybrid embryos respond best to Norstog’s modified medium if they are allowed to develop 12–14 days in vivo. Raina (1984) followed these suggestions but realized that 12–14 day old embryos were too young and found that best seedling development was obtained with 20–22 day old embryos. In contrast to this, Sirkka and Immonen (1993) found that embryo rescue 15–17 day after pollination was optimal both for callus and embryo culture of primary triticale. Based on these results we used two time points for rescuing wheat-rye hybrid embryos—16 and 20 DAP. In our experiment, 77.9 – 95.1% of embryos 16 DAP and 75 – 100% (Table 1) of embryos 20 DAP from control crosses CS × CS and CS × L6 formed shoots on the fourth day after start of cultivation on rescue medium. In contrast, none of the abnormal embryos from cross CS × L2 formed shoots even after 20 days of cultivation on Kruse medium (Table 2). Some of these (17.6 –80%) formed a short primary roots 1–3 mm long before dying without shoot formation. In our case, the intraspecific pollination of wheat and self-fertile rye L2 results in normal caryopsis development. However, histological analyses showed that after interspecific crosses between wheat CS with rye L2, which carries the Eml mutation, all hybrid embryos have differentiated scutellum and root meristems but no shoot apical meristem and no coleoptile (Fig.1e, f). In control crosses of CS with rye L6, which carries wild type allele of Eml, all hybrid embryos have a differentiated scutellum and normal meristem development (Fig.1d). The histological analysis suggests that younger hybrid embryos from cross CS × L2 may also be cultivated on Kruse medium.
The phenotype of embryo lethality caused by the rye Eml gene is similar to, segregating mutations of maize and rice (Clark 1996; review Itoh et al. 2006). It also resemble the maize mutants dek1 and dek23 which have an atypical development of endosperm and embryo, and the maize mutant emb12 in which only embryo development is altered (Sheridan and Neuffer 1981). The dek1 mutant embryos possess a root but no shoot meristem while embryos of dek23 reach the coleoptilar stage with root meristem and elongated scutellum but never develop a shoot apex. Clark and Sheridan (1986) proposed that the failure of normal function of the dek23 locus results in a loss of vitality of the cells at that central site. This locus may control the synthesis of a substance in this region that is essential for its differentiation into a shoot apex. Embryos of emb12 maize mutants, which are blocked primarily during morphogenesis and early maturation, have a distorted embryonic axis and an inverted shoot apical meristem similar to that of dek23. A lesion in ZmPRPL35-1, probably coding for protein L35 of the large subunit of plastid ribosomes, is responsible for the phenotypic changes observed in emb12 maize mutants (Magnard et al. 2004). In another case, Pilu et al. (2002) have shown that mutations in two independent genes in maize, SML and DGR, lead to suppression of the shoot apical meristem (SAM) development during embryogenesis and produce a similar phenotype to shl in rice and Eml in wheat-rye hybrid. This indicates that the observed defect in wheat-rye hybrid embryos could be connected with meristem development abnormalities. But it is unclear which gene(s) controls SAM development in wheat-rye hybrid embryos. Also in rice, a group of mutants with abnormal SAM development during embryogenesis has been reported (see review Itoh et al. 2006). They carried several shootless mutations and so far four shootless loci have been identified (Satoh et al. 1999). Thus, SHOOTLESS genes are thought to be directly involved in the formation of the SAM.
Therefore, the abnormal embryo phenotype is a result of the wide hybridization of the rye line L2 with common wheat. Furthermore, the monogenic control of embryo lethality has been confirmed by crossing CS with a set of rye recombinant inbred lines (RIL). Gene Eml has been located on rye chromosome 6R applying molecular markers (Tikhenko et al. 2006).
Sears (1940) was the first to report on monofactorially conditioned inviability of an intergeneric hybrid in the Triticinae. He identified two alleles in Triticum monococcum which act as dominant lethals in hybrids with Aegilops umbellulata, but which are without effect in T. monococcum itself. The alleles differ in the time, i.e. the developmental stage, at which they cause death. A third, normal allele is present in the closely related T. aegilopoides. Multiple hybrids combining the normal allele and one of the inviability factors with the Ae. umbellulata genome were viable (Sears 1944). In his work, Sears suggested two mechanisms by which a factor might cause inviability of a hybrid: (1) by failure to produce some substance essential to the proper development of the hybrid plant, or (2) by some positive action antagonistic to the foreign genome. The viability of the multiple hybrids (T. monococcum × Ae. uniaristata 4n) × Ae. umbellulata and (T. aegilopoides x Ae. uniaristata 4n) × (T. monococcum × Ae. uniaristata 4n) prevents the antagonism between an inviability gene and the Ae. umbellulata genome. This antagonism might be expected to cause lethality regardless of what additional genomes were present. The absence of an antagonistic action suggests that hybrid death is due to a deficiency of some essential substance. Recent evidence has emerged that the combination of two unrelated genomes may give rise to novel or suppressed gene expression. In general, polyploid species often display new traits and genetic variability (Levin 1983; Feldman et al. 1997; Comai et al. 2000; Liu et al. 1998a, b; Ma et al. 2004; Rapp and Wendel 2005).
