Abstract
Forests serve as a sink and source of carbon and play a substantial role in regional and global carbon cycling. The Himalayan forests act as climate regulators of the Hindukush region, which is experiencing climate change at a high pace, and a proper understanding of these systems is necessary to mitigate this problem. We hypothesize that the variance of abiotic factors and vegetation will influence the carbon sink and source function of the different forest types of the Himalayas. Carbon sequestration was computed from the increment of carbon stocks estimated allometrically using Forest Survey of India equations, and soil CO2 flux was determined by the alkali absorption method. The carbon sequestration rate and CO2 flux by the different forests exhibited a negative relation. The carbon sequestration rate was highest with minimum emission in the temperate forest, while the tropical forest recorded the least sequestration and maximum carbon flux rate. The Pearson correlation test between carbon sequestration and tree species richness and diversity revealed a positive-significant influence but negative relation with climatic factors. An analysis of variance indicated significant seasonal differences between the rate of soil carbon emissions due to variations in the forest. A multivariate regression analysis of the monthly soil CO2 emission rate shows high variability (85%) due to fluctuations of climatic variables in the Eastern Himalayan forests. Results of the present study revealed that the carbon sink and source function of forests respond to changes in forest types, climatic variables, and edaphic factors. Tree species and soil nutrient content influenced carbon sequestration, while shifts in climatic factors influenced soil CO2 emission rate. Increased temperature and rainfall may further change the soil quality by enhancing soil CO2 emission and reducing soil organic carbon, thereby impacting this region’s carbon sink and source function. Enhancing tree diversity in the forests of this region may be beneficial for retarding this impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The earth is experiencing various climate change-related impacts such as drought, floods, rise in sea level, warming, erratic weather, and rainfall patterns, (Ades et al., 2019; Moomaw et al., 2020). Carbon sequestration is one of the promising and cheap solutions to combat these problems (Singh, 2013). Natural climate solutions (NCS) through the conservation, restoration, and improvement of ecosystems such as forests, wetlands, grasslands, and agricultural lands can effectively mitigate greenhouse gases by stabilizing warming (Griscom et al., 2017). Among the ecosystems, the role of forests in carbon mitigation is substantial and important due to the presence of trees, but forest ecosystems are at threat due to deforestation activities especially tropical forests in low-income countries (Kirilenko & Sedjo, 2007). Climate change-related atmospheric temperature increases and fluctuations in precipitation patterns often lead to the shifting of vegetation towards the poles or the boreal zones (Allen & Kirilenko, 2016; Cramer et al., 2001; Foley et al., 1998) and migration of tree species at a rapid rate towards the north and higher altitude (Clark, 1998; Liang et al., 2018; You et al., 2018).
Increased levels of CO2 in the atmosphere enhanced net primary productivity (NPP) and soil carbon (Chertov, 2010; Ge et al., 2013; Kirschbaum et al., 2012; Poulter et al., 2013; Wang et al., 2012), alter precipitation patterns and growing seasons of plants (Rustad et al., 2012; Schindlbacher et al., 2012), change the soil and plant respiration, nitrogen mineralization, and dominance of commercial trees in natural and managed forests as a result of the adoption of different management practices (Ge et al., 2013; Noormets et al., 2015). Moreover, variance in soil water content due to changes in precipitation pattern and increase in temperature affects the species distribution (Petrie et al., 2015) and carbon allocation pattern in the plants (Ogaya et al., 2014), impacting the vegetation and soil carbon dynamics and nutrient status of forest ecosystems (Reich et al., 2006). For example, the litter of broadleaved species dominant in tropical areas with warm temperatures and high rainfall decomposes and releases faster nutrients than temperate coniferous species in the cold and less rainfall area (Priha et al., 2001).
The capability of carbon storage in the soil varies with a variance in forest types and vegetation types, for example, boreal forests can store 60% carbon, while tropical forests store only 32% carbon in soil (Pan et al., 2011). But an increased temperature from global warming might dry up the soil faster leading to the enhancement of litter decomposition rate and faster nutrient release (Santonja et al., 2015), thereby stimulating root growth (Norby & Zak, 2011) and ectomycorrhizal carbon accumulation in the temperate and boreal forests (Hobbie, 2006).
The carbon sink and source function of vegetation and soil is influenced by the carbon source, tree species composition, and removal of understory species (Winsome et al., 2017) and soil microorganisms and macrofauna (Lepcha & Devi, 2020). Several researchers have raised concerns that increased temperature accelerates soil C release (Davidson et al., 2000; Trumbore et al., 1996), while other studies reported enhanced-temperature-induced declination of primary production of plants leading to a low soil carbon content (Epstein et al., 1998; Giardina & Ryan, 2000). In addition, precipitation pattern also plays a substantial role in regulating decomposition and microbial activity in an ecosystem and soil C dynamics (Schindlbacher et al., 2012), which indicates a significant effect of climate change on forest ecosystems. Besides these, the amount and frequency of precipitation influence temperature-regulated organic matter decomposition due to the close interaction of plants, soil, and microorganisms (Davidson et al., 2006; Gu et al., 2004; Högberg & Read, 2006). Also, a study on climate change (Mahlstein et al., 2013) concluded that mountain ecosystems show early signs of climate change due to the shrinkage of the polar and alpine regions. A variation in geographical and climatic patterns affects the patterns of the monsoon of the Indian subcontinent and other neighboring countries (Qiu, 2008) and the “Third Pole” or the Hindukush Himalayan region (Cook et al., 2010). Additionally, around 915 million people depend on mountains for their livelihoods (Sharma et al., 2019) as mountain ecosystems provide various services such as food security, biodiversity conservation, climate regulation, water provision, timber, electricity, and habitat to wildlife (Molden & Sharma, 2013), and many more which may be affected by the climate change. Hence, studies on the impact of climate change on the mountain ecosystems are needed for a timely response to secure the lives of the people as well.
Sikkim, an Eastern Himalayan mountainous state of India situated at an ecologically important location, reported signs and evidence of global warming and climate change such as a change in day and night temperature (Seetharam, 2008), phenological and distribution change of plant species (Telwala et al., 2013), glacial changes, and changes in the cropping pattern of the higher altitude (Raina, 2004). Since mountain ecosystems are sensitive and vulnerable to climate change, therefore a study on the carbon sink and source function of the Himalayan forest ecosystems with different climatic conditions, edaphic factors, and vegetation types can help in understanding how climate change will impact the functions of the forests ecosystem for a timely response. In the recent past, several studies have been conducted in the Himalayas that highlight the impact of climate change on different aspects such as ecosystem services and biodiversity (Kattel, 2022), carbon storage in different forest types (Gogoi et al., 2022), and net carbon exchange in the pine forest of Western Himalayas (Singh et al., 2019). However, all these studies were limited to a single forest or process such as either carbon storage or carbon release. None of these studies addressed the carbon assimilation and release process in multiple forest types which is important for understanding the impact of abiotic and biotic variables on the carbon cycle. We hypothesize that a change in climatic variables and its induced effects such as species range shifts influence the carbon sink and source function of the forests, and through this study, we want to explore how a change in abiotic and biotic factors, climate, and its induced effect will impact the carbon source and sink function of forests. Therefore, we investigate (i) how the carbon sequestration (vegetation and soil) and soil CO2 emission varies across forests in the different agroclimatic zone of Eastern Himalaya (ii) and identify influential drivers of carbon sequestration and emission and possible implications of climate change in this region.
