Abstract
The present study was undertaken in moist temperate forest of Mandal-Chopta area in the Garhwal region of Uttarakhand, India. The aim of the present study was to assess the chemical properties of soils in relation to the forest structure and composition. Twelve forest types according to the altitude, slope aspect, and species composition were selected for the study. Chemical properties of the soil, i.e., total nitrogen (N), available phosphorus (P), available potassium (K), organic carbon (C), soil organic matter (SOM), pH and C:N ratios were analyzed for three different depths viz., (i) ‘Upper’ (0–10 cm), (ii) ‘Middle’ (11–30 cm), and (iii) ‘Lower’ (31–60 cm) in all the selected forest types. Phytosociological and diversity parameters viz. total basal cover (Gha−1), stem density (Nha−1), tree species richness (SR), Simpson concentration of dominance and Shannon–Wiener diversity index (\( \overline{\rm H} \)) were also calculated for each forest type. Values of N, P, K, C, SOM, C:N ratio and pH ranged from 0.17 to 0.45 %, 2.73 to 20.17 ppm, 40.67 to 261.17 ppm, 2.29 to 4.31 %, 3.95 to 7.43 %, 8.12 to 14.49 and 5.47 to 6.67, respectively. N showed a positive relationship with C and K. P was positively correlated with C and negatively correlated with altitude. P was higher in the soil of lower horizons of all the forest types. K was found to be positively correlated with altitude, C and N. pH of all the forest types was slightly acidic. C was comparatively higher in mixed broad-leaved forest types. No relationship between altitude and C was observed, which may be due to different composition of forest types along the altitudinal gradient and their differential decomposition rates. C content decreased with the depth of the soil. C showed positive correlation with N, P, and K. N showed a positive correlation with Gha−1. K showed a negative correlation with SR and \( \overline{\rm H} \). P showed no relationship with the phytosociological parameters. This study also provides the comparisons between the results of chemical analysis of the present study with numerous other previous studies in the temperate Himalayan region of the Uttarakhand. The values of soil chemical properties of the present study in most of the forest types are on the higher end than the values previously recorded for the other similar forests of the region. The possible reason being luxuriant vegetation and undisturbed nature of these forest types, which is evident from higher values of diversity and other phytosociological parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The growth and reproduction of forest cannot be understood without the knowledge of soil. The soil and vegetation have a complex interrelation because they develop together over a long period of time. The vegetation influences the chemical properties of soil to a great extent. The selective absorption of nutrient elements by different tree species and their capacity to return them to the soil brings about changes in soil properties (Singh et al. 1986). Concentration of elements in the soils is a good indicator of their availability to plants. Their presence in soil would give good information towards the knowledge of nutrient cycling and bio-chemical cycle in the soil–plant ecosystem (Pandit and Thampan 1988). Forests in general have a greater influence on soil conditions than most other plant ecosystem types, due to a well-developed “O” horizon, moderating temperature, and humidity at the soil surface, input of litter with high lignin content, high total net primary production, and high water and nutrient demand (Binkley and Giardina 1998). Moreover, different tree species can differ significantly in their influence on soil properties as well as soil fertility (Augusto et al. 2002). The properties of the soil are the important factor for the growth of the plants. Among them, the most important factor is soil fertility, i.e., the essential nutrients available in the soil, for the growth of plants. The adequate theoretical and practical knowledge of various forest soils and the complex relationship between the life of various trees and other plants of the forest is therefore necessary to study.
Himalayan forests play an important role in tempering the inclemency of the climate, in cooling and purifying the atmosphere, in protecting the soil, in holding the hill slopes in position, and in buffering up huge reserves of soil nutrients. The Garhwal Himalaya has vast variations in the climate, topography, and soil conditions, which form a very complex ecosystem. The knowledge of chemical properties of soils of different forest types of temperate region of Garhwal Himalaya is meager. In our earlier studies on forests of Garhwal region relationships between forest dependency and socio-economic status of rural people (Sharma et al. 2009), the role of physiographic factors (Sharma et al. 2010a), tree diversity and C stocks (Sharma et al. 2010b, 2011; Gairola et al. 2011a), regeneration status (Gairola et al. 2012), effects of slope aspects (Sharma et al. 2010c), physical properties of the soils (Sharma et al. 2010d), and structure and composition (Gairola et al. 2011b) were worked out. However, the present study was undertaken with the aim of assessing the chemical properties of soils in relation to the forest structure and composition in different forest types of moist temperate Mandal-Chopta forest of Garhwal Himalaya.
