Abstract
Due to increasing awareness about the environmental impacts of oil-based synthetic dyes used in textile coloration, the research on natural dyes from sustainable resources has gained importance again. In this study, the ultrasound-assisted extraction (UAE) technique was employed for the extraction process of colorants from Hawthorn fruits. Various solvents (e.g., methanol, acetone, ethanol, water, a mixture of ethanol and water) were used for the extraction process. Compared to the conventional extraction technique, the UAE technique was found to improve the extraction efficiency between 20 and 70% depending on the type of solvent. The synergistic dye extraction efficiency was observed when a mixture of water and ethanol was used for conventional and UAE processes, and consequently, W1/4E was selected as the most efficient mixture. The important parameters, such as dried fruit powder concentration, initial pH, extraction time and temperature, were optimized using the response surface methodology (RSM). A second-order polynomial model was developed for estimating the extraction efficiency based on the important initial parameters. The maximum extraction yield was achieved at the initial dried fruit powder of 8 g/L, at pH 5, 60 °C after 40 min. Three main flavonoids (i.e., quercetin, rutin, kaempferol) were detected in the extracted solvent from Hawthorn fruits by FTIR and HPLC techniques. Polyamide (nylon 6) fabric was successfully dyed by the extracted natural colorants with relatively high colorfastness properties. The dyed fabric also showed remarkable antimicrobial properties against E. coli and S. aureus. This study indicated that the natural colorants extracted from Hawthorn fruits were promising for textile coloration with noticeable antioxidant and antimicrobial properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
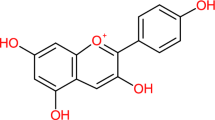
Hawthorn (Crataegus elbursensis) has been used as medicine and food for centuries. Many medicinal properties of Hawthorn are originated from the presence of proanthocyanidin and flavonoids (Liu et al. 2010). Hawthorn fruits are rich in (a) sugar and sugar alcohols such as fructose and glucose, (b) organic and phenolic acids such as citric and gallic acids, (c) essential oils like monoterpenoids and sesquiterpenoids, and (d) phenylpropanoids such as hydroxycinnamic acids, monolignols, and flavonoids (Urbonaviciute et al. 2006; Arslan et al. 2011; Edwards et al. 2012). Epicatechin, hyperoside, and chlorogenic acid are 3 components of this fruit with free radical scavenging property (Kwok et al. 2010; Jurikova et al. 2012). Flavonoids are responsible for Hawthorn’s color, and more than 50 flavonoids (e.g., quercetin, kaempferol and their derivatives as quercitrin, hyperoside) have already been isolated from the fruit so far (Fig. 1) (Edwards et al. 2012; Jurikova et al. 2012; Yang and Liu 2012; Kumar et al. 2012). Some of these flavonoids also have gastroprotective, anti-inflammatory, and antimicrobial properties. Hawthorn shrubs with small black-colored fruits can be found in the forested regions in the north of Iran.
Chemical structures of the main flavonoids in hawthorn fruits (Lucconi et al. 2014)
Traditionally, natural dyes are extracted from plants or animals with hot/boiling water. Such extraction process may have limited yield due to the high polarity of the water and hydrolyze some of the chemical structures, especially at elevated temperatures. Thus, it is essential to find more advanced extraction methods with higher efficiency, less environmental impact, and lower energy consumption. Natural colorants are often firmly integrated to the cell wall of plants, and an ideal extraction method should be able to efficiently facilitate the release and transport of the colorants into the solvent (Ali et al. 2009; Santis and Moresi 2007; Sivakumar et al. 2011). In recent years, various new technologies such as microwave and ultrasound have been employed for the enhancement of the extraction efficiency (Ali et al. 2009; Santis and Moresi 2007). The use of ultrasonic-assisted extraction (UAE) of colorants has been a subject of research since 1963 (Lott and Demaggio 1963; Djilani et al. 2006; Chen et al. 2007; Melecchi et al. 2006). The high power of ultrasound (20–100 kHz) can be used for extraction intensification and enhancement. Upon ultrasound irradiation of liquids, micro-bubbles grow, vibrate very quickly, and even erupt aggressively at high pressures. These exciting micro-bubbles can damage the solid surface and initiate some micro-channels and pores (Feng et al. 2011). Moreover, the heat and mass transfer is promoted in the liquid phase near the particles and through the channels by disrupting the cell walls (Sivakumar et al. 2009; Mason et al. 1996).
