Abstract
In this communication, experiments have been performed to check the capability of the newly formed composite desiccant material (CaCl2/floral) for the extraction of freshwater from atmospheric air. Three numbers of solar glass desiccant box type system (SGDBS) with a captured area of 0.36 m2 each, have been used. The design parameters for the water production are height of glass from desiccant bed at 0.22 m, inclination in angle as 30°, the effective thickness of glass as 3 mm and number of glazing as single. The maximum yield by the new composite desiccant material is 0.35 ml/cm3/day. The efficiency of the system SGDBS with 37 % concentration of CaCl2 is 76.44 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Drinkable water is one of the major challenges in front of developed and developing countries. Valipour et al. (2015) stated that there are enough water resources, but their unequal distribution has led to the situation of scarcity and need of fresh water. Also the practice of efficient use of water is not followed properly in areas such as agriculture, industry and domestic activities. Valipour (2015) stated that the there are many countries which are facing water crisis currently. Many attempts have been made for the production of freshwater from atmospheric air. Alayli et al. (1987) conducted the experiments on the new developed composite material, which has S-shaped isotherms, to obtain freshwater from the wet air. It was stated that for the collection of 1 l of water, the temperature required during nocturnal phase was 20 °C, humidity required as 50 % and clear sky during diurnal phase per m2. Abualhamayel and Gandhidasan (1997) studied the methods for the production of freshwater by using liquid desiccant for the summer climate of Saudi Arabia. It was proposed that for a given set of operating condition, it is possible to obtain 1.90 kg of water per m2 of the unit. Aristov et al. (1998) performed the experiments by developing the new selective water sorbent (SWS). The test results demonstrated that the freshwater of 3–5 tonnes can be produced by using 10 tonnes of dry SWS per day. Hamed (1999) theoretically analyzed the adsorption and desorption rate for the production of water. It was found that by taking the strong solution concentration, the value of cyclic efficiency could be obtained more than 90 %. Gad et al. (2000) used two host materials, namely mesoporous silica gel and alumina with the two composites SWS-1L and SWS-1A which were formed by impregnating two host matrices with CaCl2 in order to study the kinematics of water vapor sorption. The result showed that there was an increase in water loading of both SWS as compared to the other material used for experimental work. Dawoud and Aristov (2002) manufactured two samples of selective water solvent SWS-2C and SWS-2EG by using mesoporous synthetic carbon sibunit and macroporous expanded graphite impregnated with lithium bromide. It was found that water sorption capacity of novel composite desiccant is 0.75 g/1 g of dry sorbent which is higher than that of the conventional silica gel or zeolite. Gordeeva et al. (2002) experimentally investigated the integrated desiccant collector system for the production of water from the air. A thick corrugated layer of cloth was used, which acts as a bed in order to enhance the mass transfer surface, and CaCl2 was used as a desiccant material. It was found that the system can produce 1.5 l of freshwater per square meter per day. Sultan (2002) studied a non-conventional method of water production from atmospheric air by using calcium chloride (CaCl2) as desiccant material. It was observed that the system efficiency increases with the initial concentration and decreases with the increase in regeneration air velocity and absorption temperature. Hamed (2003) experimentally investigated the adsorption of water vapor on the horizontal surface of a sandy layer impregnated with calcium chloride (CaCl2). It was found that rate of absorption decreased with the decrease in mixing ratio, and mass transfer coefficient was highly affected by the desiccant concentration in the bed. Bar (2004) studied the extraction of water from air by using patented technology based on extraction of air humidity into water streams. By this technology, adsorption/desorption ratio is 2:1 and it can be operated at ambient temperature range between 5 and 45 °C and at RH of 20 %, whereas at RH 60 %, system achieves maximum capacity. Kabeel (2007) worked experimentally on the glass pyramid shape with a multi-shelf solar system to extract water from