Abstract
Estimation of whole root biomass including coarse and larger roots and root balls can provide better understanding of carbon and nitrogen stocks in floodplain forests. Whole root systems of nine ash trees (Fraxinus angustifolia Vahl.) and six alder trees (Alnus glutinosa L.) trees ranging in diameter breast height (dbh) from 29.1 to 72.0 cm for ash and from 29.1 to 44.3 cm for alder were excavated, and their small < 1 cm, medium 1–4 cm, larger > 4 cm and root-ball biomass, and root carbon and nitrogen stocks were determined in Karacabey floodplain forest in Bursa, Turkey. In addition, for the method comparison, small root biomass (< 1 cm) was also determined using soil-core method. The whole root biomass of ash trees varied from 167.7 to 186.8 Mg ha−1. Alder trees had lower whole root biomass than ash trees ranging from 49.0 to 63.6 Mg ha−1. The determination of small root biomass by soil excavation method was nearly two-fold higher than by soil core method. Both root carbon and nitrogen stocks showed an increase with increasing root diameter. Among the tree characteristics (dbh, age, height, and volume), the dbh showed the highest correlation with whole root biomass and root carbon and nitrogen stocks for both tree species. It is concluded that young trees can have higher small, medium, and large root biomass and store more C and N in those roots, whereas older trees can have higher root-ball biomass and root-ball carbon and nitrogen stocks in Karacabey floodplain forests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands cover about 5–8% of the total land surface on earth (Dayathilake et al., 2020; Gardner & Finlayson, 2018). Although they cover a small percentage of area on land, many studies have reported that wetland ecosystems can store much higher carbon than terrestrial ecosystems (Liu et al., 2013). However, when considering global averages reported from forested wetlands such as mangroves, riparian, and floodplain forests, mean carbon stocks are generally so much higher compared to tropical and temperate forests (Adame et al., 2017; Shupe et al., 2021). In addition to higher carbon storage capacity, forested wetlands are also important areas where other nutrients, such as nitrogen (N), are stored in high amounts (Valiela & Cole, 2002). On the other hand, the potential of forested wetlands as C and N stocks is still under discussion due to the differences in data and the belowground root estimation methods (Abdul Malak et al., 2021).
In general, the amount of belowground organic carbon in forest ecosystems is higher than the amount of aboveground organic carbon (Dantas et al., 2020). Among the belowground carbon resources, tree roots account for between 10 and 65% of a tree’s total biomass, depending on factors such as age, species, nutrient availability, competition, and water (Brunner & Godbold, 2007). Despite this, most studies have generally focused and assessed carbon resources of aboveground biomass and soil organic matter in forested wetland ecosystems (Cierjacks et al., 2011; de Assis et al., 2019). Thus, studies on the determination of belowground root biomass and C and N stocks are very few, and leading to some uncertainties in the precise determination and modeling of the global C and N budgets in forested wetlands (Adame et al., 2017; Alongi, 2014).
On the other hand, reviews and studies on belowground root biomass have highlighted the limited understanding of whole tree root dynamics, especially in forested wetlands (Njana et al., 2015; Addo-Danso et al., 2016). Thus, analyzing the whole root biomass and carbon and N stocks in different biomass compartments is a promising approach to understand the formation of belowground C and N stocks. In general, small roots < 10 mm), coarse roots (> 10 mm), and root balls are main components to whole root biomass of both terrestrial forests and forested wetlands. Those root components play an important part in understanding the cycle and allocation of C and nutrients in forest ecosystems (Raich et al., 2014). Although both root balls and coarse roots may account for as much as 90% of the belowground total root biomass (Huynh et al., 2021), the focus of belowground root studies is typically on the fine-root component, which is responsible for only 1% of the total root biomass of trees (Jagodzinski et al., 2016). This could be mainly attributed to two reasons: Firstly, it is well known that fine roots are very important component of belowground C and N cycling and stocks in any forest ecosystem (Baker et al., 2001; Neumann et al., 2020); secondly, dealing with large roots is expensive and laborious and requires digging and opening large soil trenches or pits by using an excavator (Addo-Danso et al., 2016).
On the other hand, most of the methods of determining root biomass in forested wetlands carry uncertainties (Addo-Danso et al., 2016; From et al., 2021). Adame et al. (2017) stated that using small diameter soil cores resulted in estimating a lower amount of root biomass in mangrove forests. They attributed this to (1) using arrow root cylinders, (2) obstruction of larger roots close to the tree trunk, and (3) sediment cores only allow for roots smaller than 20 mm in diameter to be sampled. Since there is no consensus on which method to use in root biomass determination studies, it is recommended to use more than one method in studies (Addo-Danso et al., 2016; Vogt et al., 1998). However, the number of studies in which root mass was determined using different methods at the same sites and at the same sampling time is very few (e.g., Girardin et al., 2013; Sun et al., 2015), and no study available on the subject from Turkish floodplain forest so far.