2 Materials and methods
2.1 Study area
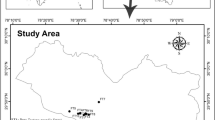
The present study was conducted in Mandal-Chopta forest area, which forms a large (nearly 1,100 ha), prestigious, and botanically valuable reserve complex (Trishula Reserve forest of Kedarnath forest division) in the Garhwal region (western Himalaya) of Chamoli district of Uttarakhand state, India (Fig. 1). It occurs at 30°27.560′N latitude and 79°15.234′E longitude along an altitudinal gradient of 1,500–2,900 m a.s.l. It is a rich moist temperate forest situated 12 km away from the district headquarters, Gopeshwar. Recently, Gairola et al. (2010) have recorded 338 species of vascular plants (334 angiosperms and four gymnosperms) belonging to 93 families (91 angiosperms and two gymnosperms) and 249 genera (246 angiosperms and three gymnosperms) from the study area. The study area is characterized by undulating topography with gentle slopes on the northern, northeastern, and northwestern faces, and somewhat steep slopes on southern and southwestern directions. The soil types found in the region are brown and black forest soils and podzolic soils. Soils are generally gravelly and large boulders are common in the area. Numerous high ridges, deep gorges and precipitous cliffs, rocky crags and narrow valleys are part of the topography of the region. The topography of the area has also been influenced by landslides, which are common during the rainy season. Meteorological details (1998–2007) of the study area are given in Fig. 2. Mean annual maximum and minimum temperature were recorded as 16.41 ± 3.60 and 6.14 ± 1.98 °C, respectively. Mean annual rainfall was recorded as 2,044.47 ± 476.01 mm. Mean relative humidity throughout the year ranged from 15 to 86 %.
2.2 Sampling and data analysis
During the reconnaissance survey of the study area, 12 forest types according to altitude, slope aspect, and species compositions were selected for the study (Table 1). Each forest type was named according to the composition of dominant tree species as per Prakash (1986), viz., ≥75 % as pure forest; 50–75 % as mainly single species dominant forest; 25–50 % as mixed forest, and <25 % miscellaneous forest. Physiographic factors (i.e., altitude) and aspect across different forest types were measured by GPS (Garmin, Rino-130).
2.2.1 Vegetation analysis
The composition of the forest types was analyzed by using nested quadrat method as per Kent and Coker (1992). Trees (≥10 cm dbh) were analyzed by 10 × 10 m-sized quadrats as proposed by Curtis and McIntosh (1950) and Phillips (1959). The basal cover was calculated by dividing the square of Cbh (circumference at breast height, i.e., 1.37 m) by 4π. The basal cover was multiplied with respective densities of the species to obtain total basal cover (Gha−1). For each species, values of frequency, density, and abundance were calculated following Curtis and McIntosh (1950). The importance value index (IVI) was calculated by summing up relative density, relative frequency, and relative dominance, following Phillips (1959). Species richness (SR) was simply taken as a count of total number of species in that particular forest type. Shannon–Wiener diversity index was calculated as per Shannon and Weaver (1963) and concentration of dominance (Cd) was calculated as per Simpson (1949):
where, \( \overline{\rm H} \) is Shannon–Wiener diversity index, N i is IVI of a species, N is total IVI of all the species, Cd is the concentration of dominance.
2.2.2 Soil sampling and analysis
Composite soil samples were collected from three different depths viz., (i) “upper” (0–10 cm), (ii) “middle” (11–30 cm) and (iii) “lower” (31–60 cm) for assessing the chemical properties of the soil in all the selected forest types. The soil tests were conducted at Central Soil and Water Conservation Research and Training Institute (CSWCRTI), Dehradun, India. The pH of soil was determined directly with the help of control dynamics digital pH meter (model Ap + 175E/C). Walkley and Black’s rapid titration method as modified by Walkley (1947) was adopted for organic carbon estimation. The factor of 1.724 was used to convert the organic carbon (%) into soil organic matter (%). Available phosphorus was determined in the soil by Olsen et al. (1954) method. Potassium was extracted by neutral normal ammonium acetate method (Morwin and Peach 1951) and was determined by the flame photometer (Evans Electro Selenium Ltd; Holsted Essex, England). Total nitrogen was measured by using the standard Kjeldhal procedure (Bremner and Mulvaney 1982). Total carbon (%) was divided by total nitrogen (%) to get values of C:N ratio. A single-tailed Pearson correlation coefficient was calculated between various phytosociological and soil chemical parameters using SPSS-16 software (SPSS Inc., Chicago, IL, USA).