the atmospheric air. Two beds formed by using cloth and saw wood were used. Beds were saturated with 30 % concentration of CaCl2 solution. It was found that 2.5 l/day/m2 freshwater can be produced. Ji et al. (2007) experimentally investigated composite adsorbent MCM-41 (Mobile Composite Material) for solar-driven freshwater generation from atmospheric air. Adsorption capacity was found to be as high as 1.75 kg/kg dry adsorbent. This new composite showed a good possibility for the production of 1.2 kg freshwater per day per m2 of solar collector. Hamed et al. (2011) performed the experiments on a sandy bed impregnated with calcium chloride for recovery of water from atmospheric air. It was found that 1.0 l/m2 of water can be produced after regeneration of the liquid desiccant material. Audah et al. (2011) studied the feasibility of solar-powered liquid desiccant system for the production of freshwater and cooling requirement of the building by using parabolic solar concentrators. It was found that 15 l of freshwater can be produced and air conditioning of the building can be done at minimum energy cost. Kumar and Yadav (2015a, b) performed experiments on the composite desiccant material CaCl2/saw wood for the water production from the atmospheric air. Results show that 180 ml/kg/day of water can be produced by the regeneration of the composite desiccant material. Kumar and Yadav (2015a, b) performed experiments for the investigation of design parameters of solar glass desiccant box type system for the production of freshwater from atmospheric air. It was found that for the production of water, height of glass from the desiccant material bed is 0.22 m; inclination in angle is 30°; the effective thickness of glass is 3 mm; and number of glazing is single. By using these parameters, the maximum quantity of water produced by using silica gel was 200 ml/kg/day.
Some of the researchers have developed composite material and used their adsorption and regeneration properties in the applications like production of dry air, desiccant cooling system and water production from atmospheric air. But none of them have used excellent absorption property of floral foam for the formation of composite desiccant material. Also the new composite desiccant material takes less time for the regeneration. The objective of this manuscript is the production of water by using the new composite desiccant material named CaCl2/floral foam in Indian climatic conditions.
2 Experimental setup
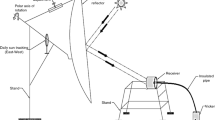
The experimental setup is shown in Fig. 2. It consists of solar glass desiccant box type system (SGDBS). The solar glass desiccant box type system (SGDBS) consists of the following main parts:
-
(1)
FRP container
-
(2)
Glazing
-
(3)
Wire mesh tray
-
(4)
Water-measuring cylinder
-
(5)
Connecting pipe
-
(6)
Composite desiccant material
-
(1)
FRP container Three containers of fiber-reinforced plastic (FRP) have been used because of good strength and long life. The size of each box is 0.6 m × 0.6 m × 0.3 m as shown in Figs. 1 and 2. Two windows of size 0.3 m × 0.3 m have been provided for the process of adsorption in the night time. This design is an advantage over the other setup designs in which for the adsorption process, glass has to be removed and for regeneration, again glass had to be fixed. A water-collecting tray with a slight slope has been provided on the front side for the collection of drops coming along the glass after condensation.
-
(2)
Glazing A glass of 3-mm thickness is used for the purpose of glazing which also acts as a condenser for the regeneration process. Glass allows sun rays of shorter wavelength to pass inside the SGDBS and trap the rays of longer wavelength which comes after heating the composite desiccant material. It is used to create the greenhouse effect inside the SGDBS.
-
(3)
Wire mesh tray A wire mesh of steel wires is used inside the SGDBS for holding the solid desiccant material. The dimension of the wire mesh is 3 mm × 3 mm. Wire mesh is screwed on the frame of plastic.
-
(4)
Connecting pipe Connecting pipe is attached between water-collecting tray and bottle in order to provide a path for the flow of condensed water droplets.
-
(5)
Water-measuring cylinder A water-measuring cylinder is used outside the SGDBS for the collection of water. The water is coming directly from the water-collecting tray through the connecting pipe to the bottle. There is provision for measuring marks on the cylinder which indicates the quantity of the water collected. The minimum quantity of water that can be measured is 5 ml.