We, therefore, set up a study to estimate the total root biomass and root carbon and nitrogen stocks of key tree species (ash and alder) using the root-excavation method and the soil-core method in ash and alder-dominated floodplain forest ecosystem which shows similar site conditions to mangrove forest of tropical regions as described by the Republic of Türkiye Ministry of Agriculture and Forestry. Our aims were to (1) investigate the variation in root biomass and C and N content and stocks of belowground root components (small Ø < 1 cm, medium Ø = 1–4 cm, larger Ø > 4 cm, and root ball) of two common tree species in floodplain forests, Karacabey, Turkey, (2) to understand the relationships between the total root biomass and stand characteristics (diameter breast height, age, height, and volume of trees) in floodplain forests under temperate climate conditions, (3) to find the better method for estimating smaller (Ø < 1 cm) root biomass and compare the accuracy of the most used two root sampling methods (root-excavation vs soil-core method), and (4) to provide first-time and unique data on whole root biomass and root carbon and nitrogen stocks from Turkish floodplain forest ecosystems.
Materials and methods
Description of the study site
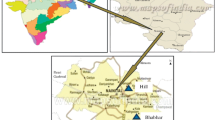
This study was carried out in ash (Fraxinus angustifolia Vahl.) and alder- (Alnus glutinosa L.) dominated floodplain forest (approximately 3800 ha) near the Karacabey town in Bursa, Turkey (Fig. 1). Most stands in the Karacabey floodplain forest are subjected to waterlogging or complete submersion for 9–10 months in every year. This unique floodplain forest ecosystem receives its water not only from river, sea, and rainfall but also from ground water. The region is characterized by a semi humid climate. The last 15 years (2007–2022) of annual precipitation and temperature are 719 mm and 15.5 °C, respectively. The floodplain forest in Karacabey plain is in the group of alluvial filled rift valley caused by tectonic movements. It was formed as a result of the collapses that took place during the Quaternary period. There are limeless brown forest soils, brown forest soils, alluvial, colluvial, alluvial coastal soils, and redzines throughout the Kocaçay delta. In the area where the study was carried out, there were alluvial and colluvial soils.
Selection of trees to sample
At the floodplain forest, ash tree stands ranged in diameter at the breast height (dbh) from 20.0 to 35.9 cm, from 36.0 to 51.9 cm, and from > 52 cm, while alder trees ranged from 20.0 to 35.9 cm and from 36.0 to 51.9 cm. Three trees were selected in each dbh group (total 9 trees for ash and 6 trees for alder). Around the selected trees, all small vegetation was cut off using an axe and removed away by hand. After that, their stems were cut off from nearly soil surface. Characteristics of the cutoff trees (age, dbh, height, and volume) are shown in Table 1. Each annual growth ring in the cutoff trees were used to determine tree age. Tree height was measured using a Blume-Leiss height indicator, with 0.5 m precision. Diameter at breast height (DBH) was measured using a caliper, but a diameter tape was also used much larger tree, especially for ash trees. The Huber’s formula was used to determine the volume of sample trees as VH = h × π × Dm2/40,000, where VH was the volume using the Huber’s formula (m3), h was the stem height or bole (m), and Dm denoted the diameter (cm) halfway along the log (Brack, 2006).
The stem was firstly removed from the site and then the remaining part of the tree was excavated using a mini excavator (Fig. 2a, b). Secondly, a 2 × 2 m square was dug around each tree. The depth of the trench was about 1.5 m and there were no roots visible inside the trench. Thirdly, the root ball was pulled out by a truck and cleaned by removing any remaining soil of the root ball. It left in the field to dry for 2 weeks. Finally, the dried root balls were weighted in the field by digital crane scales lifted using a mini excavator (Fig. 2a, b). Herbaceous roots (grasses, weeds, and herbs) were also distinguished from the live tree roots. The herbaceous roots formed masses that were clearly distinguishable from the tree roots.
Steps for root excavation in ash tree (a) and alder (b) sites. A trench was dug around the rootball monolith of the tree. Soil sieved to collect medium roots from the trench, excavator pushed the tree over and removed soil from the rootball, a measuring tape was used to measure the belowground hole dimensions, and all roots removed from the excavated area. Root ball measured on cattle scales in the field. The other root components were collected by hand and placed in the bags
The root samples of the excavated method were brought to the laboratory and categorized into three root classes as small roots (Ø < 10 mm) (SR), medium roots (Ø = 10–40 mm) (MR), and larger roots (Ø > 40 mm) (LR). The root samples were then dried until they reached constant weight. After that, they were weighed to determine the dry root biomass of each sample tree (kg root biomass per tree) in relation to root diameter classes.
