Abstract
This experiment examined variation of iron (Fe) concentration in grain of local Thai rice varieties from Huai Tee Cha village, in northern Thailand. Seeds of 17 named varieties were collected from farmers in 66 accessions. Brown rice from each accession was stained individually with Perls’ Prussian blue, with IR68144 (high grain Fe) and KDML105 (low grain Fe) as checks. A wide range of grain Fe contents were found among different varieties, different seed lots with the same name and within individual accessions, notably BB, BGU and BK. The Fe concentrations in these varieties were confirmed by chemical analysis. Two accessions of each variety with most intense staining and highest average concentration of grain Fe were selected, and 20–40 seeds from each were sown for subsequent progeny testing. There was morphological variation between plants in 5 out of 6 accessions in characteristics such as pigmentation of the apiculus, pericarp and seed coat, while the sixth was externally uniform. Analysis for Fe concentration in seeds of individual lines found that the highest Fe lines were 40% to 50% higher than the lowest Fe lines. This range of grain Fe was found in all six accessions that were progeny tested, including BGU5 which was externally uniform in appearance (H′ = 0). We have shown that variation in grain Fe concentration in local Thai rice may be found between different seed lots bearing the same name and within individual seed lot as well as between varieties. This level of genetic variation may need to be considered when assessing for traits such as grain Fe for breeding programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron deficiency anemia (FDA) is widespread, affecting two thirds of the world’s population (IFPRI 1999; WHO 2002). In humans, FDA impairs growth, cognitive development and immunity (WHO 2002). The effect is especially critical in infants and young children and harmful in preschool children and pregnant women (IFPRI 1999). Increasing daily Fe intake in human nutrition is one way to solve the problem. Iron supplementation in the form of tablets is suggested but it has not been successful in developing countries as it is costly and requires considerable efforts in logistics and distribution (Bouis et al. 1996). Interestingly, it has been reported in Philippines that 50% of Fe intake in human diet comes from cereals such as rice (Senadhira et al. 1998) which is the staple food crop in Asia. Rice, unfortunately, has especially low grain Fe concentration compared with other cereals (Juliano 1993; Senadhira et al. 1998). Increasing Fe in rice grain may help to alleviate the incidences of FDA in the rice consuming regions.
Molecular and conventional breeding have been suggested as strategies to increase grain Fe concentration in rice (Graham and Welch 1996; Graham et al. 1999; Gregorio 2002). It has been possible to increase the Fe concentration in the endosperm two to three folds by inserting the ferritin gene from Fe-rich plant such as soybean and French bean into rice (Goto et al. 1999; Lucca et al. 2002; Vasconcelos et al. 2003). However, genetically modified rice may not be acceptable to all consumers. Ecological concerns have also been raised regarding release of genetically modified rice in to the Oryza gene pool (Lu and Snow 2005). Conventional breeding seems to offer a manageable and non-controversial solution. Therefore, varieties with high grain Fe content are required to be the genetic source for a breeding program and they can be identified under a screening process for different rice regions, especially in the regions, which have high genetic diversity of rice varieties. Previous studies reported that there was a wide variation of Fe concentration in brown rice grain among different varieties (Prom-u-thai and Rerkasem 2001; Prom-u-thai et al. 2007; Senadhira et al. 1998). Grain Fe concentration ranges from 7.5 mg Fe kg−1 to 24 mg Fe kg−1 among 939 varieties tested in IRRI, which included IR68144, an improved variety with high grain Fe concentration (Senadhira et al. 1998). The variation of grain Fe concentration in brown rice among Australian and Thai rice varieties was found to be in a similar range, from 10 to 20 mg Fe kg−1 (Prom-u-thai et al. 2007) and from 7 mg Fe kg−1 to 22 mg Fe kg−1 (Prom-u-thai and Rerkasem 2001), respectively. Most of the popular Thai rice varieties have been found to have low grain Fe concentration, however, high grain Fe varieties were found in many local upland rice varieties in Thailand (Prom-u-thai and Rerkasem 2001).