3 Results and discussion
The information about dominant and associated tree species in different forest types is shown in Table 1. Results of forest composition and diversity analysis are given in Table 2. The values of total nitrogen (N), available phosphorus (P), and available potassium (K) at various depths in the different forest types are given in Table 3 and of organic carbon (C), soil organic matter and C:N ratios are given in Table 4. The results of Pearson correlation coefficients between different vegetation and soil parameters are given in Table 5. Comparisons between chemical properties of soils at different altitudes and forest types in different parts of the Uttarakhand Himalaya (earlier reported values) with the study area have been shown in Table 6. The results of the present findings are within the reported range of values for chemical properties of soils in similar forest types at same altitudes elsewhere in the Uttarakhand Himalaya (Table 6).
3.1 Nitrogen
Nitrogen is an essential element for all growth processes in plants, especially in cold regions. If it is not available, the plant remains stunted and comparatively undeveloped. Ecologists have long considered temperate forests systems to be limited by N availability (Mitchell and Chandler 1939). Soil N is supposed to be the most limiting nutrient in a majority of ecosystems (Fenn et al. 1998). The values of total N varied significantly in different forest types. Values of total N in the study area ranged between 0.17 % (FT12) and 0.45 % (FT6) (Table 3). The values of total N in the present study are higher than the values recorded by Khera et al. (2001), Srivastava et al. (2005), and Semwal (2006) and lower than the values recorded by Pande et al. (2001) and Sharma et al. (2010a), but similar to the values recorded by Thadani and Ashton (1995), Kumar et al. (2004), Nazir (2009), and Sharma et al. (2010c) in other parts of Uttarakhand Himalaya (Table 6). Although N is mostly present in the form of nitrates in the soil, which is very mobile and get moved freely with moisture (Gupta and Sharma 2008), the values of total N in the study area were higher in upper layers as compared to lower layers. This could be attributed to higher water holding capacity and the presence of heavy litter and humus contents in the upper layers of the studied forest types. The availability of N depends to a large extent on the amount and properties of organic matter (de Hann 1977). Therefore the high amount of organic matter in the forest types in the upper layers may also be the reason for richness of N in the upper layers as compared to lower layers. As C and N are intimately linked and primary source of C and N is found in the soil as an organic matter, in the form of plants and animal’s debris (Aber and Melillo 1991), total N showed a significantly positive relationship with organic C (0.840). Total N also showed a significantly positive relationship with available K (0.569) (Table 5). Gupta and Sharma (2008) also showed that N and K were positively correlated chiefly because all these attributes are intimately linked with soil humus.
3.2 Phosphorus
Available P is inevitable for the vital growth processes in plants. It is observed that P is found in all terrestrial systems in the form of organic and inorganic matter, while organic P forms are the major available source of phosphorus. Soil organic matter has the inorganic form of P transformed into insoluble form in many soils. The rates of weathering also control phosphorus availability to plants. Phosphorus in turn controls the input levels of plant residues (Brown et al. 1994). The carbon–phosphorus and nitrogen–phosphorus ratios vary according to the parent material, which depends upon degree of weathering and by other means (Paul and Clark 1996). The amount of P indicates the character of soil to allow specific plants to grow at a particular site, which is also useful to identify the vegetation type of the area. It has been reported that a large proportion of P is stored in the forms that are unavailable to plants (Murphy 1958), for example, H2PO4, which becomes available at low pH values and suffers from fixation by hydrous oxides and silicate minerals (Soromessa et al. 2004). Values of available P in the study area varied between 2.73 ppm in Conifer mixed broad-leaved (FT5) and 20.17 ppm in mixed broad-leaved (FT9) forest type (Table 3). The values of available P in the present study are similar to those recorded by some other investigators in other parts of the Uttarakhand Himalaya, but in general varied considerably (Table 6). Available P was positively correlated with organic C (0.520). Gupta and Sharma (2008) also showed that organic C and P were positively correlated chiefly because all these attributes were intimately linked with soil humus. Available P showed a negative correlation with altitude (−0.407) (Table 5). The P was also found higher in the lower horizons of all the forest types, which may be due to the leaching properties of the soils.