Natural dyes are considered environmentally friendly and sustainable materials with minimum harmful chemicals during their photosynthesis. Some of these natural dyes have noticeable antimicrobial and antioxidants activities. Therefore, they can be used for simultaneous coloration and antimicrobial finishing of textiles (Safapour et al. 2018; Arslan et al. 2011). Compared to the production cost of petroleum-based synthetic dyes, the extraction of natural dyes can be relatively cheaper. If the extraction and application of these natural dyes can be optimized with promising results, the mass production (scaling-up) can be easily justified due to sustainability, biodegradability, waste minimization, and saving the environment.
To the best of our knowledge, the current research is the first study on using natural dyes extracted from Hawthorn fruits for the coloration and antimicrobial finishing of polyamide fabric. This study presents the optimization of UAE extraction using the RSM method. The UV–visible, FTIR, and HPLC techniques are used for the characterization of the extracted colorants. The extracted colorants are used for dyeing polyamide fabrics. The color strength and colorfastness properties (i.e., washing, light, rubbing) of the dyed polyamide samples are determined according to the standard test methods.
2 Experimental
2.1 Materials and equipment
Ripe Hawthorn fruits were harvested from Galugah (Mazandaran, Iran) in October 2018. Ethanol, methanol, acetone, sulfuric acid, and sodium hydroxide were of analytical grade from Merck Company. Polyamide 6 knitted fabric (101 g/m2) was purchased from the local market and scoured by a nonionic detergent (Lotensol, Hansa). α,α-diphenyl-β-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria and the antimicrobial testing kits were obtained from Iranian Research Organization for Science and Technology (IROST).
Ultrasonic extraction was conducted in a double-jacketed cell within a 3L ultrasonic cleaning bath (Sonorex DT/DL/RK100/102(H)-UK). Fourier transform infrared (FTIR) spectra were recorded by Nicolet (USA) using KBr pellets. The extracted colorants from hawthorn fruit were analyzed by HPLC (Merck-Hitachi with diode array detector Hitachi L-2450). A sample dyeing machine (Smart dyer rapid sd-16, India) was used for sample dyeing. A UV–Vis spectrophotometer (Cecil 9200 double-beam, the UK) was used for assessing the extraction yield of the colorants by measuring the absorbance of the solution. The surface reflectance of the dyed samples was determined by a color-eye portable spectrophotometer (X-Rite Inc., USA, D65 illumination, 10° Standard Observer coordinates).
2.2 Extraction process
Hawthorn fruits were chopped into several pieces, and their seeds were removed. The pulp, along with the skin, was dried at 40 °C for 24 h, powdered, and sieved to an average size of about 20 μm. The powder was used for further extraction study using various solvents under the same conditions. The absorbance of the solvent was measured using a UV–Vis spectrophotometer after appropriate dilution.
2.3 Optimization of the UAE process
A mixture of ethanol and water (W1/4E) was selected as the most efficient medium for the extraction of natural colorants from the fruits. The response surface methodology (RSM) with central composite design (CCD) was used for the optimization of the UAE conditions using “MiniTab” statistical software (Ver. 4). Some preliminary screening tests were carried out to determine the appropriate range of the effective parameters on extraction (i.e., initial concentration of dried fruit in the solvent, extraction time, temperature, and pH). Four independent variables at 5 levels were selected for further RSM analysis, as shown in Table 1.
The extraction efficiency was calculated by a second-order polynomial model (Eq. 1) (Sivakumar et al. 2009; Sinha et al. 2012):
where ηi is the absorbance value, b0 is the constant coefficient, bi is the linear coefficients, bii is the quadratic coefficients, bij is the interaction coefficients, and xi, xj are the coded values of the variables.
Analysis of the variance (ANOVA) was used for statistical analysis of the model and determining the relationship between a response variable and 1 or more independent variables. The squared multiple correlation coefficient (R2), also known as the proportion of the variance, was obtained for the model. This coefficient was used to evaluate the selected model and to calculate the error between the real model and the theoretical one. A model with perfect predictability would have R2 = 1. The optimum conditions were selected by examining the surface plots to achieve the highest response level (i.e., maximum extraction efficiency) and suggested factor levels (Sivakumar et al. 2009; Sinha et al. 2012).