Previous studies reported that seed of local rice varieties maintained by farmers are genetically diverse (Chang 1976; Dennis 1987). Different varieties can be given similar names and different names can be applied to the same varieties (Harlan 1992). Diversity analyses may be based on morphological characteristics such as shape, size and pigmentation of plant parts that local farmers use to distinguish different rice varieties (Morishima et al. 1980; Watabe 1967). Molecular techniques such as AFLP, ISSR and SSR (Saini et al. 2004; Shishido et al. 2006; Zhang et al. 1992) allows variation to be evaluated between individual plants. However, for grain Fe and many other useful traits molecular markers are as yet unavailable to detect differences between individuals. Chemical analysis for grain Fe represents the average content in 50 grains or more. A possibility for determining variation in grain Fe between individual rice grains has been provided by the method for assessing Fe in individual rice grains by staining with Perl’s Prussian blue (Prom-u-thai et al. 2003). In this study, we used the staining technique and chemical analysis to examine the range of grain Fe concentration between the different varieties, different seed lots with the same name and within each seed lot. Seed lots selected by this first screening were grown for progeny testing. We expected to illustrate that useful traits such as grain Fe may be found with varying frequencies in farmers’ seed lots of local rice varieties and that individuals with higher Fe concentration in their grains than that detected by chemical analysis may be identified.
Materials and methods
Sample collection
Sixty six accessions, individual rice samples from different farmers, with 17 named varieties of upland rice (Oryza sativa) were collected from Huai Tee Cha village, Sob Moei District, Mae Hong Son, Thailand (17°51′ N and 97°54′ E). Among accessions collected from different farming households in the village, the number of accessions bearing the same name ranged from 1 to 15 (Table 1).
Perls’ Prussian blue staining and chemical analysis
The Perls’ Prussian blue reaction was determined on individual grains of brown rice. Check varieties included IR68144 (a high Fe content variety from IRRI, 16 mg Fe kg−1) and KDML105 (a low Fe content variety from Thailand, 8 mg Fe kg−1) (Prom-u-thai et al. 2003). After the husk was removed by hand, 20 seeds for each accession were imbibed in distilled water for 4–5 h, and were cut in half lengthwise through the embryo with a Teflon knife (Advanced Personna Brand) in a Petri dish. The specimens were submerged in freshly prepared Perls’ Prussian blue (Perls 1867) solution (2% hydrochloric acid mixed with 2% potassium ferrocyanide) for 10 min. The seed were then gently washed continuously in distilled water for 2 min. The ferric Fe is released from any attachments to protein by treatment with dilute hydrochloric acid and then reacts with a dilute solution of potassium ferro cyanide to produce the Prussian blue color of insoluble ferric ferro cyanide (Doucet and Viel 2002). The intensity of staining was rated from 0 (no staining), + (low staining) to +++ (most intense) under a stereo microscope. Grain Fe concentration were determined on samples of about 40 to 50 grains by dry-ashing and atomic absorption spectrophotometry, AA (Delhaize et al. 1984) in mature grain as whole grain brown rice (palea and lemma removed, complete with embryo) with two replications.
Progeny testing
Two seed lots, individual rice samples with the same variety name, from three varieties: BB, BGU and BK (six in total), which showed variation of Perls Prussian blue staining and high average grain Fe concentration by chemical analysis were selected for progeny testing. From seed lot of each accession 20–40 plants were grown as upland rice, i.e. in well-drained soil. Each plant was characterized (IRRI-IBPGR 1980) for pigmentation (of leaf, leaf sheath, ligule, auricle, node, internode, apiculus, sterile lemma), culm angle, flowering date, plant height at maturity, and number of tillers per plant. Seeds from the individual plant in progeny testing were collected at the maturity. Grain Fe content was measured again by Perls’s Prussian blue technique and chemical analysis as previously described.
Statistics and equations
Standard deviation (SD) and coefficient of variation (CV) were used to evaluate the variation of grain Fe concentrations and plant phenology among different accessions. Shannon–Weaver index was used to detect the variation of the staining intensity and plant phenology among different accessions. The correlation analysis was used to test the significance of the staining intensity of Perls Prussian blue reaction and grain Fe concentration in brown rice among different accession of farmer seed lots.