Materials and methods
Study site
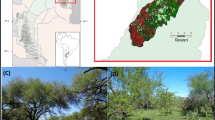
The study sites are at Sikkim, a mountainous Indian state in the Eastern Himalayan region (Fig. 1) with a diverse agro-climatic zone. The tropical forest for the present study is at South Sikkim district (27°6.842′ N, 88°21.392′ E) at an elevation of 320–875 m a.m.s.l, while the subtropical forest is at Dzongu, North Sikkim district (27°31.543′ N, 88°29.704′ E) located at an altitude of 1400–1700 m a.m.s.l, and the temperate forest at Maenam (27°19.407′ N, 88°22.498′ E) is part of Maenam Wildlife Sanctuary, South Sikkim district, at an altitude of 2300–3263 m a.m.s.l. The tropical forest and temperate forest are reserve-protected forests of the Government of Sikkim, but the former forest is subjected to burning of forest floor litter mass as a management practice to remove shrubs which compete with the trees for resources. The subtropical forest is young and converted from paddy fields about 20 years ago. All the study sites have a monsoonal climate which can be categorized into four seasons: summer (March–May), rainy (June–August), autumn (September–November), and winter season (December–February). However, summer is mild, the rainy season is very wet, autumn is cool and moist, and winter is dry and cold. The annual rainfall was highest for the tropical forest (2867 mm), followed by the subtropical forest (2787 mm), and the least for the temperate forest (2699 mm). Climatic data of the study sites (Source: State Meteorological Department) is placed (Fig. 2). Soils in the tropical and temperate forests originated from feldspathic greywacke, while the parent rock of the soil in the subtropical forest is gneissic (Sikkim ENVIS, 2017). All the study sites lie on the slope surface which has loamy and acidic soil and a soil pH ranging from 4.2 to 6.2.
Vegetation
Vegetation in the tropical forest dominates with Sal (Shorea robusta. Roth), but very few other trees of Schima wallichii (D.C) Korth and Pinus roxburghii (Sarg) were also recorded. The dominating tree species in the subtropical forest is Alnus nepalensis (D. Don) along with other tree species such as Macaranga pustulata (King ex Hook. f), Schima wallichii (D.C) Korth, and Lyonia ovalifolia (Wall). The temperate forest represents a mixed oak forest dominated by Quercus lamellosa Sm., Quercus lanceifolia Roxb., Symplocos theifolia D. Don., Quercus pachyphylla (Kurz), and Daphniphyllum himalayense Benth. Shrubs were removed from all the forests as a management practice, but herbs were present.
Diversity and other indices of vegetation
Diversity indices such as Simpson (D) and Shannon Weiner (H) along with evenness and species richness (d) indices were estimated using the formula of Simpson (1949), Shannon (1948), Pielou (1969), and Margalef (1957), respectively.
where n is no. of individual of each species and N is the total number of individuals of all the species, Shannon Weiner,
where Pi is the proportion of individuals in the i.th species, i.e., (ni / N)
where H is the Shannon index and N is the number of species, and
where S is the number of species and N is the number of individual species.
Experimental design
Each of the forest types under study was demarcated into five different plots across the altitudinal gradient, and in each of the plots, two permanent quadrats of 0.1 ha were laid. Ten permanent quadrats were used for the sampling of vegetation and soil in each of the forest types.
Soil analyses
Soil samples for analysis of physical and chemical properties were collected (five replicates each from each of the five plots of every forest type) from the 0–45 cm soil layer of each of the vegetation sampling plots from each forest type. Soil pH was measured by using a pH meter (1:5 soil water suspension), soil moisture, and bulk density by oven drying a known amount of soil at 80 °C till weight becomes constant. Soil temperature was recorded during the time of sampling at the study sites with a soil thermometer (Devi & Yadava, 2006).
Determination of soil organic carbon (SOC) content was by colorimetric method (Anderson & Ingram, 1993), total N by using Kjeltec 8500 (FOSS), and available phosphorus (AP) by ammonium molybdate stannous chloride method (Allen et al., 1974). Analysis of microbial biomass carbon was done by using the chloroform-fumigation extraction method (Anderson & Ingram, 1993).
Carbon sequestration
For the computation of carbon sequestration, five plots at different elevations were demarcated in each forest type, and two quadrats of 0.1 ha were laid in each plot. The aboveground biomass of trees in the forests was computed by using species-specific volume equations and general equations of FSI (1996) and converted into carbon stock using the wood densities of tree species determined by the moisture content method (Kanawjia et al., 2013) and biomass expansion factor (BEF) of IPCC (2008). The estimation of annual carbon sequestration for each forest type was from the difference in carbon stock measured in 2016 and 2018 divided by 2. Belowground biomass was estimated using a standard root-to-shoot ratio and the default value of 0.26 (Ravindranath & Ostwald, 2008). Computation of herbaceous and litter biomass was carried out through a complete harvest of herbs and collection of litter biomass from ten quadrats of 1 × 1m2 in each of the forest sites. Biomass values were then converted to carbon stock values using the carbon default fraction 0.47 of the Intergovernmental Panel on Climate Change (IPCC). Carbon content and stock in soil were estimated colorimetrically by using the procedures of Anderson and Ingram (1993) and Ravindranath and Ostwald (2008), respectively.
CO2 flux from soil
Monthly soil CO2 emission of the study sites was determined by the alkali absorption method (Anderson & Ingram, 1993). Six open-ended cylinders of 13 cm diameter and 25 cm height were inserted in each of the demarcated vegetation sampling plots in every forest site, and one out of six cylinders served as a blank sample in each of the plots. Of the total 30 cylinders used in each of the study sites, 5 cylinders serve as blank samples in every forest site. A known volume of 0.25 N NaOH was kept overnight in vials inside the airtight cylinder after removing all green herbaceous vegetation within the cylinder. After 24 h, NaOH in each of the vials was titrated with a standard HCL solution of 0.25 N concentration using phenolphthalein as an indicator. CO2 absorbed by the alkali (NaOH) is then calculated by using the procedure of Anderson and Ingram (1993)
Statistical analyses of data
Tukey’s HSD test was used to test the significant differences between means. Pearson correlation test was used to test the influential factors of carbon sequestration and emission in the forests. A multivariate regression test was performed to test the variance of soil CO2 flux from the study sites due to climatic variables such as air temperature and rainfall. Two-way ANOVA was carried out to understand the influence of seasons and forest types on CO2 flux.