Two models could explain the regulatory mechanisms in newly formed hybrids. The first model proposed by Dobzhansky (1937) suggests the existence of complementary genes that determine lethality or sterility in distant hybrids. Each gene has at least two different alleles: ‘normal’ (non-complementing) and ‘abnormal’ (as Eml rye gene; complementing). Altered phenotypes only occur with a combination of ‘abnormal’ alleles of both complementary genes in F1 hybrids. ‘Abnormal’ alleles in either of the two genes may be fixed in different taxons or forms of autogamous species, or be present in all possible combinations in plants of allogamous species. In the latter case, segregation of abnormal phenotypes in a population may be observed. According to this model, any abnormal (novel) trait revealed in the wide hybrids is the result of interaction of at least two complementary genes.
The second model is based on the changes in gene expression through increased variation in dosage-regulated gene expression, altered regulatory interactions, and rapid genetic and epigenetic changes (Sears 1944, for review Osborn et al. 2003). More studies are necessary to obtain further insights into embryo lethality and a better understanding of genome interaction in polyploids. Consequently, Eml appears to be a valuable mutation for investigating the organization of the shoot meristem in monocots as well as a means to study the relationships between wheat and rye genes involved in apical meristem development during embryogenesis.
References
Baum M, Lagudah ES, Appels R (1992) Wide crosses in cereals. Annu Plant Physiol Plant Mol Biol 43:117–143
Burke JM, Arnold Ml (2001) Genetics and fitness of hybrids. Ann Rev Genet 35:31–52
Clark JK (1996) Maize embryogenesis mutants. In: Wang TL, Cuming A (eds) Embryogenesis: the generation of a plant. Bios Scientific Publishers, Oxford, pp 89–112
Clark JK, Sheridan WF (1986) Developmental profiles of the maize embryo-lethal mutants dek22 and dek23. J Hered 77:83–92
Clark JK, Sheridan WF (1988) Characterization of the two maize embryo-lethal defective kernel mutants rgh*-1210 and fl*-1253B: Effects on embryo and gametophyte development. Genetics 120:179–290
Comai L, Tyagi AP, Winter K, Holmes-Davis R, Reynolds SH, Stevens Y, Byers B (2000) Phenotypic instability and rapid gene silencing in newly formed Arabidopsis allotetraploids. Plant Cell 12:1551–1567
Dobzhansky TH (1937). Genetics and the origin of species. Columbia University Press, New York
Feldman M, Liu B, Segal G, Abbo S, Levy AA, Vega JM (1997) Rapid elimination of low-copy DNA sequences in polyploid wheat: possible mechanism for differentiation of homeologous chromosomes. Genetics 147:1381–1387
Gernand D, Rutten T, Varshney A, Rubtsova M, Prodanovic S, Brüß C, Kumlehn J, Matzk F, Houben A (2005) Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell 17:2431–2438
Gerstel DU (1954) A new lethal combination in interspecific cotton hybrids. Genetics 39:628–639
Gerstel DU, Burns JA, Burk LG (1979) Interspecific hybridizations with an African tobacco, Nicotiana africana Merxm. J Hered 70:342–344
Hermsen JGTh (1963) The genetic basis of hybrid necrosis in wheat. Genetica 33:445–487
Hermsen JGTh (1967) Hybrid dwarfness in wheat. Euphytica 16:134–162
Hollingshead L (1930) A lethal factor in Crepis effective only in an interspecific hybrid. Genetics 15:114–140
Inoue E, Marubashi W, Niwa M (1994) Simple method for overcoming the lethality observed in the hybrid between Nicotiana suaveolens and N. tabacum. Breed Sci 44:333–336
Inoue E, Marubashi W, Niwa M (1996) Genomic factor controlling the lethality exhibited in the hybrid between Nicotiana suaveolens Lehm. and N. tabacum L. Theor Appl Genet 93:341–347
Inoue E, Marubashi W, Niwa M (1997) Improvement of the method for overcoming the hybrid lethality between Nicotiana suaveolens and N. tabacum by culture of F1 seeds in liquid media containing cytokinins. Breed Sci 47:221–216
Inoue E, Marubashi W, Niwa M (2000) Characterization and overcoming of temperature-dependent lethality exhibited by hybrid embryos from the cross Nicotiana suaveolens × N. sylvestris. Breed Sci 50:297–302