3.3 Potassium
The mineral potassium (exchangeable K) is found in soluble form in all parts of plants, and is responsible for the carbohydrate and protein formations. Potassium activates the enzymes of the plants, which in turn help in the metabolism of the plants, starch synthesis, nitrate reduction, and also plays a role in sugar degradation. Potassium performs very vital processes like regulating transpiration and respiration, influencing enzyme action, and synthesis of carbohydrates and proteins, etc. (Brady 1996). The decrease of K is caused by leaching and drainage, which results in the destruction of vegetation (Basumatary and Bordoloi 1992). Values of available K in the study area varied between 40.67 ppm in mixed Quercus leucotrichophora (FT12) and 261.17 ppm in mainly Quercus semecarpifolia (FT1) forest type (Table 3). The values of available K in the present study are similar to those recorded by some other investigators in other parts of the Uttarakhand Himalaya, but in general varied considerably (Table 6). Since exchangeable K in soil largely depends on the composition of parent rock material, no specific reason can be assigned to its differential quantities at different altitudes and sites in various parts of Uttarakhand Himalaya. Available K was found to be significantly and positively correlated with altitude (0.538). Total soil K varied on different altitudes and the higher content of available K was found in surface soil in the form of exchangeable K, which may later convert into the soil solution (Dimri et al. 2006). Although Gupta and Sharma (2008) argued that the K is not much influenced by soil organic matter because it is not the direct supplier of K, in our study area, available K showed a significant positive correlation with organic matter (0.520) (Table 5). This was supported by the findings of Basumatary and Bordoloi (1992) and Boruah and Nath (1992), who reasoned that a layer of organic matter significantly improves the retention of K in the soils. Available K showed a positive correlation with total N (0.569). Gupta and Sharma (2008) also showed that N and organic C were positively correlated chiefly because all these attributes are intimately linked with soil humus.
3.4 pH
It has been reported that forest soils should be slightly acidic for nutrient supply to be balanced (Leskiw 1998). A fertile soil generally has a pH range between 5.5 and 7.2, which makes the essential elements and nutrients available to the flora. Values of pH in the study area varied between 5.47 (FT5) and 6.67 (FT1) (Table 3). Most of the forest types had soils with pH below 6.0, except Quercus semecarpifolia, Quercus floribunda, and Aesculus indica forest types. This may be due to high organic matter content and the undisturbed nature of the soils in the study area. Robertson and Vitousek (1981) and Adams and Sidle (1987) have also recorded low pH in undisturbed natural forests as compared to disturbed ecosystems. The reduction in pH can be attributed to accumulation and subsequent slow decomposition of organic matter, which releases acids (de Hann 1977). The values of pH in present study are lower than the values of pH recorded by Khera et al. (2001) for Kumaun Himalaya and are similar to most other studies in the Uttarakhand Himalaya (Table 6).
3.5 Organic C and soil organic matter (SOM)
SOM can absorb and hold substantial quantities of water, up to 20 times its mass (Stevenson 1994). SOM in the form of surface residues can also influence water retention directly by reducing evaporation rates and increasing the infiltration of water. Its impact on soil biology is even more enormous. Most soil organisms are heterotrophic and gain their energy from the decomposing SOM. The quality and quantity of SOM therefore determine the production potential of the soil. The values of organic C (Table 4) in the study area ranged between 2.29 % in mixed Abies pindrow (FT7) and 4.31 % in mainly Aesculus indica (FT6) forest type, which were more or less similar to the values recorded by other workers in similar forest types at similar altitudes in other parts of the Uttarakhand Himalaya (Table 6). Organic C was also comparatively higher in mixed broad-leaved forest types (FT8, FT9, and FT10). SOM is responsible for building a major portion of the soil organic C pool, which regulates the soil properties viz., physical, chemical, and biological properties (Woomer et al. 1994). In fine-textured soils, the content of SOM usually varies from 3 to nearly 10 % by weight (Wilde 1946). In the present study, values of SOM varied between 3.95 (FT7) and 7.43 % (FT6) (Table 4). These values are also within the range of earlier recorded values by different investigators in different parts of Uttarakhand Himalaya (Table 6). The soil C concentration varies across the landscape but more soil C variability is found at varying elevations (Powers and Schlessinger 2002). Generally, the increase in SOM with increasing altitude is due to the addition of leaf litter annually and slow decomposition rates of organic residues under low temperature (Dimri et al. 1997). However, no relationship between altitude and organic C concentration was observed in this study, which may be due to different composition of forest types along the altitudinal gradient and their differential decomposition rates. The organic C content decreased with the depth of the soil in all the forest types, which may be due to the fact that humus formation and decomposition of organic matter takes place in the upper layers. Organic C showed a positive correlation with total N (0.840), available P (0.520), and available K (0.520) (Table 5). This finding was also supported by Gupta and Sharma (2008), who also recorded that these parameters were positively correlated and reasoned that all these attributes were intimately linked with soil humus. According to Jha et al. (1984), if the soil is rich in SOM contents, it is definitely rich in total N contents also.