Result
Bio-climatic factors of the study sites
Tree density was the highest in the tropical forest, followed by the temperate forest, and least in the subtropical forest. But the basal area of trees was maximum in the temperate forest and least in the subtropical forest, while litter biomass was least in the temperate forest and the maximum in the tropical forest. Shannon Wiener’s diversity index, Pielou’s evenness index, and Margalef’s species richness index were highest for the temperate forest, followed by the subtropical and tropical forests. The trend of Simpson’s dominance index was temperate > tropical > subtropical forest (Table 1). The annual mean minimum and maximum air temperature varied across the study sites with the least in the subtropical forest (9.89 °C and 16.92 °C) followed by the temperate forest (13.83 °C and 17.53 °C) and tropical forest (17.69 °C and 27.59 °C). Variations of the monthly temperature and rainfall in the different forest types are in Fig. 2. Annual rainfall was highest for the tropical forest (2867 mm), followed by the subtropical forest (2787 mm) and the temperate forest (2699 mm). The minimum rainfall was during December, and the peak rainfall occurred during July in all the study sites (Fig. 2).
Edaphic factors of the study sites are listed in Table 1. All the forests have acidic soil, with the highest soil pH in the tropical forest and the least in the temperate forest. The soil moisture in the different forest types follows the trend of tropical > subtropical > temperate forest, while that of soil temperature is subtropical > temperate > tropical forest. The tropical forest exhibited a maximum bulk density of soil, while the minimum was in the sub-tropical forest. Both the temperate and tropical forests exhibited the highest concentration of soil organic carbon (SOC) and available phosphorus (AP), but the peak total nitrogen (TN) concentration was in the subtropical forest. However, the concentration of SOC and TN was least in the tropical forest and that of AP in the subtropical forest.
Carbon sequestration in different forest types
Carbon sequestration of both the vegetation and soil exhibited the same trend with the highest in the temperate forest, followed by subtropical forest and tropical forest; however, soil carbon was not sequestered but mineralized/lost from the soil in the tropical forest (Table 2). Also, a two-tailed Pearson correlation test revealed a significant positive relationship between soil carbon sequestration and tree species diversity (p < 0.01) and species richness of the site (p < 0.05), but vegetation C sequestration did not show any significant relationship with any of the bio-edaphoclimatic factors (Table 3).
CO2 flux from soils of the different forests
The monthly soil CO2 evolution was highest in the tropical forest 156.54 mg CO2 m−2 h−1 (January)–260.94 mg CO2 m−2 h−1 (March), followed by subtropical forest 114.58 mg CO2 m−2 h−1 (January)–245.03 mg CO2 m−2 h−1 (July), and temperate forest 30.32 mg CO2 m−2 h−1 (February)–177.6 mg CO2 m−2 h−1 (June) in the present study (Fig. 3). Tropical and subtropical forests have a similar seasonal trend of CO2 emission, i.e., rainy > summer > autumn > winter, but in the temperate forest, the trend was rainy > winter > summer > autumn (Table 4). The Tukey test revealed that both the tropical and temperate forests exhibited significant seasonal differences while the subtropical forest failed to exhibit significant seasonal differences from other forest types except in the autumn season (Table 4). Two-way ANOVA (analysis of variance) indicated a significant difference in the rate of soil CO2 emission due to the influence of forest types and seasons (Table 5). Multivariate regression analysis of soil CO2 emission (Y) and climatic variables, i.e., minimum temperature (X1), maximum temperature (X2), and rainfall (X3), indicates no autocorrelation (Durbin-Watson = 2.8) and revealed 85% variability of soil CO2 emission in the study sites due to the climatic factors (Eq. 1). The rate of CO2 emission in the different forests exhibited significant positive influence due to rainfall (p < 0.05) and was negatively influenced by carbon sequestration (p < 0.05) (Table 3).
Discussion
Carbon sequestration in the different forest types
The insignificant correlation between the vegetation carbon sequestration and the bio-edaphoclimatic factors of the present study indicates that any bio-edaphoclimatic variances may not substantially impact the rate of carbon sequestration of the forests in this region. However, a positive and significant relationship between soil carbon sequestration and plant species richness and tree diversity of the study sites (Table 3) implies a significant alteration in soil carbon due to a variance in vegetation/species diversity and richness, thereby explaining higher carbon sequestration in the temperate forest with higher plant diversity, species richness, and soil moisture content. A higher tree species diversity in the temperate forest with a higher elevation than that of tropical forests (Joshi et al., 2022) increases soil carbon sequestration (Chen et al., 2018; Valentini et al., 2000), which conforms with our findings. Moreover, higher tree density, basal area, and microbial biomass C in the temperate forest in contrast to the other type of forests could be another reason for more vegetation C sequestration in this forest (Kothandaraman et al., 2020). Also, a higher diversity of tree species or species richness in the temperate forest reduces carbon loss as plant diversity enhances belowground carbon, microbial activity, and diversity, thereby increasing soil carbon storage and sequestration (De Deyn et al., 2011; Fornara & Tilman, 2008; FSI, 1996; Lange et al., 2015). Our results extend the findings of other previous studies that reported enhancement of carbon storage due to an increase in tree basal area, plant growth, higher soil water availability (Alvarez Davila et al., 2017), and other bio-environmental factors in different forest ecosystems (Poorter et al., 2018; Vayreda et al., 2012).
The lower rate of C sequestration in the present tropical and subtropical forests despite higher rainfall could be related to the higher temperature in these sites making the soil unable to retain moisture, thereby reducing the rate of carbon sequestration in these sites (Brienen et al., 2010; Dai et al., 2015). The negative but insignificant relationship between carbon sequestration and average temperature and rainfall of the present study (Table 3) supports the finding that increased rainfall, temperature, and extreme events lead to the reduction of carbon stock both in vegetation and soil (Baker et al., 2004; Souza & Longhi, 2019). An extremely wet climatic condition in this region could have led to exceeding the critical point of carbon sequestration, beyond which an increase in C is negligible (Dai et al., 2015). However, other studies reported a positive relationship between C sequestration and rainfall (Li et al., 2019; Toledo et al., 2012), which is contrasting with our result. Carbon mineralization or loss instead of C sequestration in the soil of the tropical forest corresponds to the highest soil pH and temperature in this site which reduces the carbon storage and nutrient supply in soil by enhancing the rate of decomposition, thereby affecting the rate of soil respiration and belowground biomass (Weil & Brady, 2016). Moreover, despite more forest litter biomass in the tropical forest, the dominating species in this site are deciduous trees with more readily degradable labile C fractions compared to the tough recalcitrant carbon fractions in the temperate species (Wiemann & Williamson, 2002). In contrast, the highest soil C sequestration in the temperate forest is because of higher tree diversity which promotes C accumulation (Chapin III, 2003), low rainfall by preventing loss of soil nutrients (Lukina et al., 2019), and less temperature by retarding decomposition and enhancing subsequent C addition through the litter. Our result conforms with the findings of other studies that reported a decline in soil organic carbon sequestration due to an increase in soil pH and increased temperature (Chen et al., 2018; Devi, 2021) in different forests and biomes.