Itoh J, Sato Y, Nagato Y, Matsuoka M (2006) Formation, maintenance and function of the shoot apical meristem in rice. Plant Mol Biol 60:827–842
Kaltsikes PJ (1974) Methods for triticale production. Z Pflanzenzücht 71(3):264–286
Kobori S, Marubashi W (2004) Programmed cell death detected in interspecific hybrids of Nicotiana repanda × N. tomentosiformis expressing hybrid lethality. Breed Sci 54:347–350
Krowlow KD (1970) Untersuchungen über die Kreuzbarkeit zwischen Weizen und Roggen. Z Pflanzenzüchtg 64:44–72
Kruse A (1974) An in vivo/vitro embryo culture technique. Hereditas 2:219–224
Larter E, Tsuchiya T, Evans LE (1968) Breeding and cytology of triticale. Proceeding of 3rd Inter. Wheat Symp., Canberra, 1968, pp 213–221
Laurie DA, Bennett MD (1986) Wheat × maize hybridization. Can J Genet Cytol 28:313–316
Laurie DA, Bennett MD (1988a) The production of haploid wheat plants from wheat x maize crosses. Theor Appl Genet 76:393–397
Laurie DA, Bennett MD (1988b) Cytological evidence for fertilization in hexaploid wheat × sorghum crosses. Plant Breed 100:73–82
Lee JA (1981) Genetics of D3 complementary lethality in Gossypium hirsutum and G. barbadense. J Hered 72:299–300
Levin DA (1983) Polyploidy and novelity in flowering plants. Am Nat 122:1–25
Liu B, Vega JM, Segal G, Abbo S., Rodova M, Feldman M (1998a) Rapid genomic changes in newly synthesized amphiploids of Triticum and Aegilops. I. Changes in low-copy noncoding DNA sequences. Genome 41:272–277
Liu B, Vega JM, Feldman M (1998b) Rapid genomic changes in newly synthesized amphiploids of Triticum and Aegilops. II. Changes in low-copy coding DNA sequences. Genome 41:535–542
Ma X-F, Fang P, Gustafson JP (2004) Polyploidization-induced genome variation in triticale. Genome 47:839–848
Magnard JL, Heckel T, Massonneau A, Wisniewski JP, Cordelier S, Lassagne H, Perez P, Dumas Ch, Rogowski PM (2004) Morphogenesis of maize embryos requires ZmPRPL35-1 encoding a plastid ribosomal protein. Plant Physiol 134:649–663
Marubashi W, Yamada T, Niwa M (1999) Apoptosis detected in hybrids between Nicotiana glutinosa and N. repanda expressing lethality. Planta 210:168–171
Masuda Yu, Yamada T, Marubashi W (2003) Time course analysis of apoptotic cell death during expression of hybrid lethality in hybrid tobacco cells (Nicotiana suaveolens × N. tabacum) Plant Cell Physiol 44(4):420–427
Matzk F, Mahn A (1994) Improved techniques for haploid production in wheat using chromosome elimination. Plant Breed 113(2):125–129
May CE, Appels R (1980) Rye chromosome translocation in hexaploid wheat: a re-evaluation of the loss of heterochromatin from rye chromosomes. Theor Appl Genet 56:17–23
May CE, Appels R (1984) Seedling lethality in wheat: a novel phenotype associated with a 2RS/2BL translocation chromosome. Theor Appl Genet 68:163–168
Müntzing A (1979) Triticale: Results and Problems. J Plant Breed Suppl 10:1–103
Oettler G (1983) Crossability and embryo development in wheat-rye hybrids. Euphytica 32:593–600
Oka H (1957) Phylogenetic differentiation of cultivated rice. XV. Complementary lethal genes in rice. Jpn J Genet 32:83–87
Orr HA, Presgraves DC (2000) Speciation by postzygotic isolation: forces, genes and molecules. Bioessays 22:1085–1094
Osborn TC, Pires JC, Birchler JA, Auger DL, Chen ZJ, Lee HS, Comai L, Madlung A, Doerge RW, Colot V, Martienssen RA (2003) Understanding mechanisms of novel gene expression in polyploids. Trend Genet 19:141–147
Pilu R, Consonni G, Busti E, MacCabe AP, Giulini A, Dolfini S, Gavazzi G (2002) Mutations in two independent genes lead to suppression of the shoot apical meristem in maize. Plant Physiol 128:502–511
Raina SK (1984) Crossability and in vitro development of hybrid embryos of Triticum durum × Secale cereale. Indian J Genet 44(3):429–437
Rapp RA, Wendel JF (2005) Epigenetics and plant evolution. New Phytol 168:81–91
Rieseberg LH, Carney SE (1998) Plant hybridisation. New Phytol 140:599–624
Satoh N, Hong SK, Nishimura A, Matsuoka M, Kitano H, Nagato Y (1999) Initiation of shoot apical meristem in rice: characterization of four SHOOTLESS genes. Development 126:3629–3636