2.4 HPLC analysis
HPLC was used to analyze the flavonoids compounds present in hawthorn fruits. The mobile phase deionized water/methanol/acetonitrile/acetic acid/phosphoric acid with fraction 139.5: 70: 50: 0.25: 0.25 mL (V/V) was injected into the HPLC column (Waters Nova-Pak C18 columns, 8 × 100 mm) and eluted at a flow rate of 0.6 mL/min with a mobile gradient phase (pH 2.9). The extracted dye (20 μl) was injected into the column of HPLC, and the amount of quercetin, rutin, and kaempferol was characterized via comparison of the retention time and the surface area under the peaks with standard samples and finally expressed in mg/g of the dry weight of fruit.
2.5 Dyeing and fastness properties
The polyamide fabric was scoured with 5 g/L nonionic detergent at 60 °C for 20 min, liquor-to-goods ratio (L.R) = 40:1, and then rinsed and air-dried. The fabric samples (2 g) were dyed at different initial dye concentrations (25–100%o.w.f.), at 100 °C, L.R of 50:1, at pH 5 for 1 h. Finally, the dyed samples were removed, rinsed with water, and then dried at room temperature.
The Kubelka–Munk equation (Eq. 2) was used to determine the color strength of the dyed samples by measuring the reflectance of the dyed samples (Sadeghi-Kiakhani and Safapour 2016; Wyszecki and Stiles 2000).
where K, S, and R are the light absorption coefficient, the scattering coefficient, and the reflectance of the dyed samples, respectively. The colorimetric data were measured 3 times to have a standard deviation of ≤ 4%.
Colorfastness properties of the dyed samples were measured according to the ISO standard methods. Gray scales were used for the assessment of change in color or staining, on a scale of 1–5 (with 4 half steps), with 5 being excellent and 1 being poor. The transfer of color from the test specimen to an adjacent white fabric was evaluated by an observer (human expert) by placing the samples in a light cabinet under the D65 daylight illumination. Standard ISO 105 C06 C2S:1994 (E) method was used to measure the washing fastness properties after washing the dyed samples at 60 °C for 30 min. The change in color of the dyed samples and staining on adjacent white samples were analyzed by the gray scales. The rubbing fastness property of the dyed samples was determined according to the ISO105-X12:1993 (E) standard method. The gray scale was used to measure the staining on the adjacent fabrics. The lightfastness of the dyed samples was evaluated by the standard ISO 105 B02:1994 (E) method. The samples were radiated under xenon arc lamp, and a blue scale on a scale of 1–8 was used for the assessment of the change in color, with 8 being excellent (no fading) and 1 being very poor (severe fading). The perspiration fastness properties of the samples in acidic and alkali solutions were measured according to standard ISO 105—E04:1994 (E) method using the gray scales (Sadeghi-Kiakhani and Safapour 2016; Sadeghi-Kiakhani et al. 2015).
2.6 Antimicrobial test
According to AATCC 100–2004 standard test method, Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria were used for measuring the antimicrobial activity of the samples. The antimicrobial efficiency was determined by comparing the reduction in the number of bacterial colonies of the treated sample with that of the untreated control after incubation at 37 ± 2 °C for 18 h. The microbial inhibition was determined by the reduction in the number of colony-forming units (CFU) with respect to untreated control sample using Eq. 3:
where R is the reduction percentage of the bacterial count and B and A are the surviving cells (CFU/mL) for the flasks containing dyed polyamide samples and raw polyamide as control, respectively.
2.7 Antioxidant activity test
α,α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging test was used to evaluate the antioxidant activity of the raw and treated samples (Rabiei et al. 2012; Kedare and Singh 2011). Briefly, a fabric sample (2.5 cm2) was immersed in DPPH methanol solution (0.15 mM, 40 mL) and dark-incubated at room temperature for 30 min. The color change from violet to pale yellow was observed, and the absorbance of the solution was measured with a UV spectrophotometer. The DPPH free radical scavenging activity was calculated using Eq. 4.
D and C are the absorbance values of the dark-incubated solutions at λ = 517 nm for the dyed and undyed samples, respectively.