CO2 flux from soils of the different forests
The significantly higher rate of soil CO2 flux in the tropical forest than that of the temperate forest coincides with a higher amount of litter biomass, temperature, and more intense rainfall with longer duration in the former site which might have enhanced the microbial activity and decomposition (Chen et al., 2013b; Ding et al., 2007; Jia et al., 2006; Patil et al., 2014). A positive significant relationship between the rate of CO2 emission from the soil and rainfall in the present study (p < 0.05) indicates that rainfall is one of the influential factors of soil CO2 emission and an erratic rainfall pattern, duration, and amount will significantly impact the carbon source function of an ecosystem (Table 3). Our result supports the findings of other studies that conclude short-duration intense rainfall lowers soil respiration (Jeong et al., 2018), while uninterrupted and precise rain can stimulate soil respiration (Davidson et al., 1998; Liu et al., 2002; Raich & Schlesinger, 1992), and soil moisture conditions can regulate the response of temperature to soil CO2 emission (Joo et al., 2012). Temperate ecosystems due to low root respiration (Groffman et al., 2006) and less litter biomass (Luo et al., 2013) reduce carbon emissions from soils. The amount of organic matter in an ecosystem influences soil respiration in an ecosystem by altering the water retention capacity, pore space, and microbial activity in the soil (Moyano et al., 2013). Multivariate regression analysis between monthly soil CO2 emission rates and climatic variables, i.e., minimum and maximum temperature and rainfall, revealed 85% variability of soil CO2 emission due to these factors, and among the climatic variables, rainfall is the most influential climatic driver that regulates the rate of carbon sequestration of the present forests (p < 0.05) (Table 3). This implies that in the Eastern Himalayan forests, climatic factors are more influential drivers of soil CO2 flux than biotic and edaphic factors such as root biomass, litter biomass, stand age, vegetation type, soil pH, moisture, and nutrient content. However, the influence of tree species and the age of vegetation on soil respiration has been reported by several studies (Gong et al., 2012; Oertel et al., 2016; Saiz et al., 2006) in different forest ecosystems. The two-way ANOVA of soil CO2 emission across seasons (p < 0.001) and forest types (p < 0.001) indicates a significant temporal variation due to different species in the present study. Rainy season recorded peak CO2 emission due to high rainfall and warmer temperature triggers microbial activity and decomposition during this season in all the study sites. Moreover, the increase in soil CO2 emission during the rainy season can be attributed to the growth and respiration of plant roots as it is the growing season of plants. An increase in carbon emission due to microbial and vegetation root respiration during the growing season of plants has been reported by various earlier studies (La Scala et al., 2010; Norman et al., 1992; Ray et al., 2020; Ren et al., 2017) which concurred with our result. Also, many studies from different forest types revealed higher soil CO2 emission in the wet season, for example, tropical forest (Makita et al., 2018), subtropical (Devi & Yadava, 2009), temperate (Laishram et al., 2002), warm temperate (Mo et al., 2005), mixed forest (Chen et al., 2013a; Takahashi et al., 2011), and Afromontane forest (Yohannes et al., 2011) which conforms with our result. The least soil CO2 emission rate during the winter season in all the forest sites coincides with the least amount of precipitation and cold climate that reduces soil moisture inhibiting cell metabolism and respiration of the microbes (Moyano et al., 2013). In the monsoonal areas of Asia, rainy days during the summer season accelerate microbial decomposition while drier months inhibit the process (Cook et al., 2010), and temperature change due to seasonal variation and water stress resulted in the fluctuation of soil respiration rates (Makita et al., 2018).
The annual mean CO2 emission from soils of the present forests was lower than the emission rates from different forests of the world. For example, CO2 fluxes from the tropical forest of Hawaii, 26.34 Mg CO2 ha−1y−1 (Townsend et al., 1995); tropical monsoon forest of Thailand, 25.6 Mg CO2 ha−1y−1 (Hashimoto et al., 2004); a subtropical moist forest of Queensland, Australia, 51.6 Mg CO2 ha−1y−1 (Butterbach-Bahl et al., 2004); tropical forest of South America, 36.94–52.68 Mg CO2 ha−1y−1 (Garcia-Montiel et al., 2004; Sotta et al., 2007); subtropical broad-leaved forest and tropical monsoon forest of China, 34.54–35.40 Mg CO2 ha−1y−1 (Fang et al., 2010); and temperate and boreal forests 11.59–40.15 Mg CO2 ha−1y−1 from all over the world (Falk et al., 2005; Fang et al., 2010; Sulzman et al., 2005; Zerva & Mencuccini, 2005). The reason for a low average annual soil CO2 flux in our forests could be related to the rainfall pattern, amount, intensity, and temperature extremes in these sites, i.e., high and intense rainfall during the rainy season and dry and cold severe winter season that limits microbial activity.
Implications of change in climate and species composition to carbon dynamics
Our results revealed that the tree species diversity and carbon sequestration potential of forests changes along an altitude gradient of the Eastern Himalayas due to the variance in plant species, forest types, and other edaphic and environmental variables. A higher carbon sequestration rate with the least CO2 flux from the cold temperate forest in the present study suggests that a vertical expansion of tropical ecosystems due to species range shift or migration may reduce the species diversity and carbon sequestration potential of the forests in this region. In the present study, sites with a higher temperature and rainfall (tropical ecosystems) exhibited enhanced soil carbon flux and reduced C storage and sequestration, suggesting that global warming and climate change may decline the carbon sequestration and storage in the soil by reducing the tree species richness and enhancing soil organic matter decomposition. Also, an erratic rainfall pattern such as rainfall in the dry season which has become a frequent trend in tropical ecosystems may further increase the rate of carbon emission from the soil in this region. Previous studies have shown that climatic factors, temperature, and rainfall regulate the distribution of species more than the edaphic factors of the sites (Holmgren & Poorter, 2007; McKenzie et al., 2003; Toledo et al., 2012), affecting the species distribution pattern. Tropical species are sensitive to temperature change, and a slight change in temperature results in drastic effects resulting in a shift in distribution patterns for these species (Wright, 2010). Also, shifting the tropical and subtropical forests along the altitudinal gradient will enhance soil CO2 flux from the forests of this region and transform the quality of the soil. Apart from the decrease in carbon storage, the transformation of soil quality will also reduce soil fertility which might contribute to further loss of species diversity. Present findings extend the results of other studies that the adoption of mixed tree species or high tree species diversity improves the soil organic matter and quality of soil (Chapin III, 2003; Andivia et al., 2016), C storage, and sequestration. Our results also corroborate the findings of earlier studies that soil organic carbon is susceptible to changes in climate (Tian et al., 2015), altitude (Tashi et al., 2016), and temperature (Sun & Liu, 2019). Therefore, enhancing species richness in tropical forests might boost more C sequestration and slow the impact of climate change. However, the present study is limited to the three forests of Eastern Himalaya only, and more studies in this context from different geographical areas, climates with diverse species, stand age, and management practices need to be carried out for a better understanding of the change in carbon sink and source function of forests in response to climate change. Also, the findings of the present study are limited to data collected from 2 years only, and long-term studies might provide better and clear results of the studied parameters.
Conclusion
The present study concludes that a forest with low temperature and rainfall, high tree diversity, and species richness, i.e., temperate forest, can sequester more carbon and emit less soil CO2 as compared to tropical forests. Carbon sequestration increases with an increase in plant species diversity and reduces with an increase in temperature and rainfall. In the present study, soil CO2 flux exhibits seasonality and variation with forest types and is influenced by the variation in climatic factors, especially rainfall. Additionally, it may be concluded that changes in the climatic variables and warming will not only affect the vegetation but may also lead to the degradation of soil which may affect the productivity and functionality of ecosystems risking the lives of the mountain people.