-
(6)
Composite desiccant material A composite material formed by using CaCl2/floral foam, has been used as desiccant material. A cube of floral foam having volume 30 cm3 is used as a host material and CaCl2 as a salt. Six samples have been formed by using different concentrations of CaCl2 as shown in Table 1. For the preparation of the sample 1, 10 cubes of floral foam having volume 30 cm3 each were dipped in an aqueous solution of CaCl2 having 9 % concentration. The preparation of composite includes three steps: (a) dehumidification of host matrix at 70 °C, (b) its impregnation with aqueous solution of calcium chloride at room temperature and (c) heating up to 80 °C in an oven for 3 h to remove water. Similarly, five other samples were prepared by using the aqueous solution of CaCl2 having 16, 23, 28, 33 and 37 % concentration, respectively, with floral foam. The same procedure is adopted for the preparation of other five samples.
Table 1 Six different adsorbent samples
3 Measuring devices and instruments
Different parameters were measured during experiments as follows:
-
Temperature of material, inner side of glass, outer side of glass and inside space temperature of SGDBS
-
Ambient temperature
-
Solar radiation intensity
-
Weighing machine
Temperature of material, inner side of the glass, outer side of the glass and inside temperature of SGDBS was measured with the help of RTD PT100 thermocouples which were connected with a digital temperature indicator which shows the temperature with a resolution of 0.1.
Dry-bulb temperature and wet-bulb temperature of ambient air were measured with the help of sling Psychrometer.
The solar radiation intensity was measured during the day time with a Pyranometer-Model CM11, of Kipp and Zonen, Holland.
Adsorption of moisture during the experiment was measured with the help of a digital weighing machine having maximum capacity of 50 kg, minimum capacity of 1 g and a resolution of 0.001 kg. Weighing machine was the make of “THOMSON Electronic weighing scales.”
The experiment for the production of water was carried out in the month of October 2014. Adsorption started during the night time and regeneration in the day time. The weight evolution during adsorption process and data during regeneration process were monitored manually in 30-min intervals.
4 System operation
For the working of the experimental setup, the composite desiccant material, i.e., CaCl2/floral foam, is placed on the wire mesh tray. In the adsorption process, the side windows of SGDBS are opened during the evening time at 6:00 p.m. When the vapor pressure on the surface of the composite desiccant material (adsorbent) is lower than the atmospheric air, then the adsorption process starts. The process is continued till the equilibrium condition is attained, i.e., when the vapor pressure on the adsorbent surface is same as that of atmospheric air. For the start of the regeneration process, side windows of SGDBS are closed in the morning at 7’o clock and exposed to the sun. With the rise of the sun, the desiccant material temperature rises and the vapor pressure difference between the surface of the desiccant material and the air of the inner space increases. With the increase in the solar intensity, mass transfer of the vapor increases and reaches to the saturation condition. The condensation of water vapors starts on the inner side of the glass and gets collected in the water-collecting tray. Due to the slop in the water-collecting tray, the water flows to the water-measuring cylinder by connecting pipe. The amount of collected water is measured after regular intervals of 30 min.
5 Analysis of experimental data
The absorption rate of quantity of desiccant is the amount of water content absorbed by the composite desiccant per unit time and is given by Kumar et al. (2014):
The empirical relation used for the calculation of the amount of salt required to prepare a solution is given by Hamed (2002):
and
where M s and M w are mass of salt and mass of distilled water, respectively.
The instantaneous value of X can be calculated as:
where subscripts “o” and “i” are the initial and instantaneous values of X and M sol.