Sample trees for each tree species were then used to develop allometric equations (Feng et al., 2021). Tree diameter (DBH) and height (H) were tested as independent variables in allometric equations. Preliminary analysis of alternative equations indicated that the allometric equation y = a + b (x1) + c (x2), where y is the biomass (kg), x1 is a tree size parameter DBH (cm), and x2 H (m), and a, b, and c are coefficients estimated by regression, best fitted the data.
We further calculated small, medium, larger, root ball, and total root biomass of all trees in each sample plot using the developed allometric equation, and then the obtained values were converted to hectares.
In order to compare the effects of root sampling methods (the root-excavation vs the soil-core method) on estimate of root biomass, five trees were chosen according to DBH and height. A soil core with 6.4 cm diameter and 34 cm height was used to get root samples. Five soil cores were sampled around each tree and at 1.5 m distance away from tree trunk. The soil cores were put in plastic bags, brought to the laboratory, and dried until they reached constant weight. After that, the root samples less than 1 cm diameter were separated, weighed, and scaled Mg root biomass ha−1.
Mean root C and N concentrations were analyzed by using a CNH-S elementary analyzer (Vesterdal & Raulund-Rasmussen, 1998). The biomass of each tree diameter class was then converted to mean root C and N stocks of the sample trees.
Soil samples and analysis
Soils were sampled from the depths of 0 to 10 cm, 10 to 20 cm, and 20 to 30 cm around the sample trees. The moist field soils were dried under laboratory conditions, crushed, and after that hand-sieved through a less than 2 mm screen, and thus, all stones, roots, large organic particles, and macro fauna were removed. Finally, the soil samples of each soil depth were mixed to have a single representative soil sample.
Soil pH was determined by a combination glass electrode in H2O (soil-solution ratio 1:2.5) (Kaçar, 2016). Electrical conductivity (EC) was determined in 1:1 soil water extract by using conductivity meter and expressed as dS/m (Allen, 1989). Soil texture was determined by Bouyoucos’ hydrometer method (Bouyoucos, 1962). Soil bulk density was determined by the undisturbed core sampling method (Kaçar, 2016).
Statistical analysis
ANOVA was used to statistically analyze the variations in whole root biomass and root C and N stocks between two tree species (ash and alder) and among the different diameter classes in Karacabey floodplain forests using the SPSS. The significance of regression for each root class was also determined by calculating the coefficient of determination (R2) using the SPSS. Tukey’s honestly significant difference (HSD) test (α = 0.05) was used to see the differences between mean values. Differences were considered significant at P < 0.05. Correlations between tree-stand characteristics and the root biomass and root carbon and nitrogen stocks of ash and alder tree species were determined using MS Excel 2000.
Results
General characteristics of sample trees and soil properties
Characteristics of each sampled trees are shown in Table 1. Age of ash trees ranged from 73 to 109 years old, while alder trees ranged from 42 to 54 years old. Tree diameters were varied from 29.1 to 72 cm for ash and from 28.7 to 44.3 cm for alder trees. Minimum tree height was 12.3 m for as and 20.4 m alder trees, while maximum tree height was 16.5 m for ash and 23.4 m for alder trees. Tree volume ranged from 0.62 to 2.79 m3 for ash and from 0.48 to 1.17 m3 for alder trees.
Soil characteristics of the studied ash and alder stands are given in Table 2. Soil properties did not vary significantly between the two tree species. For both tree species, soil pH, EC, and bulk density increased with soil depth, but soil texture did not vary much with soil depths (Table 2). Soil pH was slightly alkaline. Soil texture type was sandy clay loam.
Root carbon and nitrogen concentrations
Carbon and nitrogen concentrations in the small, medium, larger, and root balls of ash and alder trees are given in Table 3. Mean carbon concentration showed an increase with root diameters for both tree species. The small roots of ash tree had the lowest C concentration (45.5%), followed by the medium (48.4%), the larger (49.7%), and the root balls (50.5%). Alder tree roots had higher C concentration than ash tree for each tree diameter (46.3%, 49.7%, 51.2%, and 51.6% respectively).