Data availability
All data are included in the MS; however, raw data if required will be available on request to the corresponding author.
References
Allen, S., & Kirilenko, A. (2016). Climate change and terrestrial biomass : What if trees do not migrate ? Global Ecology and Biogeography Letters, 6, 139–148.
Ades, M., Adler, R., Aldeco, L. S., et al. (2019). State of the climate in 2018. Bulletin of the American Meteorological Society, 100(9), SIS305.
Allen, S. E., Grimshaw, H. M., Parkinson, J. A., & Quarmby, C. (1974). Chemical analysis of ecological materials. Blackwell Scientific Publications.
Alvarez Davila, E., Cayuela, L., González-Caro, S., et al. (2017). Forest biomass density across large climate gradients in Northern South America is related to water availability but not with temperature. PLoS One, 12, e0171072. https://doi.org/10.1371/journal.pone.0171072
Anderson, J. M., Ingram, J. S. I. (1993) Tropical soil biology and fertility: A handbook of methods (2nd ed., p. 221). Wallingford: C.A.B. International.
Andivia, E., Rolo, V., Jonard, M., et al. (2016). Tree species identity mediates mechanisms of top soil carbon sequestration in a Norway spruce and European beech mixed forest. Annals of Forest Science, 73, 437–447. https://doi.org/10.1007/s13595-015-0536-z
Baker, T. R., Phillips, O. L., Malhi, Y., et al. (2004). Increasing biomass in Amazonian forest plots. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359, 353–365. https://doi.org/10.1098/rstb.2003.1422
Brienen, R. J. W., Lebrija-Trejos, E., Zuidema, P. A., & Martinez-Ramos, M. (2010). Climate-growth analysis for a Mexican dry forest tree shows strong impact of sea surface temperatures and predicts future growth declines. Global Change Biology, 16, 2001–2012. https://doi.org/10.1111/j.1365-2486.2009.02059.x
Butterbach-Bahl, K., Kock, M., & Willibald, G., et al. (2004). Temporal variations of fluxes of NO, NO2, N2O, CO 2, and CH4 in a tropical rain forest ecosystem. Global Biogeochemical Cycles, 18: https://doi.org/10.1029/2004GB002243.
Chapin, F. S., III. (2003). Effects of Plant Traits on Ecosystem and Regional processes: A conceptual framework for predicting the consequences of global change. Annals of Botany, 91, 455–463. https://doi.org/10.1093/aob/mcg041
Chen, S., Wang, W., Xu, W., et al. (2018). Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences U S A, 115, 4027–4032. https://doi.org/10.1073/pnas.1700298114
Chen, W., Jia, X., & Zha, T., et al. (2013a). Soil respiration in a mixed urban forest in China in relation to soil temperature and water content. European Journal of Soil Biology, 54:. https://doi.org/10.1016/j.ejsobi.2012.10.001.
Chen, W., Zheng, X., & Chen, Q., et al. (2013b). Effects of increasing precipitation and nitrogen deposition on CH4 and N2O fluxes and ecosystem respiration in a degraded steppe in Inner Mongolia, China. Geoderma, 192: https://doi.org/10.1016/j.geoderma.2012.08.018.
Chertov, O. (2010). Impact of temperature increase and precipitation alteration at climate change on forest productivity and soil carbon in boreal forest ecosystems in Canada and Russia: Simulation approach with the EFIMOD model. In S. Simard (Ed.), Climate change and variability, London, p. 502.
Clark, J. S. (1998). Why trees migrate so fast: Confronting theory with dispersal biology and the paleorecord. The American Naturalist, 152, 204–224. https://doi.org/10.1086/286162
Cook, E. R., Anchukaitis, K. J., & Buckley, B. M., et al. (2010) Asian monsoon failure and megadrought during the last millennium. Science (80- ) 328:486 LP – 489. https://doi.org/10.1126/science.1185188.
Cramer, W., Bondeau, A., Woodward, F. I., et al. (2001). Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Global Change Biology, 7, 357–373. https://doi.org/10.1046/j.1365-2486.2001.00383.x
Dai, Z., Johnson, K. D., Birdsey, R. A., et al. (2015). Assessing the effect of climate change on carbon sequestration in a Mexican dry forest in the Yucatan Peninsula. Ecological Complexity, 24, 46–56. https://doi.org/10.1016/j.ecocom.2015.09.004
Davidson, E. A., Trumbore, S. E., & Amundson, R. (2000). Soil warming and organic carbon content. Nature, 408, 789–790. https://doi.org/10.1038/35048672
Davidson, E. A., Belk, E., & Boone, R. D. (1998). Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Global Change Biology, 4: https://doi.org/10.1046/j.1365-2486.1998.00128.x.
Davidson, E. A., Janssens, I. A., & Lou, Y. (2006). On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Global Change Biology, 12: https://doi.org/10.1111/j.1365-2486.2005.01065.x.
Devi, A. S. (2021). Influence of trees and associated variables on soil organic carbon: A review. Journal of Ecology and Environment, 45, 5. https://doi.org/10.1186/s41610-021-00180-3
Devi, N. B., & Yadava, P. S. (2006). Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Applied Soil Ecology, 31, 220–227. https://doi.org/10.1016/j.apsoil.2005.05.005
Devi, N. B., & Yadava, P. S. (2009). Emission of CO2 from the soil and immobilization of carbon in microbes in a subtropical mixed oak forest ecosystem, Manipur, Northeast India. Current Science, 96(12), 1627–1630.
De Deyn, G. B., Shiel, R. S., & Ostle, N. J., et al. (2011). Additional carbon sequestration benefits of grassland diversity restoration. Journal of Applied Ecology, 48: https://doi.org/10.1111/j.1365-2664.2010.01925.x
Ding, W., Meng, L., & Yin, Y., et al. (2007). CO2 emission in an intensively cultivated loam as affected by long-term application of organic manure and nitrogen fertilizer. Soil Biology and Biochemistry, 39: https://doi.org/10.1016/j.soilbio.2006.09.024.
Epstein, H. E., Lauenroth, W. K., Burke, I. C., & Coffin, D. P. (1998). Regional productivities of plant species in the Great Plains of the United States. Plant Ecology, 134, 173–195. https://doi.org/10.1023/A:1009732800810
Falk, M., Wharton, S., & Schroeder, M. (2005).s Is soil respiration a major contributor to the carbon budget within a Pacific Northwest old-growth forest? Agricultural and Forest Meteorology, 135:. https://doi.org/10.1016/j.agrformet.2005.12.005.
Fang, H. J., Yu, G. R., Cheng, S. L., et al. (2010). Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences, 7, 395–407. https://doi.org/10.5194/bg-7-395-2010
Foley, J. A., Levis, S., Prentice, I. C., et al. (1998). Coupling dynamic models of climate and vegetation. Global Change Biology, 4, 561–579. https://doi.org/10.1046/j.1365-2486.1998.t01-1-00168.x
Fornara, D. A., & Tilman, D. (2008). Plant functional composition influences rates of soil carbon and nitrogen accumulation. Journal of Ecology, 96, 314–322. https://doi.org/10.1111/j.1365-2745.2007.01345.x
FSI. (1996). Volume equations for forests of India, Nepal, and Bhutan. Forest Survey of India, Ministry of Environment & Forests, Govt. of India, Dehradun, India, p. 249.