3.6 C:N ratio
The carbon:nitrogen ratio indicates the availability of carbon and nitrogen (C/N) in the soil (Miller 2001). When fresh organic material undergoes decomposition in soil, the rate of decomposition and the amount of humus formed are related to the C:N ratio of the residue. When the proportions are equal, the rate of decomposition increases as the C:N ratio narrows in the soil. The C and N cycles in the soil are linked through processes of N assimilation, N mineralization, denitrification, and organic matter decomposition (Yano et al. 2000). Apart from the importance of C and N, separately, the relation between them is interesting in ecological terms. The C:N ratio is widely used, when researching on the ecology of the forest. This ratio indicates the availability of N in floor material, rate of decay of the forest floor (Fisher and Binkley 2000), and the quality of the organic matter under the canopy (Cote et al. 2000), which can also be linked to the soil microbial biomass (Hogberg 2004). Johnson and Ball (1996) considered the ratio as a key variable through which all ecosystem perturbations act. The C:N ratio reflects the release of N in the soil through organic matter decomposition and therefore indicates the degree of decomposition of organic matter in the forest soils (Ulrich 1971). The vegetation influences the C:N ratio and the C:N ratio determines the stand composition (Fisher and Binkley 2000).
Values of the C:N ratio in the study area ranged between 8.12 (FT3) and 14.49 (FT12) (Table 4). The values of C:N ratio in the present study were more or less similar to the values recorded by other workers in similar forest types at similar altitudes in other parts of the Uttarakhand Himalaya, except those recorded by Khera et al. (2001) for Kumaun Himalaya (Table 6). As all the forest types were mature in nature and were undisturbed and established, C:N ratios were near or above 10. Kawahara and Tsutsuni (1972) have reported that generally the soil of a forest stand attains the steady state when the C:N ratio reaches 10 and at that level the release of nutrients is rapid due to mineralization. The C:N ratio was found to be significantly and negatively correlated with total N (−0.753) (Table 5), which is obvious as increasing N content will automatically decrease C:N ratio. There is considerable evidence that temperate broad-leaved and conifer tree species growing in a single species dominant stands alter soil C and N dynamics (Binkely 1995). A study by Cote et al. (2000) concluded that both organic C and N were significantly affected by factors such as stand type, stand age, and the interaction between age, species composition, and soil properties.
3.7 Relationship between chemical properties of soils and phytosociological parameters
The highest total basal cover (Gha−1) was recorded in FT6 (mainly Aesculus indica: 86.56 m2 ha−1) followed by FT10 (mixed broad-leaved: 84.25 m2 ha−1) and FT7 (mixed Abies pindrow: 84.03 m2 ha−1), whereas the lowest Gha−1 was recorded in FT11 (mainly Alnus nepalensis: 32.77 m2 ha−1). The highest stem density was observed in FT10 (mixed broad-leaved: 1,470 Nha−1) and the lowest in FT4 (mainly Abies pindrow: 380 Nha−1). Species richness ranged between 5 (mainly Quercus semecarpifolia: FT1, mainly Quercus floribunda: FT2, mainly Abies pindrow: FT4 and mainly Aesculus indica: FT6) and 16 (mixed broad-leaved: FT8). Shannon-Wiener diversity index (\( \overline{\rm H} \)) was recorded to be the highest in FT8 (mixed broad-leaved: 3.33), followed by FT7 (mixed Abies pindrow: 3.19), and FT9 (mixed broad-leaved: 3.14), whereas the lowest \( \overline{\rm H} \) was recorded in FT4 (mainly Abies pindrow: 1.45). Values of concentration of dominance (Cd) ranged between 0.129 (mixed broad-leaved: FT8) and 0.467 (pure Acer acuminatum: FT3). Nitrogen showed a positive correlation with total basal cover (0.391). The total N (0.45 %) and organic C (4.31 %) were highest in mainly Aesculus indica forest type (FT6), where Gha−1, was also highest. A higher concentration of total N and organic C in soil may have given rise to higher productivity (Gha−1) of trees in this forest type. The lowest value of total N (0.17 %) was recorded for mixed Quercus leucotrichophora forest type (FT12), where the value of total basal cover was also very low (35.08 m2 ha−1). Potassium showed a negative correlation with species richness (−0.594) and \( \overline{\rm H} \) (−0.556). Phosphorus showed no correlation with the phytosociological parameters. The variation in values of phosphorus in different forest types may be attributed to the variation in composition of parent rock material of the particular site. pH had a positive correlation with total basal cover. High values of stem density and species diversity were recorded in mixed broad-leaved forest types growing at middle elevations (1,600–2,400 m a.s.l.), whereas forest types growing at higher altitudes (>2,400 m a.s.l.) had low species richness and stem density. Tree density showed a positive relationship with species richness and \( \overline{\rm H} \), and a negative relationship with Cd. The high K contents were reported under mainly Quercus semecarpifolia and mainly Quercus floribunda forest types between 2,550 and 2,850 m a.s.l. Tomlinson and Tomlinson (1990), Sharpe et al. (1992), and Srivastava et al. (2005) are of the view that the oak individuals are related with higher K release, which may be the main reason for the higher content of K in the soils of these forest types. High values of phytosociological parameters and chemical properties of the soils complement each other and show that these forest types are very fertile. The values of soil chemical properties of the present study in most of the forest types are on the higher end than the values previously recorded for the other similar forests of the region. The possible reason for this is the luxuriant vegetation and undisturbed nature of these forest types, which is evident from the higher values of diversity and other phytosociological parameters.