The equation 1 was used to calculate the average staining intensity within each of accession among different seed lots as following:
Y = Average staining intensity within each of accession among seed lots
a = 3; Intense intensity (+++)
b = 2; Intermediate intensity (++)
c = 1; Weak intensity (+)
n = Number of accession
The equation 2 was used to calculate the variation of staining intensity and plant phenology within each of accession among different seed lots (Shannon–Weaver diversity index, H′) (Power and McSorley 2000; Shannon and Weaver 1949) as following:
H′ = Shannon–Weaver diversity index
s = number of characters found
pi = Ratio of number of characters found and total number of characters examined
Results
Variation in grain Fe concentration in farmers’ rice seed
Chemical analysis, averaging over 40–50 grains per sample used, found the variation of Fe concentration in brown rice among different seed lots within and between named varieties, to range from 7.5 mg Fe kg−1 to 14.4 mg Fe kg−1, with considerable variation among accessions (Table 1). Coefficient of variation (%CV) for the grain Fe content between accessions was especially high in some varieties: 17.4% in PAC, 19.2% in BGU and 23.6% in BB. Up to three folds difference of grain Fe content was measured in accessions sharing the name BB that came from different farmers (data not shown). The staining intensity of the standard check varieties averaged 2.51 in IR68144 and 1.11 in KDML105. The average staining intensity among different accessions of some seed lots was closer to the high grain Fe check variety (IR68144); 2.55 in BC, 2.47 in BKK, 2.24 in BPOL, 2.18 in BGO and BK and 2.11 in BB. Whereas, some seed lots were closer to the low grain Fe check variety (KDML105); 0.80 in BS, 1.12 in PAKP, 1.15 in PAS, and 1.35 in BKCM (Table 1). Shannon–weaver diversity index (H′) showed the variation of the staining intensity among different accessions within each of the seed lot. The H′ value for the staining intensity was 0.38 in KDML105 and 0.69 in IR68144, and it ranged from 0.18 in the variety BS to 7.61 in BB. The H′ value for Fe staining that was 3 times or more that in IR68144 was found in seven varieties (Table 1).
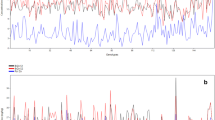
The rapid detection of grain Fe by Perls’ Prussian blue staining was confirmed by chemical analysis in this study (r = 0.74*, P < 0.01) (Fig. 1). The staining, which evaluates the Fe in individual grains, also revealed variation in Fe content within each accession (Table 2). Staining that ranged over 3 levels of intensity, +/++/+++, was found in 42% of the 66 accessions of upland rice from Huai Tee Cha. Another 45% had two levels, +/++ or ++/+++, with uniform reaction of + in the remaining 13%. Within the variety BB, +++/++/+ and +++/++ accounted for 40% of the seeds, with the remainder in ++/+. In BTL, 33% was +++/++/+ and 67% in ++/+. Of the check varieties, the high Fe IR68144 varied between two levels of intensity: half and half at +++ and ++, but low Fe KDML105 was 90% in + and only 10% in ++ (Table 2).
Progeny testing
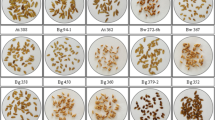
The six accessions of farmers’ upland rice evaluated showed varying degrees of uniformity in appearance of the plants and grain (Fig. 2). Completely uniform in appearance was BGU5 (H′ = 0), with most variation in BB3 (H′ = 2.9) and BK1 (H′ = 4.0), and some variation in BB4, BGU6 and BK2 (H′ = 1.6−2.0). The results indicated by the %CV for days to flowering of 3% to 6%, phenology was uniform within each accession (Table 3). Plant height was found to vary more than days to flower, with CV of 7% to 15%, but BGU5 was still the least variable, 3.1% CV in days to flower and 6.8% CV in plant height compared with the others. Concentration of Fe in brown rice from individual plants covered a range of 40–54% difference between the lowest and highest lines in each accession (Table 4). Some lines were found to have higher Fe concentration in brown rice than the original seed lots. For example, one line from progeny testing of BB3 had grain Fe concentration 16.2 mg Fe kg−1, while the original farmers seed lot had 14.1 mg Fe kg−1. No correlation was found between variation in the grain Fe and morphological diversity in each accession. Indeed, visually uniform BGU5, with the range of grain Fe from 10.0 mg Fe kg−1 to 15.4 mg Fe kg−1, had the highest %CV at 12% for grain Fe among the lines.