The efficiency of the SGDBS is calculated by the following formula:
Here, M w is the mass of water (kg); L is the latent heat of water at an average bed temperature (J/kg); H is the solar intensity in W/m2; A is the area (m2); (τα) is the transmissivity absorptivity product
6 Result and discussion
In this paper, experimental investigation has been done on six samples of the composite desiccant materials. The first three samples 1, 2 and 3 having 9, 16 and 23 % concentration of CaCl2 in the floral foam, respectively, are tested on October 20, 2014, and other three samples 4, 5 and 6 having 28, 33 and 37 % concentration of CaCl2 in floral foam, respectively, are tested on October 22, 2014. The maximum solar intensity is 822 W/m2 on the first day and 798 W/m2 on the second day.
6.1 Adsorption rate of composite desiccant material
The experiment for the adsorption process was started at 6:00 p.m. At the initial stage, maximum adsorption rates for sample 1, sample 2, sample 3, sample 4, sample 5 and sample 6 are 0.02156, 0.02921, 0.040528, 0.033184, 0.039664 and 0.043992 kg/h, respectively. This is because at that time pores were empty and with the progression of time, they started to fill and adsorption rate started to decrease. In the sample 6, adsorption rate is maximum because it has the maximum concentration of CaCl2 so it is adsorbing more moisture as compared to the other samples. Composite materials in all six boxes got saturated at 1:30 a.m. as shown in Figs. 3 and 4.
6.2 Variations in material temperature and solar intensity with time
Figures 5 and 6 show the material temperature distribution of six samples of composite desiccant material. Results from Fig. 5 show that the sample 3 has a maximum material temperature, i.e., 73.0 °C during the experimental day as compared to the samples 1 and 2, i.e., 64.7 and 70.1 °C, respectively. Similarly, in Fig. 6, sample 6 has a maximum material temperature, i.e., 78.8 °C as compared to samples 4 and 5, i.e., 74.1 and 76.9 °C, respectively.
6.3 Variations in internal space temperature and solar intensity with time
The temperature distribution of internal space temperature is shown in Figs. 7 and 8. Maximum temperature gained by samples 1, 2, 3, 4, 5 and 6 is 66.1, 73.2, 74.3, 70.4, 69.1 and 60.5 °C, respectively.
6.4 Variations in water productivity
As discussed earlier, adsorption performance of sample 6 is more as compared to the other samples. It means the moisture content in sample 6 is higher which is responsible for the maximum productivity of water. The maximum production of water by the samples 1, 2, 3, 4, 5 and 6 is 0.25, 0.20, 0.13, 0.26, 0.3 and 0.35 ml/cm3/day, respectively, as shown in Figs. 9 and 10.
7 Efficiency of the SGDBS
The efficiency of the system can be calculated as:
Result from Fig. 11 shows that the maximum percentage efficiency of SGDBS is from the sample having 37 % concentration of CaCl2. This is 3.14 times of sample having 9 % concentration of CaCl2, 2.09 times of sample having 16 % concentration of CaCl2, 1.67 times of sample having 23 % concentration of CaCl2, 1.31 times of sample having 28 % concentration of CaCl2 and 1.16 times of sample having 33 % concentration of CaCl2. The efficiency of the system with 37 % concentration of CaCl2 is 55.66 % more as compared to the solar collector system with horizontal and corrugated bed.
8 Experimental error
The measured values during the experiment are not accurate. These are affected by the deviations caused by various errors. There are two types of errors, namely systematic error and random error. Systematic errors occur due to instrument and environmental errors. Random errors arise due to random and unpredictable fluctuation in the experimental conditions, e.g., unpredictable fluctuation in temperature, voltage supply, mechanical vibrations in the experimental setup, etc., and personal errors by the observer taking the readings.