References
Aber JD, Melillo JM (1991) Terrestrial ecosystems. Saunders College Publishing, Philadelphia
Adams PW, Sidle RC (1987) Soil conditions in three recent landslides in southeast Alaska. For Ecol Manag 18(2):93–102
Augusto L, Ranger J, Binkely D, Rothe A (2002) Impact of several common tree species of European temperate forests on soil fertility. Ann For Sci 59(3):233–253
Basumatary A, Bordoloi PK (1992) Forms of potassium in some soils of Assam in relation to soil properties. J Indian Soc Soil Sci 40(3):443–446
Binkely D (1995) The influence of tree species on forest soils: processes and patterns. In: Mead DJ, Cornforth IS (eds) Proceedings of the trees and soil workshop, Lincoln University, agronomy society of New Zealand special publications number 10. Lincoln University Press, Canterbury, pp 1–33
Binkley D, Giardina C (1998) Why do species affect soils? The warp and woof of tree-soil interaction. Biogeochemistry 42(1–2):89–106
Boruah HC, Nath AK (1992) Potassium status in three major soil orders of Assam. J Indian Soc Soil Sci 40(3):559–561
Brady NC (1996) The nature and properties of soil, 10th edn. Prentice Hall, New Delhi
Bremner JM, Mulvaney CS (1982) Nitrogen total. In: Miller RH, Kieney DR (eds) Method of soil analysis- part 2: chemical and microbiological methods, 2nd edn. Agronomy series No. 9. American Society for Agronomy and Soil Sciences, Madison, pp 595–624
Brown S, Anderson JM, Woomer PL, Swift MJ, Barrios E (1994) Soil biological processes in tropical ecosystems. In: Woomer PL, Swift MJ (eds) The biological management of tropical soil fertility. Wiley, Chichester, pp 15–46
Cote L, Brown S, Pare D, Fyles J, Bauhus J (2000) Dynamics of carbon and nitrogen mineralization in relation to stand type, stand age and soil texture in the boreal mixedwood. Soil Biol Biochem 32(8–9):1079–1090
Curtis JT, McIntosh RP (1950) The interrelations of certain analytic and synthetic phytosociological characters. Ecology 31(3):434–455
de Hann S (1977) Humus, its formation, its relation with the mineral part of the soil and its significance for soil productivity. In: Organic matter studies, vol 1. International Atomic Energy Agency, Vienna, pp 21–30
Dimri BM, Jha MN, Gupta MK (1997) Status of soil nitrogen at different altitudes in Garhwal Himalaya. Van Vigyan 359(2):77–84
Dimri BM, Jha MN, Gupta MK (2006) Soil potassium changes at different altitudes and seasons in upper Yamuna Forests of Garhwal Himalayas. Indian For 132(5):609–614
Fenn ME, Poth MA, Aber JD, Boron JS, Bormann BJ, Johnson DW, Lenly AD, McNulty SG, Ryan DF, Stottlemeyer R (1998) Nitrogen excess in North American ecosystems: predisposing factors, ecosystem responses and management strategies. Ecol Appl 8(3):706–733
Fisher RF, Binkley D (2000) Ecology and management of forest soils, 3rd edn. Wiley, New York, p 489
Gairola S, Sharma CM, Rana CS, Ghildiyal SK, Suyal S (2010) Phytodiversity (angiosperms and gymnosperms) in Mandal-Chopta forest of Garhwal Himalaya, Uttarakhand, India. Nat Sci 8(1):1–17
Gairola S, Sharma CM, Suyal S, Ghildiyal SK (2011a) Composition and diversity of five major forest types in moist temperate climate of the western Himalayas. For Stud China 13(2):139–153
Gairola S, Sharma CM, Ghildiyal SK, Suyal S (2011b) Live tree biomass and carbon variation along an altitudinal gradient in moist temperate valley slopes of the Garhwal Himalaya (India). Curr Sci 100(12):1862–1870
Gairola S, Sharma CM, Ghildiyal SK, Suyal S (2012) Regeneration dynamics of dominant tree species along an altitudinal gradient in a moist temperate valley slopes of the Garhwal Himalaya. J For Res 23(1):53–63
Gupta MK, Sharma SD (2008) Effect of tree plantation on soil properties, profile morphology and productivity index I. Poplar in Uttarakhand. Ann For 16(2):209–224
Hogberg NM (2004) Carbon and nitrogen relations among soils, microbes and plants in boreal forests. PhD Thesis, Swedish University of Agricultural Sciences
Jha MN, Dimri BM (1991) Soil nutrients and forest productivity in four natural stands. J Indian Soc Soil Sci 39:735–738