3 Results and discussion
3.1 Solvent extraction efficiency
One of the most important factors on the yield of extraction and solubility of dyes is the polarity of solvents (Ong et al. 2000; Tehrani-Bagha et al. 2012). Table 2 shows that the extracted dyes in a very polar solvent like water have λmax. = 490 nm, while the λmax of the extracted dyes in a less polar solvent like ethanol is about 537 nm. This shows that a range of flavonoids (Fig. 1) with different polarities can be extracted with various λmax (Urbonaviciute et al. 2006; Arslan et al. 2011). Interestingly, the λmax of the extracted dyes in acetone, with very close polarity index to those of methanol and ethanol, is 447 nm, which is 90 nm less than that in ethanol. The extraction temperature was kept as low as 40 °C to protect the susceptible colorants against degradation at elevated temperatures (Chen et al. 2007). Table 2 shows that the sonication had enhanced the extraction efficiency noticeably in the presence of various solvents. The ultrasound waves exert negative pressure on the liquid, pulling the molecules away from one another. Above a certain threshold, the pressure overcomes the attractive intermolecular forces between solvent molecules, and micro-bubbles are formed, which enhances the dye extraction from plants (Chen et al. 2007; Ou et al. 1997).
In conventional and UAE methods, the mixture of water and ethanol showed significantly higher extraction efficiency than the sum extracted by each of the individual solvents. Although the extraction of flavonoids from Hawthorn fruit had been previously reported in a mixture of ethanol and water (Rabiei et al. 2012), the current study is probably the first report showing the synergistic effect of the binary solvents. The flavonoids are tightly bound inside the cellular matrix of the fruit. Probably, the mixture of water and ethanol can extract a broader range of flavonoids and open more channels (i.e., facilitate the solvent penetration and perturb the complex structure) within the cellular matrix of the fruit, which enhances the extraction efficiency.
Figure 2 shows the concentration of the extracted dyes using various solvents as a function of time, presenting the kinetic of extraction as well as the time needed for reaching the equilibrium. The complete extraction achieved for various solvents after 1 h. The highest dye extraction was achieved in the presence of water 1:4 ethanol (W1:4E).
3.2 Response surface analyses
Since W1/4E was the best solvent for the extraction of the colorants from Hawthorn fruits, the response surface method with CCD (Sect. 2.3) was used for the optimization of the UAE conditions. Accordingly, 31 extraction recipes were tested, and the absorbance of the filtered solutions was obtained (Table 3). The maximum extraction yield was achieved at pH 5 at 60 °C after 40 min. Also, the effects of the mass of fruits, time pH, temperature, and time extraction on dye extraction are investigated in the following.
3.2.1 Effect of the mass of fruit on extraction
Figure 3 shows the 3D plots of the selected variables on the extraction process of natural colorants. As shown in Fig. 3a, the absorbance increased by increasing the fruit mass up to 8.0 g/L. The amount of available dye molecules for extraction increases by increasing the initial concentration of dried fruit powder (Spyroudis 2000; Ho et al. 2008).
3.2.2 Effect of initial pH
The effects of initial pH (X1) and time contact (X3) on the extraction process can be seen in Fig. 3b. The absorbance of the solution after extraction sharply was increased by reducing the pH value. The addition of acid serves to increase the polarity of the solvent and to degrade the cellulosic cellular matrix of the plants. This improves the accessibility of the solvents to the interior of the matrix and enhances the colorants extraction, especially for anthocyanins with very polar structures (Wrolstad 2004). The acidic extraction medium is also favorable to protect the susceptible extracted species (e.g., anthocyanin, polyphenols, flavonoids, and hydroxyl acids) against the chemical hydrolysis at elevated pH. As an example, the absorption maximum of anthocyanins in the most stable form (oxonium or flavylium cation) is at pH 1.0 (Fig. 4), and a bright orange–red color is produced by the pigment. By increasing the pH to 4.5, anthocyanin turns into colorless chalcone/hemiketal forms, and a cyanidin-3-glucoside molecule generates an oxonium ion with orange–red color at pH 3.0 (Wrolstad 2004). The color of anthocyanins changes to blue–green at pH above 7 (in their quinonoidal form), as can be seen in Fig. 4.
The chemical structures of anthocyanin at various pH values (Wrolstad 2004)
3.2.3 Effect of temperature
By increasing the temperature, the fruit powder swallows more, and the solvent can penetrate and diffuse inside the microstructure of the powder. The higher permeability of the cell walls of the fruit and lower viscosity of the solvent at elevated temperatures are 2 reasons that enhance the extraction efficiency from the fruits (Tan et al. 2011; Al-Farsi and Lee 2008). Moreover, the solubility of the colorants increases in solvents by temperature, which results in higher extraction efficiency (Fig. 3c). It should be noted that the extracted colorants are prone to chemical degradation/oxidation with the change of color at higher temperatures (> 60 °C) (Chen and Wu 2009; Dai and Mumper 2010; Rhim 2002; Dyrby et al. 2001) due to the presence of glycosidic bond in their chemical structures. The thermo-degradation of anthocyanins has been primarily reported to follow first-order kinetics (Ahmed et al. 2004; Wallace and Giusti 2008).