The errors are calculated for the measuring instruments during the experiments as follows:
-
% error in measuring intensity = 2 %
-
% error in measuring temperatures = 0.3 %
-
% error in measuring water quantity = 0.025 %
-
Total % error for the SGDBS = 1 × 2 + 4 × 0.3 + 1 × 0.025 = 3.225 %
-
% of error in weighing machine = 0.1 %
9 Testing of water sample
A number of tests have been carried out in order to check the quality of the water sample collected from the composite desiccant material, floral foam/CaCl2. The tests were performed at CSSRI lab, Karnal, Haryana, India, on November 18, 2014. The results of physical and chemical tests are shown in Table 2.
10 Cost analysis of solar glass desiccant box type system (SGDBS)
The use of SGDBS depends upon the cost-effectiveness. Investment is made on the SGDBS in order to reduce the dependency on the conventional system for the production of water, as the demand for the water is increasing day by day.
A comprehensive study of cost analysis is presented by Govind and Tiwari (1984).
If P is the initial investment of SGDBS, r % as annual rate of interest, n as number of useful years to which system will perform and S as salvage value of the SGDBS then,
10.1 For SGDBS
The cost breakup for the SGDBS has been given in Table 3:
-
P = Rs. 3000
-
S = Rs. 250
Assuming n = 15 years, r = 12 % and maintenance cost = 10 % of the total cost.
The cost calculation can be done as follows:
-
CRF = 0.1467
-
SFF = 0.0268
-
Final annual cost of the system = CRF × P = 0.1467 × 3000 = Rs. 440.26
-
Annual salvage value = SFF × S = 0.0268 × 250 = Rs. 6.70
-
Annual maintenance cost = 10 % = 0.10 × 440.26 = Rs. 44
-
Annual cost = 440.26 + 44 − 6.70 = Rs. 477.56
-
Annual yield = 0.35 × 365 = 382.746 ml/cm3/year
11 Conclusion
A new composite material is formed by using floral foam as host material and calcium chloride as a hygroscopic salt. Six samples of different concentrations of calcium chloride have been prepared.
The following conclusion can be drawn as:
-
1.
Results show that the sample having 37 % concentration of CaCl2, has maximum adsorption rate as 0.043992 kg/h.
-
2.
Results show that maximum production of water is from the sample having 37 % concentration of CaCl2. This is 1.4 times of sample having 9 % concentration of CaCl2, 1.75 times of sample having 16 % concentration of CaCl2, 2.69 times of sample having 23 % concentration of CaCl2, 1.34 times of sample having 28 % concentration of CaCl2 and 1.16 times of sample having 33 % concentration of CaCl2.
-
3.
The maximum amount of water produced during the experimental days has been reached up to 0.35 ml/cm3/day.
-
4.
The maximum efficiency for samples 1, 2, 3, 4, 5 and 6 is 24.36, 36.48, 45.60, 58.24, 65.52 and 76.44 %, respectively.
-
5.
Results show that with the increase in the concentration of calcium chloride in floral foam, the water production rate can be increased.
12 Recommendations
During the experiments, it was observed that a theoretical model can be developed for the prediction of water production from the atmospheric air in different climatic conditions. Further, the properties of the air inside the SGDBS may be measured.
Abbreviations
- CRF:
-
Capital recovery factor
- G a :
-
Adsorption rate (kg/h)
- L :
-
Latent heat of water at average bed temperature (J/kg)
- M sol :
-
Mass of solution (kg)
- M s :
-
Mass of salt (kg)
- M w :
-
Mass of water (kg)
- m ws :
-
Weight of desiccant on wet basis (kg)
- n :
-
Number of useful years
- P :
-
Initial investment
- r%:
-
Annual rate of interest
- S :
-
Salvage value
- SFF:
-
Sinking fund factor
- w :
-
Moisture content in desiccant (kgwater vapor/kgdesiccant)
- X :
-
Concentration of solution
References
Abualhamayel, H. I., & Gandhidasan, P. (1997). A method of obtaining fresh water from humid atmosphere. Desalination, 113, 51–63.
Alayli, Y., Hadji, N. E., & Leblond, J. (1987). A new process for extraction of water from air. Desalination, 67, 227–229.