Sawant AC (1956) Semilethal complementary factors in a tomato species hybrid. Evolution 10:93–96
Sears ER (1940) Monofactorially conditioned inviability of an intergeneric hybrid in the Triticinae. Genetics 25:133
Sears ER (1944) Inviability of intergeneric hybrids involving Triticum monococcum and T. aegilopoides. Genetics 29:113–127
Shao ZZ, Taira T (1990) Production of primary triticale from calluses of immature abnormal hybrid embryos between Triticum durum and Secale cereale. Plant Breed 105:81–88
Sheridan WF, Neuffer MG (1981) Maize mutants altered in embryo development. In: Subtelney S, Abbott U (eds) Levels of genetic control in development. Alan R Liss, New York, pp 137–156
Shii CT, Mok MC, Temple SR, Mok DWS (1980) Expression of developmental abnormalities in hybrid of Phaseolus vulgaris L. Interaction between temperature and allelic dosage. J Hered 71:218–222
Sirkka A, Immonen T (1993) Comparison of callus culture with embryo culture at different times of embryo rescue for primary triticale production. Euphytica 70:185–190
Sitch LA, Snape JW (1987) Factors affecting haploid production in wheat using the Hordeum bulbosum system. 1. Genotypic and environmental effects on pollen germination, pollen tube growth and the frequency of fertilization. Euphytica 36:483–496
Smirnov VG, Sosnikhina SP (1984) Genetika rzhi (Genetics of rye). Leningr Gos Univ., 264 pp
Snape JW, Chapman V, Moss J, Blanchard CE, Miller TE (1979) The crossabilities of wheat varieties with Hordeum bulbosum. Heredity 42:29–298
Stebbins GL (1957) The inviability, weakness and sterility of interspecific hybrids. Adv Genet 9:147–215
Taira T, Larter EN (1978) Factors influencing development of wheat-rye hybrid embryos in vitro. Crop Scien 18:348–350
Takahashi R, Hayashi J, Moriya I (1970) Studies on lethal seedling of barley by complementary genes. I. Mode of inheritance and the geographical distribution of the lethal genes. Nogaku Kenkyu 53:197–204
Tezuka T, Marubashi W (2006) Hybrid lethality in interspecific hybrids between Nicotiana tabacum and N. suaveolens: evidence that the Q chromosome causes hybrid lethality based on Q-chromosome-specific DNA markers. Theor Appl Genet 112:1172–1178
Tikhenko ND, Pyukkenen VP, Voylokoy AV (2003) Specific of embryo development in hybrid wheat-rye corn seeds. Proceeding of 5th international symposium “New and nontraditional plants and prospects of their use”. Moscow 2003, vol 2, pp 441–443
Tikhenko ND, Tsvetkova NV, Voylokoy AV (2005) Genetic control of embryo lethality in crosses between common wheat and rye. Russ J Genet 41(8):877–884
Tikhenko N, Tsvetkova N, Börner A, Voylokov V (2006) Genetic study of embryo lethality in wheat-rye hybrids. In: Book of Abstracts International Symposium on Rye Breeding & Genetics, Groβ Lüsewitz, 28–30 June 2006, p 69
Voylokov AV, Tikhenko ND (2002) Triticale as a model for study of genome interaction and genome evolution in allopolyploid plants. Proceeding of 5th international Triticale symposium, Radzikow, 30 June–5 July 2002, vol 1, pp 63–69
Worland AJ, Law CN (1980) The genetics of hybrid dwarfing in wheat. Z Pflanzenzüchtg 85:28–39
Yamada T, Marubashi W, Niwa M (2000) Apoptotic cell death induces temperature-sensitive lethality in hybrid seedlings and calli derived from the cross of Nicotiana suaveolens × N. tabacum. Planta 211:614–622
Acknowledgments
We thank Dr F. Matzk for providing the facilities for embryo rescue and tissue culture together with Dr Richard Pickering for critical reading of the manuscript. We are grateful to K. Kumke, M. Hantschmann, M. Pürschel, H. Büchner and H. Block for excellent technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft and the Russian Foundation for Basic Research: project 06-04-48758a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tikhenko, N., Rutten, T., Voylokov, A. et al. Analysis of hybrid lethality in F1 wheat-rye hybrid embryos. Euphytica 159, 367–375 (2008). https://doi.org/10.1007/s10681-007-9528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9528-x