Garcia-Montiel, D. C., Melillo, J. M., & Steudler, P. A., et al. (2004). Emissions of N2O and CO2 from terra firme forests in Rondônia, Brazil. Ecological Applications, 14: https://doi.org/10.1890/01-6023.
Ge, Z. M., Kellomäki, S., & Peltola, H., et al. (2013). Impacts of climate change on primary production and carbon sequestration of boreal Norway spruce forests: Finland as a model. Climatic Change, 118: https://doi.org/10.1007/s10584-012-0607-1.
Giardina, C. P., & Ryan, M. G. (2000). reply: Soil warming and organic carbon content. Nature, 408, 790–790. https://doi.org/10.1038/35048675
Gogoi, A., Ahirwal, J., & Sahoo, U. K. (2022). Evaluation of ecosystem carbon storage in major forest types of Eastern Himalaya: Implications for carbon sink management. Journal of Environmental Management, 302, 113972. https://doi.org/10.1016/j.jenvman.2021.113972
Gong, J., Ge, Z., & An, R., et al. (2012). Soil respiration in poplar plantations in northern China at different forest ages. Plant and soil, 360: https://doi.org/10.1007/s11104-011-1121-3.
Griscom, B. W., Adams, J., Ellis, P. W., et al. (2017). Natural climate solutions. Proceedings of the National Academy of Sciences U S A, 114, 11645–11650. https://doi.org/10.1073/pnas.1710465114
Groffman, P. M., Hardy, J. P., Driscoll, C. T., & Fahey, T. J. (2006). Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Global Change Biology, 12: https://doi.org/10.1111/j.1365-2486.2006.01194.x.
Gu, L., Post, W. M., & King, A. W. (2004) Fast labile carbon turnover obscures sensitivity of heterotrophic respiration from soil to temperature: A model analysis. Global Biogeochemical Cycles, 18: https://doi.org/10.1029/2003gb002119.
Hashimoto, S., Tanaka, N., & Suzuki, M., et al. (2004). Soil respiration and soil CO2 concentration in a tropical forest, Thailand. Journal of Forest Research, 9: https://doi.org/10.1007/s10310-003-0046-y.
Hobbie, E. A. (2006). Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology, 87: https://doi.org/10.1890/05-0755.
Högberg, P., & Read, D. J. (2006). Towards a more plant physiological perspective on soil ecology. Trends in Ecology & Evolution, 21: https://doi.org/10.1016/j.tree.2006.06.004.
Holmgren, M., & Poorter, L. (2007). Does a ruderal strategy dominate the endemic flora of the West African forests? Journal of Biogeography, 34, 1100–1111. https://doi.org/10.1111/j.1365-2699.2006.01683.x
IPCC. (2008). 2006 IPCC guidelines for national greenhouse gas inventories – a primer. In H. S. Eggleston, K. Miwa, N. Srivastava, & K. Tanabe (Eds.), Prepared by the national greenhouse gas inventories programme. Japan: IGES.
Jeong, S.-H., Eom, J.-Y., Park, J.-Y., et al. (2018). Effect of precipitation on soil respiration in a temperate broad-leaved forest. J Ecol Environ, 42, 10. https://doi.org/10.1186/s41610-018-0071-6
Jia, B., Zhou, G., & Wang, Y., et al. (2006). Effects of temperature and soil water content on soil respiration of grazed and ungrazed Leymus chinensis steppes, Inner Mongolia. Journal of Arid Environments, 67: https://doi.org/10.1016/j.jaridenv.2006.02.002.
Joo, S. J., Park, S. U., Park, M. S., & Lee, C. S. (2012). Estimation of soil respiration using automated chamber systems in an oak (Quercus mongolica) forest at the Nam-San site in Seoul, Korea. Science of the Total Environment, 416: https://doi.org/10.1016/j.scitotenv.2011.11.025.
Joshi, V. C., Bisht, D., Sundriyal, R. C., & Pant, H. (2022). Species richness, diversity, structure, and distribution patterns across dominating forest communities of low and mid-hills in the Central Himalaya. Geology, Ecology, and Landscapes, 1–11. https://doi.org/10.1080/24749508.2021.2022424.
Kanawjia, A., Kumar, M., & Sheikh, M. A. (2013). Specific gravity of some woody species in the Srinagar Valley of the Garhwal Himalayas, India. For Sci Pract, 15, 85–88. https://doi.org/10.1007/s11632-013-0109-x
Kattel, G. R. (2022). Climate warming in the Himalayas threatens biodiversity, ecosystem functioning and ecosystem services in the 21st century: Is there a better solution? Biodiversity and Conservation, 31, 2017–2044. https://doi.org/10.1007/s10531-022-02417-6
Kirilenko, A. P., & Sedjo, R. A. (2007). Climate change impacts on forestry. Proc Natl Acad Sci U S A, 104, 19697–19702. https://doi.org/10.1073/pnas.0701424104
Kirschbaum, M. U., Watt, M. S., Tait, A., & Ausseil, A. G. E. (2012). Future wood productivity of Pinus radiata in New Zealand under expected climatic changes. Global Change Biology, 18: https://doi.org/10.1111/j.1365-2486.2011.02625.x.
Kothandaraman, S., Dar, J. A., Sundarapandian, S., et al. (2020). Ecosystem-level carbon storage and its links to diversity, structural and environmental drivers in tropical forests of Western Ghats, India. Scientific Reports, 10, 13444. https://doi.org/10.1038/s41598-020-70313-6
La Scala, N., de Sá, M. E., Vanir de Souza, J., et al. (2010). Spatial and temporal variability in soil CO2–C emissions and relation to soil temperature at King George Island, maritime Antarctica. Polar Science, 4, 479–487. https://doi.org/10.1016/j.polar.2010.07.001
Laishram, I. D., Yadava, P. S., & Kakati, L. N. (2002). Soil respiration in a mixed Oak forest ecosystem at Shiroy hills, Manipur in North-Eastern India. International Journal of Ecology and Environmental Sciences, 28, 133–137.
Lange, M., Eisenhauer, N., & Sierra, C. A., et al. (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications,6: https://doi.org/10.1038/ncomms7707.
Lepcha, N. T., & Devi, N. B. (2020). Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecological Processes, 9, 1–14. https://doi.org/10.1186/s13717-020-00269-y
Li, Y., Bao, W., & Bongers, F., et al. (2019). Drivers of tree carbon storage in subtropical forests. Science of the Total Environment, 654: https://doi.org/10.1016/j.scitotenv.2018.11.024.