The exposure of plant tissue to heat weakens the phenol–protein and phenol-polysaccharide interactions; thus, more phenolic compounds can be transferred to the solvent. However, due to the instability of some of the species, the extraction temperature should be optimized and controlled during the process (Wang et al. 2007).
3.2.4 Effect of contact time
The increase in the absorbance by increasing the extraction time can be seen in Fig. 3d. However, the ultrasound power may degrade the extracted compounds over a long time, and the optimum time of extraction should be carefully selected. This effect is correlated to ultrasound power, the stability of color compounds, and solvents as a medium. Based on the trend shown in Fig. 3d, the suitable time of the UAE method was found to be 40 min. The formation and bursting of small micro-bubbles in UAE with very high pressure at the surface of the powder increase the agitation and enhance the extraction efficiency. However, extending the duration of the UAE process can increase the chance of degradation/oxidation of the extracted colorants in solution because of more exposure to oxygen, which is not favorable (Shaukat et al. 2009; Naczk and Shahidi 2004). The decrease in phenolic content could also be due to the endogenous enzymatic degradation of the phenolic compounds over a prolonged extraction period (Kuljarachanan et al. 2009).
3.2.5 Development of the regression model equation
The regression coefficients and the statistical values of the response function for the extraction process are summarized in Table 4. The regression model equation was developed based on the RSM results (Eq. 5). The validity of the response surface quadratic model was confirmed by a low error of 4.5% between the experimental and theoretical values. Also, the model validity confirmed by analysis of variance (ANOVA) is reported in Table 4. The p-values are < 0.0001 that demonstrates the selective factors on the extraction tests are very impressive.
3.2.6 Model validation
As can be seen in Table 5, the proposed second-order polynomial equation (Eq. 5) was statistically significant (P < 0.05). The model exhibited a high R2-value of 97.44% for the dye extraction, which showed its accuracy. The results indicated that the adjusted R2-value was acceptable (95.20%) and close to the R2-value of the model that verified a good fit of the polynomial model and its reliability. The plots of normal probability and residuals against the fitted values are shown in Fig. 5. The presence of data inside the red area marked in Fig. 5a and straight linear relation (Fig. 5b) showed that the normality assumption was met, and there was a meaningful relation between the predicted and experimental values by the model.
3.3 Characterization of the extracted colorants
3.3.1 FTIR analysis
To determine the functional groups of the extracted colorants, the extracted samples were analyzed by FTIR spectroscopy. Dissimilar peaks at 3333, 2923, 1732, 1230, 1037(C–O), 883, and 592 cm−1 were observed in the FTIR spectral (Fig. 6). The broad and strong bands at 3333 cm−1–2923 cm−1 were attributed to –OH groups and C–H symmetry stretching vibration, respectively. –C = O stretching in carbonyl groups appears as a strong peak at 1732 cm−1, and CH = CH stretching vibration is visible at 592 cm (Jurikova et al. 2012).
3.3.2 HPLC analysis
A large number of chemical compounds (e.g., sugar, phenolic acids, flavonoids, etc.) have already been detected and identified in the extracted solution from Hawthorn fruits with different techniques (Edwards et al. 2012; Jurikova et al. 2012; Yang and Liu 2012; Kumar et al. 2012; Lucconi et al. 2014). HPLC was used for analyzing the extracted solution, as described in Sect. 2.4. Figure 7 shows the peak positions of the extracted flavonoids; rutin was found to be the most dominant and mobile species in the extracted solution. Compared to other extracted species (Fig. 1), rutin has higher polarity and a tendency to be dissolved in the HPLC solvent and move through the column. Therefore, it appears faster than other compounds available in Hawthorn fruits. The other 2 peaks with longer retention time were assigned to quercetin and kaempferol (Jurikova et al. 2012; Kumar et al. 2012; Lucconi et al. 2014).
The results indicated that the amounts of the compounds in the extract of Hawthorn fruits were as follows: rutin > kaempferol > quercetin. It was found that the concentration of these compounds in the extract was high, so, it can be used for dyeing of textiles, antioxidants, antimicrobials, and medical textiles.