Jha MN, Rathore RK, Pande P (1984) Soil factor affecting the natural regeneration of silver fir and spruce in Himachal Pradesh. Indian For 110(3):293–298
Johnson DW, Ball JT (1996) Interactions between CO2 and nitrogen in forests: can we extrapolate from the seedling to the stand level? In: Koch GW, Mooney HA (eds) Carbon dioxide and terrestrial ecosystems. Academic Press, San Diego, p 443
Kawahara T, Tsutsuni T (1972) Studies on the circulation of Carbon and Nitrogen in forest ecosystem. Bull Kyoto Univ For 44:141–158
Kent M, Coker P (1992) Vegetation description and analysis. Belhaven Press, London
Kharkwal G (2002) Spatial pattern of plant species diversity with particular reference to forest herbs along an altitudinal transect in Central Himalaya. PhD Thesis, Kumaun University, Nainital, Uttarakhand, India
Khera N, Kumar A, Ram J, Tewari A (2001) Plant biodiversity assessment in relation to disturbances in mid-elevational forest of Central Himalaya, India. Trop Ecol 42(1):83–95
Kumar M, Rajwar GS, Sharma CM (2004) Physico-Chemical properties of forest soils along altitudinal gradient in the Garhwal Himalayas. J Hill Res 17(2):60–64
Leskiw LA (1998) Land capability classification for forest ecosystem in the oil stands region. Alberia Environmental Protection, Edmonton
Miller C (2001) Understanding the carbon-nitrogen ratio. Reprinted from ACRES. A voice for Eco-agriculture 30(4):20
Mitchell HL, Chandler RF (1939) The nitrogen nutrition and growth of certain deciduous trees of Northeastern United States. The Blackrock Forest Bulletin no. 11. Cornwall-on-the-Hudson, NY
Morwin HD, Peach PM (1951) Exchangeability of soil potassium in and, silt and clay fractions as influenced by the nature of complementary exchangeable cations. Proc Soil Sci Soc Am 15:125–128
Murphy HP (1958) The fertility status of some soils of Ethiopia. College of Agriculture, Jimma
Nazir T (2009) Estimation of site quality of important temperate forest cover on the basis of soil nutrient and growing stock in Garhwal Himalaya. D.Phil. Thesis, HNB Garhwal University, Srinagar (Garhwal), Uttarakhand, India
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with Sodium bicarbonate. Department of Agriculture Circular, US, p 939
Pande PK, Negi JDS, Sharma SC (2001) Plant species diversity and vegetation analysis in moist temperate Himalayan forest. Indian J For 24(4):456–470
Pande PK, Negi JDS, Sharma SC (2002) Plant species diversity, composition, gradient analysis and regeneration behaviour of some tree species in a moist temperate western Himalayan forest ecosystem. Indian For 128(8):869–886
Pandit BR, Thampan S (1988) Total concentration of P, Ca, Mg, N & C in the soils of reserve forest near Bhavnagar (Gujarat State). Indian J For 11(2):98–100
Paul EA, Clark FE (1996) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego
Phillips EA (1959) Methods of vegetation study. Henry Holt, New York, p 107
Powers JS, Schlessinger WH (2002) Relationships among soil carbon distributions and biophysical factors at nested spatial scales in rain forests of northeastern Costa Rica. Geoderma 109:165–190
Prakash R (1986) Forest management. International Book Distributors, Dehradun, p 214
Rawal RS (1991) Woody vegetation analysis along an elevational gradient (1600-3400 m) of upper Sarju catchment, Kumaun Himalaya. PhD Thesis, Kumaun University, Naini Tal, Uttarakhand, India
Robertson GP, Vitousek PM (1981) Nitrification in primary and secondary succession. Ecology 62:376–386
Semwal S (2006) Studies on phytosociology, diversity patterns and competition along an altitudinal gradient in a part of lesser Himalaya in Garhwal, Uttaranchal. D.Phil. Thesis, HNB Garhwal University, Srinagar (Garhwal), Uttarakhand, India
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana, p 117
Sharma CM, Baduni NP (2000) Effect of aspects on the structure of some natural stands of Abies pindrow in Himalayan moist temperate forest. Environmentalist 20(4):309–317
Sharma CM, Gairola S, Ghildiyal SK, Suyal S (2009) Forest dependent livelihood in relation to socio-economic status of the people in temperate villages of Garhwal Himalaya: a case study. Mt Res Dev 29(4):308–319