Aristov, Y. I., Tokarev, M. M., Gordeeva, L. G., Snytnikov, V. N., & Parmon, V. N. (1998). New composite sorbents for solar driven technology of fresh water production from the atmosphere. Solar Energy, 66, 165–168.
Audah, N., Ghaddar, N., & Ghali, K. (2011). Optimized solar-powered liquid desiccant system to supply building fresh water and cooling needs. Applied Energy, 88, 3726–3736.
Bar, E. (2004). Extraction of water from air—An alternative solution for water supply. Desalination, 165, 335.
Dawoud, B., & Aristov, Y. (2002). Experimental study of water vapor sorption on selective water sorbents, silica gel and alumina under typical operating conditions of sorption heat pump. International Journal of Heat and Mass Transfer, 46, 273–281.
Gad, H. E., Hamed, A. M., & Sharkawy, I. I. (2000). Application of solar desiccant/collector system for water recovery from atmospheric air. Renewable Energy, 22, 541–556.
Gordeeva, L. G., Restuccia, G., Ferni, A., & Aristov, Y. I. (2002). Water sorption on composites “LiBr in a porous carbon”. Fuel Processing Technology, 79, 225–231.
Govind, & Tiwari, G. N. (1984). Economic analysis of some solar energy systems. Energy Conversion and Management, 24, 131–135.
Hamed, A. M. (1999). Absorption–regeneration cycle for production of water from air-theoretical approach. Renewable Energy, 19, 625–635.
Hamed, A. M. (2002). Theoretical and experimental study on the transient adsorption characteristics of a vertical packed porous bed. Renewable Energy, 27, 525–541.
Hamed, A. M. (2003). Experimental investigation on the natural absorption on the surface of sandy layer impregnated with liquid desiccant. Renewable Energy, 28, 1587–1596.
Hamed, A. M., Aly, A. A., & Zeidan, B. E.-S. (2011). Application of solar energy for recovery of water from atmospheric air in climatic zones of Saudi Arabia. Natural Resources, 2, 8–17.
Ji, J. G., Wang, R. Z., & Li, L. X. (2007). New composite absorbent for solar-driven fresh water production from the atmosphere. Desalination, 212, 176–182.
Kabeel, A. E. (2007). Water production from air using multi-shelves solar glass pyramid system. Renewable Energy, 32, 157–172.
Kumar, A., Chaudhary, A., & Yadav, A. (2014). The regeneration of various solid desiccant by using the parabolic dish collector and adsorption rate: An experimental investigation. International Journal of Green Energy, 11, 936–953.
Kumar, M., & Yadav, A. (2015a). Experimental investigation of solar powered water production from atmospheric air by using composite desiccant material “CaCl2/saw wood. Desalination, 367, 216–222.
Kumar, M., & Yadav, A. (2015b). Experimental investigation of design parameters of solar glass desiccant box type system for water production from atmospheric air. Journal of Renewable and Sustainable Energy, 7, 033122.
Sultan, A. (2002). Absorption/regeneration non-conventional system for water extraction from atmospheric air. Renewable Energy, 29, 1515–1535.
Valipour, M. (2015). A comprehensive study of irrigation management in Asia and Oceania. Archives of Agronomy and Soil Science, 61, 1247–1271.
Valipour, M., Ziatabar Ahmadi, M., Raeini-Sarjaz, M., Gholami Sefidkouhi, M. A., Shahnazari, A., Fazlola, R., & Darzi-Naftchali, A. (2015). Agricultural water management in the world during past half century. Archives of Agronomy and Soil Science, 61, 657–678.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, M., Yadav, A. Solar-driven technology for freshwater production from atmospheric air by using the composite desiccant material “CaCl2/floral foam”. Environ Dev Sustain 18, 1151–1165 (2016). https://doi.org/10.1007/s10668-015-9693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-015-9693-3