3.4 Dyeing and fastness properties
The dried fruit powder without extraction (i.e., raw dye powder) and the extracted powder after extraction under optimum conditions were used for the dyeing of polyamide fabric, as explained in Sect. 2.5. The color strength (K/S) values of the dyed polyamide samples as a function of initial dye concentration are shown in Fig. 8. The results indicated that the complete color saturation of the polyamide fabric was achieved at the extracted dye powder initial concentration of 50% o.w.f. The K/S values of the samples dyed with raw dye powder were also noticeable. Because the dyeing process was carried out at boiling temperature and can be considered as an extraction bath for the dried fruit powder, both the extraction of the colorants and dyeing process can be performed simultaneously in the same bath, as can be seen in Fig. 8, which is noteworthy from energy consumption and environmental points of view.
Light-, wash-, and rub-fastness properties of the dyed polyamide fabrics were evaluated and are presented in Table 6. The wash-fastness of the dyed sample was very good (4–5), which showed the presence of hydrogen bonding, in addition to nonpolar van der Waals interactions, between the colorants and polyamide fabric (Ismal and Yildirim 2012). Rubbing fastness of the dyed samples in both dry and wet conditions was suitable. The lightfastness of fabrics was also very good to excellent (7) due to the presence of flavonoids with a free radical scavenging effect that could reduce the density of electrons in chromophore (Ghouila et al. 2012). The color change in perspiration fastness properties of samples in acidic and alkaline media was very good (4–5), and the staining of adjacent fabrics was excellent (5). The color staining on adjacent white fabrics was low and indicated the stability of the adsorbed dyes on polyamide fabric in acidic and basic media. Overall, the fastness properties of the dyed samples were satisfactory and acceptable.
3.5 Antimicrobial activity
The presence of the natural dyes on polyamide fabric noticeably enhanced the antimicrobial activity of the samples against E. coli and S. aureus bacteria (Table 7). The enhancement, proportional to the amount of the adsorbed dyes on the fabric, was attributed to the presence of high phenolic compounds in the extracted dyes from hawthorn fruits (Edwards et al. 2012; Jurikova et al. 2012).
3.6 Antioxidant property
The DPPH test was used to investigate the antioxidant activity of the dyed samples. Figure 9 shows that the antioxidant activity of the dyed samples increased to above 80% with increasing the initial dye concentration above 50% and leveled off at higher initial dye concentrations. This could be related to the radical scavenging nature of the extracted natural dyes owing to the presence of the polyphenolic compound and their active hydrogen groups (Edwards et al. 2012; Jurikova et al. 2012; Yang and Liu 2012).
4 Conclusions
Among the employed solvents for natural dyes extraction from Hawthorn fruits, a mixture of water and ethanol (W1/4E) showed a favorable synergistic extraction capacity. Compared with the conventional water-based extraction method, the UAE method was found to be more efficient and faster. Based on the RSM optimization, the optimum extraction conditions were at pH 5, temperature 60 °C, after 40 min. Rutin was the most dominant extracted flavonoids, followed by kaempferol and quercetin. The polyamide fabric was successfully colored with the extracted colorants. The dyed fabrics showed very good fastness, antimicrobial, and antioxidant properties that were attributed to the chemical structures of the natural dyes. The study showed that the extracted natural dyes have the potential to be used for textile coloration and antimicrobial finishing. The extraction, dyeing, and finishing of textile materials can be done in a single bath simultaneously that is a sustainable solution with low environmental impact and energy consumption.
References
Ahmed, J., Shivhare, U. S., & Raghavan, G. S. V. (2004). Thermal degradation kinetics of anthocyanin and visual colour of plum puree. European Food Research Technology, 218, 525–528.
Al-Farsi, M. A., & Lee, C. Y. (2008). Optimization of phenolics and dietary fibre extraction from date seeds. Food Chemistry, 108, 977–985.
Ali, S., Hussain, T., & Nawaz, R. (2009). Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. Journal of Cleaner Production, 17, 61–66.
Arslan, R., Bor, Z., Bektas, N., Meriçli, A. H., & Ozturk, Y. (2011). Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thrombosis Research, 127, 210–213.
Chen, F., Sun, Y., Zhao, G., Liao, X., Hu, X., Wu, J., et al. (2007). Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrasonics Sonochemistry, 14, 767–778.