Liang, Q., Xu, X., Mao, K., et al. (2018). Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. Journal of Biogeography, 45, 1334–1344. https://doi.org/10.1111/jbi.13229
Liu, X., Wan, S., Su, B., et al. (2002). Response of soil CO2 efflux to water manipulation in a tallgrass prairie ecosystem. Plant and Soil, 240, 213–223. https://doi.org/10.1023/A:1015744126533
Lukina, N. V., Tikhonova, E. V., Danilova, M. A., et al. (2019). Associations between forest vegetation and the fertility of soil organic horizons in northwestern Russia. Forest Ecosystems, 6, 34. https://doi.org/10.1186/s40663-019-0190-2
Luo, Y., Durenkamp, M., De Nobili, M., et al. (2013). Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biology & Biochemistry, 57, 513–523. https://doi.org/10.1016/j.soilbio.2012.10.033
Mahlstein, I., Daniel, J. S., & Solomon, S. (2013). Pace of shifts in climate regions increases with global temperature. Nature Clinical Practice Endocrinology & Metabolism, 3, 739–743. https://doi.org/10.1038/nclimate1876
Makita, N., Kosugi, Y., Sakabe, A., et al. (2018). Seasonal and diurnal patterns of soil respiration in an evergreen coniferous forest: Evidence from six years of observation with automatic chambers. PLoS One, 13, 1–16. https://doi.org/10.1371/journal.pone.0192622
Margalef, R. (1957). Memorias de la Real Academia de Ciencias y Artes de Barcelona. Real Academia de Ciencias y Artes de Barcelona, 34, 374–559.
McKenzie, D., Peterson, D. W., Peterson, D. L., & Thornton, P. E. (2003). Climatic and biophysical controls on conifer species distributions in mountain forests of Washington State, USA. Journal of Biogeography, 30, 1093–1108. https://doi.org/10.1046/j.1365-2699.2003.00921.x
Mo, W., Lee, M. S., & Uchida, M., et al. (2005). Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agricultural and Forest Meteorology, 134:. https://doi.org/10.1016/j.agrformet.2005.08.015.
Molden, D., & Sharma, E. (2013). ICIMOD’s strategy for delivering high-quality research and achieving impact for sustainable mountain development. Mountain Research and Development, 33: https://doi.org/10.1659/MRD-JOURNAL-D-13-00018.1.
Moomaw, W. R., Law, B. E., & Goetz, S. J. (2020). Focus on the role of forests and soils in meeting climate change mitigation goals: Summary. Environmental Research Letters, 15, 45009. https://doi.org/10.1088/1748-9326/ab6b38
Moyano, F. E., Manzoni, S., & Chenu, C. (2013). Responses of soil heterotrophic respiration to moisture availability: An exploration of processes and models. Soil Biology & Biochemistry, 59, 72–85. https://doi.org/10.1016/j.soilbio.2013.01.002
Noormets, A., Epron, D., Domec, J. C., et al. (2015). Effects of forest management on productivity and carbon sequestration: A review and hypothesis. Forest Ecology and Management, 355, 124–140. https://doi.org/10.1016/j.foreco.2015.05.019
Norby, R. J., & Zak, D. R. (2011). Ecological lessons from Free-Air CO 2 Enrichment (FACE) experiments. Annual Review of Ecology, Evolution, and Systematics, 42:. https://doi.org/10.1146/annurev-ecolsys-102209-144647.
Norman, J. M., Garcia, R., & Verma, S. B. (1992). Soil surface CO2 fluxes and the carbon budget of a grassland. Geophysical Research: Atmospheres, 97, 18845–18853. https://doi.org/10.1029/92JD01348
Oertel, C., Matschullat, J., Zurba, K., et al. (2016). Greenhouse gas emissions from soils—A review. Chemie Der Erde, 76, 327–352. https://doi.org/10.1016/j.chemer.2016.04.002
Ogaya, R., Llusià, J., & Barbeta, A., et al. (2014). Foliar CO2 in a holm oak forest subjected to 15 years of climate change simulation. Plant Science, 226: https://doi.org/10.1016/j.plantsci.2014.06.010.
Pan, Y., Birdsey, R. A., & Fang, J., et al. (2011). A large and persistent carbon sink in the world’s forests. Science, (80- ) 333: https://doi.org/10.1126/science.1201609.
Patil, A. A., Shewale, B. Y., & Kadam, S. R. (2014). Soil quality restoration through carbon sequestration under climate change scenario in India. An Asian Journal Of Soil Science, 9: https://doi.org/10.15740/has/ajss/9.2/311-317.
Petrie, M. D., Pockman, W. T., Pangle, R. E., et al. (2015). Winter climate change promotes an altered spring growing season in piñon pine-juniper woodlands. Agricultural and Forest Meteorology, 214–215, 357–368. https://doi.org/10.1016/j.agrformet.2015.08.269
Pielou, E. C. (1969). An introduction to mathematical ecology. USA, Wiley-Inter-science.
Poorter, L., van der Sande, M. T., Arets, E. J. M. M., et al. (2018). Biodiversity and climate determine the functioning of Neotropical forests. Global Ecology and Biogeography, 27, 389–390. https://doi.org/10.1111/geb.12721
Poulter, B., Pederson, N., & Liu, H., et al. (2013). Recent trends in Inner Asian forest dynamics to temperature and precipitation indicate high sensitivity to climate change. Agricultural and Forest Meteorology, 178–179:. https://doi.org/10.1016/j.agrformet.2012.12.006.
Priha, O., Grayston, S. J., & Hiukka, R., et al. (2001). Microbial community structure and characteristics of the organic matter in soils under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Biology and Fertility of Soils, 33: https://doi.org/10.1007/s003740000281.
Qiu, J. (2008). China: The third pole. Nature, 454, 393–396. https://doi.org/10.1038/454393a
Raich, J. W., & Schlesinger, W. H. (1992). The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B, 44: https://doi.org/10.1034/j.1600-0889.1992.t01-1-00001.x.
Raina, V. K. (2004). Himalayan glaciers: A state-of-art review of glacial studies, Glacial Retreat and Climate Change. MoEF, Govt. of India, New Delhi, p. 56.
Ravindranath, N. H., & Ostwald, M. (2008). Carbon inventory methods. Springer.
Ray, R. L., Griffin, R. W., Fares, A., et al. (2020). Soil CO2 emission in response to organic amendments, temperature, and rainfall. Science and Reports, 10, 5849. https://doi.org/10.1038/s41598-020-62267-6
Reich, P. B., Hobbie, S. E., Lee, T., et al. (2006). Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature, 440, 922–925. https://doi.org/10.1038/nature04486
Ren, F., Zhang, X., Liu, J., et al. (2017). A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Science and Reports, 7, 8123. https://doi.org/10.1038/s41598-017-07793-6
Rustad, L., Campbell, J., Dukes, J. S., Huntington, T., Fallon Lambert, K., Jacqueline, M., Nicholas, R. (2012). Changing climate, changing forests: The impacts of climate change on forests of the northeastern United States and eastern Canada. Gen. Tech. Rep. NRS-99. Newtown Square. PA: U.S. Department of Agriculture, Forest Service, Northern Research Station, p 48.
Saiz, G., Byrne, K. A., & Butterbach‐Bahl, K. L. A. U. S., et al. (2006). Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Global Change Biology, 12: https://doi.org/10.1111/j.1365-2486.2006.01145.x.