The morphological characteristics diversity expressed in Shannon-Weaver index (H′) within each of accession among different seed lots of local upland rice varieties: (1)= 0.69, (2)= 0.21, (3) = 0.41, (4) = 0.31, (5) = 0.19, (6) = 0.51, (7) = 0.34, (8) = 0.62, (9) = 0.44, (10) = 0.14, (11) = 0.46, (12) = 0.50, (13) = 0.20, (14) = 0.45, (15) = 0.42, (16) = 0.30, (17) = 0.61, (18) = 0.24, (19) = 0.59. H′ value for all characters in BGU5 = 0
Discussion
The present results have shown that considerable genetic variation in grain Fe can be expected in farmer’s seeds of local rice varieties. This is not surprising, since local rice varieties are generally recognized as genetically diverse (Chang 1976 and Dennis 1987), and variation in grain Fe concentration among rice varieties has been previously reported (Prom-u-thai and Rerkasem 2001; Prom-u-thai et al. 2007; Senadhira et al. 1998). However, the same range of grain Fe was also found among individual plants of the same accession and accessions sharing the same name that came from different farmers. The range of grain Fe contents found is comparable to the roughly 50% difference between standard low (cf. KDML105) and high (cf. IR68144) grain Fe varieties. It is as yet unclear how this variation is brought about and maintained, and the genetic control of rice grain Fe remains to be described.
Far from being random and static collections of genotypes, the seed of local rice varieties kept and perpetuated by farmers in many rice growing communities in Asia is a product of two related agronomic processes: seed exchange among the farmers and genetic selection. In agricultural systems throughout the world, the seed stock is frequently renewed among those farmers who save seed from the previous year to plant in the next (Zeven 1999). The practice of frequent seed stock renewal was reported to be common among rice farmers in Northern Thailand (Dennis 1987). In the study village, about one in five farmers do not plant the same seed they planted in the previous year (Sirabanchongkran et al. 2003). However, as new varieties are acquired to replace the ones abandoned, the number of varieties planted by each farmer and total number planted in the community was largely unchanged. Farmers’ seed selection, on the other hand, involves both the choice of new seed stock and the selection for likely plant types that can be described as mass selection (Allard 2001) that is practiced by some farmers. Growing condition of each farmer’ field imposes another set of selection pressure on the genetic system. Thus, agronomic characteristics of the rice seed maintained by farming communities can be expected to change over time, as demonstrated in a study in the Mekong Delta in Vietnam (Tin et al. 2001).
In the germplasm studied, selection for uniform development is indicated by the low CV (3–6%) of days to flower in each accession. On the other hand, uniform plant height (CV of up to 15%) appeared to be less crucial in upland rice that is normally grown on steep slopes. Many discussions with farmers in the village have not revealed any recognition of grain Fe or nutritional quality that would be linked to the trait. In contrast, variation in tolerance to soil acidity in this local rice germplasm (Phattarakul et al. 2006) is in agreement with highly variable acidity of the soil of the village’s shifting cultivation field, which range from pH 4 to pockets made alkaline with ash from slash-and-burning (Yimyam 2006).
The range of grain Fe contents in individual accessions indicated by the Perls’ Prussian blue staining was confirmed by Fe analysis of seed produced by each plant. The range from 10 to 15 mg Fe kg−1 found in the grain from progeny lines of BGU5 indicates how variation in agronomically important traits can be found in rice seeds that appear to be externally uniform in both plant and grain. Considerable molecular variation within as well as between accessions from different farmers and villages has been reported in 22 accessions of Bue Chomee, a mid-altitude local wetland rice variety in Thailand, that appeared to be outwardly uniform (Meesin 2003; Meesin et al. 2004).