Sharma CM, Suyal S, Ghildiyal SK, Gairola S (2010a) Role of Physiographic factors in distribution of Abies pindrow (Silver Fir) along an altitudinal gradient in Himalayan temperate Forests. Environmentalist 30(1):76–84
Sharma CM, Baduni NP, Gairola S, Ghildiyal SK, Suyal S (2010b) Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. For Ecol Manag 260(12):2170–2179
Sharma CM, Baduni NP, Gairola S, Ghildiyal SK, Suyal S (2010c) The effect of slope aspects on forest compositions, community structures and soil properties in natural temperate forests in Garhwal Himalaya. J For Res 21(3):331–337
Sharma CM, Gairola S, Ghildiyal SK, Suyal S (2010d) Physical properties of soils in relation to forest composition in moist temperate valley slopes of the Central Western Himalaya. J For Sci 26(2):117–129
Sharma CM, Gairola S, Baduni NP, Ghildiyal SK, Suyal S (2011) Variation in carbon stocks on different slope aspects in seven major forest types of temperate region of Garhwal Himalaya, India. J Biosci 36(4):701–708
Sharpe WE, Swistock BR, Dewalle DR (1992) A greenhouse study of northern red oak seedling growth of two forest soils at different stages of acidification. Water Air Soil Pollut 66(1–2):121–133
Simpson EH (1949) Measurement of diversity. Nature 163:688
Singh AK, Parsad A, Singh B (1986) Availability of phosphorus and potassium and its relationship with physico-chemical properties of some forest soils of Pali-range (Shahodol, M.P.). Indian For 112(12):1094–1104
Soromessa T, Teketay D, Demissew S (2004) Ecological study of the vegetation in Gamo Gofa zone, southern Ethiopia. Trop Ecol 45(2):209–221
Srivastava RK, Khanduri VP, Sharma CM, Kumar P (2005) Structure, diversity and regeneration potential of oak-dominant conifer mixed forest along an altitudinal gradient in Garhwal Himalaya. Indian For 131(12):1537–1553
Stevenson FJ (1994) Humus chemistry, 2nd edn. Wiley, New York
Thadani R, Ashton PMS (1995) Regeneration of Banj oak (Quercus leucotrichophora A. Camus) in the central Himalaya. For Ecol Manag 78:217–224
Tomlinson GH, Tomlinson FL (1990) Effects of acid deposition on the forests of Europe and North America. CRC Press, Boca Raton, FL, p 281
Ulrich B (1971) The ecological value of soil chemical data. In: Duvigneaud P (ed) Productivity of forest ecosystems. UNESCO, Paris, pp 101–105
Usman S, Singh SP, Rawat YS, Bargali SS (2000) Fine root decomposition and nitrogen mineralisation patterns in Quercus leucotrichophora and Pinus roxburghii forests in Central Himalaya. For Ecol Manag 131:191–199
Walkley A (1947) An estimation of methods for determining organic carbon and nitrogen in soils. J Agric Sci 25:598–609
Wilde SA (1946) Forest soils and forest growth. Publishers Periodical Experts Book Agency
Woomer PL, Martin A, Albrecht A, Reseck DVS, Scharpenseel HW (1994) The importance and management of soil organic matter in the tropics. In: Woomer PL, Swift MJ (eds) The biological management of tropical soil fertility. Wiley, Chichester
Yano Y, McDowell WH, Aber JD (2000) Biodegradable dissolved organic carbon in forest soil solution and effects of chronic nitrogen deposition. Soil Biol Biochem 32:1743–1751
Acknowledgments
We thankfully acknowledge the financial support provided by the Department of Science and Technology, Government of India, New Delhi, vide its Project No. SP/SO/PS-52/2004. We also thank Uttarakhand Forest Department for providing meteorological data, and CSWCRTI, Dehradun, India, for help in the soil analysis. One of the authors, Sumeet Gairola, is thankful to University Grants Commission, New Delhi, India, for providing financial support under Dr. D.S Kothari Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gairola, S., Sharma, C.M., Ghildiyal, S.K. et al. Chemical properties of soils in relation to forest composition in moist temperate valley slopes of Garhwal Himalaya, India. Environmentalist 32, 512–523 (2012). https://doi.org/10.1007/s10669-012-9420-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-012-9420-7