Santonja, M., Fernandez, C., Gauquelin, T., & Baldy, V. (2015) Climate change effects on litter decomposition: Intensive drought leads to a strong decrease of litter mixture interactions. Plant and Soil, 393: https://doi.org/10.1007/s11104-015-2471-z.
Schindlbacher, A., Wunderlich, S., Borken, W., et al. (2012). Soil respiration under climate change: Prolonged summer drought offsets soil warming effects. Global Change Biology, 18, 2270–2279. https://doi.org/10.1111/j.1365-2486.2012.02696.x
Seetharam, K. (2008). Climate change synthetic scenario over Gangtok. Mausam, 59, 361–366. https://doi.org/10.54302/mausam.v59i3.1268
Shannon, C. E. (1948). A mathematical theory of communication. Bell System Technical Journal, 27:. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x.
Sharma, E., Molden, D., Rahman, A., et al. (2019). Introduction to the Hindu Kush Himalaya assessment BT - The Hindu Kush Himalaya assessment: Mountains, climate change, sustainability and people. In A. Mishra, A. Mukherji, & A. B. Shrestha (Eds.), Wester P (pp. 1–16). Springer International Publishing.
Sikkim, ENVIS. (2017). Soils of Sikkim. Forest and Environment Department, Government of Sikkim, Gangtok, India, p. 14.
Simpson, E. H. (1949). Measurement of diversity [16]. Nature, 163, 688. https://doi.org/10.1038/163688a0
Singh, U. (2013). Carbon capture and storage: An effective way to mitigate global warming. Current Science, 105, 914–922.
Singh, N., Parida, B. R., Charakborty, J. S., & Patel, N. R. (2019). Net ecosystem exchange of CO2 in deciduous pine forest of lower Western Himalaya (p. 8). Resour.
Sotta, E. D., Veldkamp, E., Schwendenmann, L., et al. (2007). Effects of an induced drought on soil carbon dioxide (CO2) efflux and soil CO2 production in an Eastern Amazonian rainforest, Brazil. Global Change Biology, 13, 2218–2229. https://doi.org/10.1111/j.1365-2486.2007.01416.x
Souza, A. F., & Longhi, S. J. (2019). Disturbance history mediates climate change effects on subtropical forest biomass and dynamics. Ecology and Evolution, 9, 7184–7199. https://doi.org/10.1002/ece3.5289
Sulzman, E. W., Brant, J. B., Bowden, R. D., & Lajtha, K. (2005). Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry, 73: https://doi.org/10.1007/s10533-004-7314-6.
Sun, W., & Liu, X. (2019). Review on carbon storage estimation of forest ecosystem and applications in China. Forest Ecosystems, 7, 4. https://doi.org/10.1186/s40663-019-0210-2
Takahashi, M., Hirai, K., & Limtong, P., et al. (2011). Topographic variation in heterotrophic and autotrophic soil respiration in a tropical seasonal forest in Thailand. Soil Science and Plant Nutrition, 57:. https://doi.org/10.1080/00380768.2011.589363.
Tashi, S., Singh, B., Keitel, C., & Adams, M. (2016). Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data. Global Change Biology, 22, 2255–2268. https://doi.org/10.1111/gcb.13234
Telwala, Y., Brook, B. W., Manish, K., & Pandit, M. K. (2013) Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS One, 8:. https://doi.org/10.1371/journal.pone.0057103.
Tian, H., Lu, C., Yang, J., et al. (2015). Global patterns and controls of soil organic carbon dynamics as simulated by multiple terrestrial biosphere models: Current status and future directions. Global Biogeochemical Cycles, 29, 775–792. https://doi.org/10.1002/2014GB005021
Toledo, M., Peña-Claros, M., Bongers, F., et al. (2012). Distribution patterns of tropical woody species in response to climatic and edaphic gradients. Journal of Ecology, 100, 253–263. https://doi.org/10.1111/j.1365-2745.2011.01890.x
Townsend, A. R., Vitousek, P. M., & Trumbore, S. E. (1995). Soil organic matter dynamics along gradients in temperature and land use on the island of Hawaii. Ecology, 76: https://doi.org/10.2307/1939339.
Trumbore, S. E., Chadwick, O. A., & Amundson, R. (1996). Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science, (80- ) 272:393–396. https://doi.org/10.1126/science.272.5260.393.
Valentini, R., Matteucci, G., & Dolman, A. J., et al. (2000). Respiration as the main determinant of carbon balance in European forests. Nature 404: https://doi.org/10.1038/35009084.
Vayreda, J., Gracia, M., Canadell, J. G., & Retana, J. (2012). Spatial patterns and predictors of forest carbon stocks in Western Mediterranean. Ecosystems, 15, 1258–1270. https://doi.org/10.1007/s10021-012-9582-7
Wang, W., Peng, C., & Kneeshaw, D. D., et al. (2012). Quantifying the effects of climate change and harvesting on carbon dynamics of boreal aspen and jack pine forests using the TRIPLEX-Management model. Forest Ecology and Management, 281: https://doi.org/10.1016/j.foreco.2012.06.028.
Weil, R., & Brady, N. (2016). The nature and properties of soils (15th ed.). Upper Saddle River, NJ: Pearson Press, 2017, p. 1086.
Wiemann, M., & Williamson, G. (2002). Geographic variation in wood specific gravity: Effects of latitude, temperature, and precipitation. Wood and Fiber Science, 34, 96–107.
Winsome, T., Silva, L. C., & Scow, K. M., et al. (2017). Plant-microbe interactions regulate carbon and nitrogen accumulation in forest soils. Forest Ecology and Management, 384: https://doi.org/10.1016/j.foreco.2016.10.036.
Wright, S. J. (2010). The future of tropical forests. Annals of the New York Academy of Sciences, 1195, 1–27. https://doi.org/10.1111/j.1749-6632.2010.05455.x
Yohannes, Y., Shibistova, O., & Abate, A., et al. (2011). Soil CO2 efflux in an Afromontane forest of Ethiopia as driven by seasonality and tree species. Forest Ecology and Management, 261: https://doi.org/10.1016/j.foreco.2010.12.032.
You, J., Qin, X., Ranjitkar, S., et al. (2018). Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Science and Reports, 8, 5879. https://doi.org/10.1038/s41598-018-24360-9
Zerva, A., & Mencuccini, M. (2005). Short-term effects of clear felling on soil CO2, CH4, and N2O fluxes in a Sitka spruce plantation. Soil Biology and Biochemistry, 37: https://doi.org/10.1016/j.soilbio.2005.03.004.
Funding
The authors are thankful to the Department of Science and Technology (DST), Government of India, for providing financial support in the form of a project grant (DST/IS-STAC/CO2-SR-229/14(G)-AICP-AFOLU-IV) to one of the authors (NBD).
Author information
Authors and Affiliations
Contributions
N Bijayalaxmi Devi: conceptualization, methodology, resources, supervision, writing review and editing, writing original draft, visualization, fund acquisition. Nima Tshering Lepcha: formal analysis, validation, investigations.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, N.B., Lepcha, N.T. Carbon sink and source function of Eastern Himalayan forests: implications of change in climate and biotic variables. Environ Monit Assess 195, 843 (2023). https://doi.org/10.1007/s10661-023-11460-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11460-x