Chen, X., & Wu, J. (2009). Sustainable landscape architecture: Implications of the Chinese philosophy of ‘‘unity of man with nature’’ and beyond. Landscape Ecology, 24, 1015–1026.
Dai, J., & Mumper, R. J. (2010). Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules, 15, 7313–7352.
Djilani, A., Legseir, B., Soulimani, R., Dickob, A., & Younos, C. (2006). New extraction technique for alkaloids. Journal of the Brazilian Chemical Society, 17, 518–520.
Dyrby, M., Westergaard, N., & Stapelfeldt, H. (2001). Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chemistry, 72, 431–437.
Edwards, J. E., Brown, P. N., Talent, N., Dickinson, T. A., & Shipley, P. R. (2012). A review of the chemistry of the genus Crataegus. Phytochemistry, 79, 5–26.
Feng, H., Barbosa-Canovas, G., & Weiss, J. (2011). Ultrasound technologies for food and bioprocessing, food engineering series. New York: Springer.
Ghouila, H., Meksi, N., Haddar, W., Mhenni, M. F., & Jannet, H. B. (2012). Extraction, identification and dyeing studies of isosalipurposide, a natural chalcone dye from Acacia cyanophylla flowers on wool. Industrial Crops and Products, 35, 31–36.
Ho, C. H. L., Cacace, J. E., & Mazza, G. (2008). Mass transfer during pressurized low polarity water extraction of lignans from flaxseed meal. Journal of Food Engineering, 89, 64–71.
Ismal, O. E., & Yildirim, L. (2012). Almond shell as a natural colorant. Indian Journal of Fibre and Textile Research, 37(4), 358–363.
Jurikova, T., Sochor, J., Rop, O., Mlcek, J., Balla, S., Szekeres, L., et al. (2012). Review polyphenolic profile and biological activity of Chinese Hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules, 17, 14490–14509.
Kedare, S. B., & Singh, R. P. (2011). Genesis and development of DPPH method of antioxidant assay. Journal of Food Science and Technology, 48, 412–422.
Kuljarachanan, T., Devahastin, S., & Chiewchan, N. (2009). Evolution of antioxidant compounds in lime residues during drying. Food Chemistry, 113, 944–949.
Kumar, D., Arya, V., Bhat, Z. A., Khan, N. A., & Prasad, D. N. (2012). The genus Crataegus: Chemical and pharmacological perspectives. Revista Brasileira de Farmacognosia, 22, 1187–1200.
Kwok, C. Y., Wong, C. N. Y., Mabel, Y. C. Y., Yu, P. H. F., Au, A. L. S., Poon, C. C. W., et al. (2010). Consumption of dried fruit of Crataegus pinnatifida (Hawthorn) suppresses high-cholesterol diet-induced hypercholesterolemia in rats. Journal of Functional Foods, 2, 179–186.
Liu, P., Kallio, H., Lü, D., Zhou, C., Ou, S., & Yang, B. (2010). Acids, sugars, and sugar alcohols in Chinese Hawthorn (Crataegus spp.) fruits. Journal of Agricultural Food Chemistry, 58(2), 1012–1019.
Lott, J. A., & Demaggio, A. E. (1963). Continuous extraction during treatment with ultrasound. Science, 139, 825–826.
Lucconi, G., Chlapanidas, T., Martino, E., Gaggeri, R., Perteghella, S., Rossi, D., et al. (2014). Formulation of microspheres containing Crataegus monogyna Jacq. extract with free radical scavenging activity. Pharmaceutical Development and Technology, 19, 65–72.
Mason, T. J. (1999). Sonochemistry. New York: Oxford University Press.
Mason, T. J., Paniwnyk, L., & Lorimer, J. P. (1996). The uses of ultrasound in food technology. Ultrasonics Sonochemistry, 3S, 253–S260.
Melecchi, M. I. S., Perez, V. F., Darvia, C., Zini, C. A., Abad, F. C., Martinez, M. M., et al. (2006). Optimization of the sonication extraction method of Hibiscus L. flowers. Ultrasonics Sonochemistry, 13, 242–250.
Naczk, M., & Shahidi, F. (2004). Extraction and analysis of phenolics in food. Journal of Chromatography A, 1054(1–2), 95–111.
Ong, E. S., Woo, S. O., & Yong, Y. L. (2000). Pressurized liquid extraction of berberine and aristolochic acids in medicinal plants. Journal of Chromatography A, 90, 457–464.