Morphological characteristics such as shape, size and pigmentation of plant parts are used by local farmers to distinguish between rice varieties, and used in diversity analyses (Watabe 1967; Morishima et al. 1980). Molecular techniques such as AFLP, ISSR and SSR (Saini et al. 2004; Shishido et al. 2006; Zhang et al. 1992) allow variation to be evaluated between individual plants. However, molecular markers are not yet available for the concentration of Fe in rice grain. The Perls Prussian blue staining provides a simple, rapid and inexpensive test that can be used to detect variation among individual seeds within each seed sample (Krishnan et al. 2001; Prom-u-thai et al. 2003). Grain Fe generally varied much more with genotype than with environment and genotype x environment interactions. This was demonstrated by previous study that high Fe grain trait is expressed in all rice environments tested such as dry season in normal and saline soils, in acid and neutral soils (Graham et al. 1999). This study has confirmed again the correlation (r = 0.74*) between staining reaction and Fe concentration from the chemical analysis as previously reported (Prom-u-thai et al. 2003). Furthermore, we have demonstrated how the staining technique can be useful as an early assessing tool to identify seed lots that may contain individuals with exceptionally high grain Fe for further evaluation, including development and evaluation of progeny lines.
The present study has shown that individuals with a range of grain Fe concentrations, that covers the 50% difference between standard low and high grain Fe varieties, may be found together in the same farmer’s seed lot, which may or may not show obvious variation in external appearance. This suggests that closer look at variation within seed lots may be crucial in assessing for certain traits in genetically diverse local rice varieties.
Abbreviations
- Fe:
-
Iron
- FDA:
-
Iron deficiency anemia
- IFPRI:
-
International Food Policy Research Institute
- WHO:
-
World Health Organization
- AFLP:
-
Amplified fragment-length polymorphism
- ISSR:
-
Inter simple sequence repeat
- SSR:
-
Simple sequence repeat
References
Allard RW (2001) Principles of plant breeding, 2nd edn. John Wiley & Sons. 264 pp
Bouis HE (1996) Enrichment of food staples through plant breeding: a new strategy for fighting micronutrient malnutrition. Nutr Rev 54:131–137
Chang TT (1976) The origin, evolution, cultivation, dissemination, and diversification of Asian and African rices. Euphytica 25:425–441
Delhaize E, Dell B, Kirk G, Loneragan J, Nable R, Plaskett D, Webb M (1984) Manual of research procedures. 1st edn. Plant nutrition research group school of environmental and life sciences, Murdoch University, Australia
Dennis JV (1987) Farmer management of rice variety diversity in Northern Thailand. Ph.D. Thesis, Cornell University, USA
Doucet MY, Viel L (2002) Alveolar macrophage graded hemosiderin score from bronchoalveolar lavage in horses with exercise-induced pulmonary hemorrhage and controls. J Vet Int Med 16:281–286
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286
Graham RD, Senadhira D, Beebe S, Iglesias C, Monasterio I (1999) Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crops Res 60:57–80
Graham RD, Welch RM (1996) Breeding for staple-food crops with high micronutrients density. International Food Policy Research Institute. 72 pp
Gregorio G (2002) A new tool for fighting micronutrient malnutrition. J Nutr 132:500s–502s
Harlan JR (1992) Crops and man. Am Soc Agron Crop Sci Soci Am, Madison, Wis, USA
IFPI, International Food Policy Research (1999) World Food Prospects: Critical issue for the early twenty-first century
IRRI-IBPGR (1980) Descriptors for rice Oryza sativa L. IRRI, P.O. Box 933, Manila, Philippines, 21pp
Juliano BO (1993) Rice in human nutrition/Prepared in collaboration with FAO. Food and Agriculture Organization of the United Nations, Rome, 162 pp
Krishnan S, Ebenezer GAI, Dayanandan P (2001) Histochemical localization of storage components in caryopsis of rice (Oryza sativa L.). Current Sci 80:567–571
Lu BR, Snow AA (2005) Gene flow from genetically modified rice and its environmental consequences. BioSci 55:669–678
Lucca P, Hurrell R, Potrykus I (2002) Fighting iron deficiency anemia with iron-rich rice. J Amer Coll Nutr 21:184S–190S