Ou, Z. Q., Jia, L. Q., Jin, H. Y., Yediler, T. H., & Ketrrup, S. A. (1997). Ultrasonic extraction and LC determination of linear alkylbenzene sulfonate in plant tissues. Journal of Chromatography A, 44, 417–420.
Rabiei, K., Bekhradnia, S., Nabavi, S. M., Nabavi, S. F., & Ebrahimzadeh, M. A. (2012). Antioxidant activity of polyphenol and ultrasonic extracts from fruits of Crataegus pentagyna subsp. elburensis. Natural Product Research, 26, 2353–2357.
Rhim, J. (2002). Kinetics of thermal degradation of anthocyanin pigment solutions driven from red flower cabbage. Food Science Biotechnology, 11, 361–364.
Sadeghi-Kiakhani, M., Gharanjig, K., & Arami, M. (2015). Grafting of prepared chitosan-poly(propylene) imines dendrimer hybrid as a biopolymer onto cotton and its antimicrobial property. Journal of Industrial and Engineering Chemistry, 28, 78–85.
Sadeghi-Kiakhani, M., & Safapour, S. (2016). Improvement of the dyeing and fastness properties of a naphthalimide fluorescent dye using poly(amidoamine) dendrimer. Coloration Technology, 131, 142–148.
Safapour, S., Sadeghi-Kiakhani, M., & Eshaghloo-Galugahi, S. (2018). Extraction, dyeing, and antibacterial properties of Crataegus Elbursensis fruit natural dye on wool yarn. Fibers Polymers, 19, 1428–1434.
Santis, D. D., & Moresi, M. (2007). Production of alizarin extracts from Rubia tinctorum and assessment of their dyeing properties. Industrial Crops and Products, 26(2), 151–154.
Shaukat, A., Tanveer, H., & Rakhshanda, N. (2009). Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. Journal of Cleaner Production, 17(1), 61–65.
Sinha, K., Saha, P. D., & Datta, S. (2012). Extraction of natural dye from petals of flame of forest (Butea monosperma) flower: Process optimization using response surface methodology (RSM). Dyes and Pigments, 94, 212–216.
Sivakumar, V., Anna, J. L., Vijayeeswarri, J., & Swaminathan, G. (2009). Ultrasound-assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrasonics Sonochemistry, 16, 782–789.
Sivakumar, V., Vijaeeswarri, J., & Anna, J. L. (2011). Effective natural dye extraction from different plant materials using ultrasound. Industrial Crops and Products, 33, 116–122.
Spyroudis, S. (2000). Hydroxyquinones: Synthesis and reactivity. Molecules, 5, 1291–1330.
Tan, P. W., Tan, C. P., & Ho, C. W. (2011). Antioxidant properties: Effects of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). International Food Research Journal, 18, 557–562.
Tehrani-Bagha, A. R., Singh, R. G., & Holmberg, K. (2012). Solubilization of two organic dyes by cationic ester-containing gemini surfactants. Journal of Colloid and Interface Science, 376, 112–118.
Urbonaviciute, A., Jakstas, V., Kornygova, O., Janulis, V., & Maruska, A. (2006). Capillary electrophoretic analysis of flavonoids in single-styled Hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. Journal of Chromatography A, 1112, 339–344.
Wallace, T. C., & Giusti, M. M. (2008). Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. Journal of Food Science, 73, c241–c248.
Wang, S., Chen, F., Wu, J., Wang, Z., Liao, X., & Hu, X. (2007). Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. Journal of Food Engineering, 78, 693–700.
Wrolstad, R. E. (2004). Anthocyanin pigments—bioactivity and coloring properties. Journal of Food Science, 69(5), 419–421.
Wyszecki, G., & Stiles, W. S. (2000). Color science (2nd ed.). New York: Wiley.
Yang, B. R., & Liu, P. Z. (2012). Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. Journal of the Science of Food and Agriculture, 92, 1578–1590.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeghi-Kiakhani, M., Tehrani-Bagha, A.R., Safapour, S. et al. Ultrasound-assisted extraction of natural dyes from Hawthorn fruits for dyeing polyamide fabric and study its fastness, antimicrobial, and antioxidant properties. Environ Dev Sustain 23, 9163–9180 (2021). https://doi.org/10.1007/s10668-020-01017-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-020-01017-0