Meesin S (2003) The structure of genetic diversity in a local Thai rice germplasm, MSc Thesis (Agronomy). Graduate School, Chiang Mai University, Thailand
Meesin S, Jamjod S, Rerkasem B (2004) Variation within and between accessions of local rice variety from Thailand. Agric J (Chiang Mai University) Special Volume 2:317–326
Morishima H, Sano Y, Oka HI (1980). Observations on wild and cultivated rices and companian weeds in the hilly area of Nepal, India and Thailand. Report of a study tour in tropical Asia, 1979. Mishima, Japan: National Institute of Genetics, 97 pp
Perls M (1867) Nachweis von eisenoxyd in gewissen pigmentation. Virchows archive fur pathologische anatomie and physiologie and fur klinisch medizine 39:42–47
Phattarakul N, Jamjod S, Rerkasem B (2006) Genotypic variation of upland rice varieties in responses to aluminium toxicity in nutrient solution compared with a field study. Paper presented at the 2006 Annual Meeting of Thailand Research Fund’s Crop Science Meso. Chiang Mai University, 3–4 December 2006
Power LE, McSorley R (2000) Ecological principles of agriculture. Delmar, Thomson Learning. 433 pp
Prom-u-thai C, Dell B, Thomson G, Rerkasem B (2003) Easy and rapid detection of iron in rice grain. Science Asia 29:213–217
Prom-u-thai C, Fukai S, Godwin DI, Huang L (2007) Genotypic variation of iron partitioning in rice grain. J Sci Food Agric, In press
Prom-u-thai C, Rerkasem B (2001) Iron concentration in Thai rice germplasm. Dev Plant Soil Sci 92:351–352
Saini N, Jain N, Jain S, Jain RK (2004) Assessment of genetic diversity within and among Basmati and non-Basmati rice varieties using AFLP, ISSR and SSR markers. Euphytica 140:133–146
Senadhira D, Gregorio G, Graham RD (1998) Breeding iron and zinc dense rice. Paper presented at the International Workshop on Micronutrient of Rice for Developing Countries, Philippines
Shannon CE, Weaver W (1949) The mathematical theory of communication. The University of Illinois press, Urbana. 117 pp
Shishido R, Kikuchi M, Nomura K, Ikehashi H (2006) Evaluation of genetic diversity of wild rice (Oryza rufipogon Griff.) in Myanmar using simple sequence repeats (SSRs). Resour Crop Evol 53:179–186
Sirabanchongkran A, Rerkasem K, Yimyam N, Boonma W, Coffey K, Pinedo-Vasquez M, Padoch C (2003) Varietal Turnover and Seed Exchange: Implications for Conservation of Rice Genetic Diversity On-Farm. IRRN 29:18–20
Tin HQ, Berg T, Bjørnstad Å (2001) Diversity and adaptation in rice varieties under static (ex situ) and dynamic (in situ) management – A case study in the Mekong Delta, Vietnam. Euphytica 122:491–502
Vasconcelos M, Datta K, Oliva N, Khalekuzzaman M, Torrizo L, Krishnan S, Olivera M, Goto F, Datta SK (2003) Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci 164:371–378
Watabe T (1967) Glutinous rice in Northern Thailand. The Center for Southeast Asian Studies, Kyoto University, Japan. 160 pp
World Health Organization (2002) The world health report, World Health Organization, Geneva, Switzerland
Yimyam N (2006) Fallow regeneration and upland rice yield variation in a system of shifting cultivation with pada (Macaranga denticulata (Bl.) Muell. Arg) as the fallow enriching species in Northern Thailand. Ph.D. Thesis, Graduate School, Chiang Mai University
Zeven AC (1999) The traditional inexplicable replacement of seed and seed ware of landraces and cultivars: a review. Euphytica 110:181–191
Zhang Q, Saghai M, Lu TY, Shen B (1992) Genetic diversity and differentiation of indica and japonica rice detected by RFLP analysis. Theor Appl Genet 83:495–499
Acknowledgements
We acknowledge financial support from the Collaborative Crop Research Program of the McKnight Foundation and Thailand Research Fund. We thank Professor Shu Fukai for editorial suggestion and farmers of Huai Tee Cha village for making available their rice seeds for our studies. KDML105 was supplied by the Thai Rice Department, and IR68144 by IRRI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pintasen, S., Prom-u-thai, C., Jamjod, S. et al. Variation of grain iron content in a local upland rice germplasm from the village of Huai Tee Cha in northern Thailand. Euphytica 158, 27–34 (2007). https://doi.org/10.1007/s10681-007-9421-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9421-7