In contrast to C concentration, N concentration showed a decrease with root diameters for both tree species. The small roots of alder tree had the highest C concentration (1.78%), followed by the medium (1.61%), the larger (1.48%) and the root balls (1.27%). Ash tree roots had lower C concentration than ash tree for each tree diameter (1.45%, 1.33%, 1.24%, and 1.03% respectively).
Root biomass and root C and N stocks
Small, medium, larger, root ball, and whole root biomass and root C and N stocks of ash and alder trees are shown in Table 4. Whole root biomass was in the range of 669.6–900.4 kg/tree for ash and 160.3–200.9 kg/tree for alder. The developed allometric equation in our study (Table 5) was used to calculate the total root biomass of small, medium, larger, root ball, and total roots for ash, and alder showed that per unit area, ash trees also had higher whole root biomass (176.4 Mg ha−1) compared to alder trees (55.5 Mg ha−1). Among the belowground root components, the root balls had the highest root biomass, followed by the larger roots, medium roots, and small roots for both tree species (Table 4). The mean root ball and larger and small root biomass of ash trees were higher than those of alder trees, while the mean medium root biomass did not vary much between the two tree species.
The whole root C stock was in the range of 336.0–453.4 kg/tree for ash and 81.8–103.0 kg/tree for alder stands. Per unit area, ash trees had higher whole root biomass (80.3 Mg ha−1) than alder trees (25.2 Mg ha−1). The whole root N stock was in the range of 7.24–9.51 kg/tree for ash and 2.27–2.73 kg/tree for alder. Per unit area, ash trees had higher whole root biomass (2.56 Mg ha−1) than alder trees (0.81 Mg ha−1).
The root balls were mostly responsible for the whole root mass and root C and N stocks of both tree species. The responsibility of the root balls was 75.4% for ash tree, and 54.7% for alder trees. The larger roots had the second-best responsibility for the total root biomass for ash (19.9%) and alder (29.4%) trees. The third-best responsibility for the total root biomass was the medium roots with 3.0% for ash and 9.9% for alder trees. The small roots had the lowest responsibility for the total root with 1.6% for ash and 1.4% for alder trees.
Root excavation vs soil core method for smaller root biomass (Ø < 1 cm)
Root biomass less than 1 cm diameter of ash and alder trees estimated using the soil core method showed similar values for ash trees but higher values for alder trees than the root excavation method. With the soil core method, the smaller root biomass for ash trees ranged from 1.57 to 3.87 Mg ha−1 (mean 2.72 Mg ha−1), while it varied from 1.88 to 2.23 Mg ha−1 (mean 2.05 Mg ha−1) for alder trees. However, with the root excavation method, mean values for the smaller roots were 2.82 Mg ha−1 (2.63–2.91 Mg ha−1) for ash and 0.813 Mg ha−1 (0.55–1.03 Mg ha−1) for alder trees (Table 4).
Relationship between stand characteristics and whole root biomass and root C and N stocks
Stand characteristics were plotted against whole root biomass and root C and N stocks of each root diameter class. Goodness fit for linear regressions of root biomass, root C and N stocks against tree age, diamater breast height (dbh), height (H), and volume (V) are shown in Table 6. The whole root biomass and root C and N stocks from two species showed the highest correlation with db. For both tree species, the dbh was positively correlated with the whole root biomass and root-ball biomass and root C and N stocks, whereas it was negatively correlated with the small, medium, and larger root biomass and root C and N stocks. Those positive and negative relationships are illustrated in Fig. 3 for the root-ball biomass, and in Fig. 4 for the small root biomass respectively.
Discussion
In this study, we determined the whole root biomass and root C and N stocks of ash and alder trees in Karacabey floodplain forests using a mini excavator. In addition, the soil-core method was used to determine the root biomass less than 1 cm in order to compare the effects of root sampling methods (the root-excavation vs the soil-core method) on the determination of root biomass floodplain forests. Unfortunately, comparable studies on whole root biomass using the root-excavation method in the literature from forested wetlands are sparse, and most estimations on root biomass only include roots less than 20 mm. Despite this, we found a few studies using the root excavation method in the forested wetlands to compare our results, but the finding of whole root biomass estimation was highly variable. For example, mean estimate of whole root biomass reported by Adame et al. (2017) in a mangrove forest was 438 Mg ha−1, while Ahmed et al. (2021) reported for 24 species that mean whole root biomass varied from 143 to 178 Mg ha−1 in mangrove forests in Bangladesh. Ahmed et al. (2021) also found that whole root carbon stocks ranged from 143 to 180 Mg ha−1. In this present study, the average whole root biomass of ash tree (176.4 Mg ha−1) was within the ranges reported from those from mangrove forests, but the whole root carbon stocks of ash trees (80.3 Mg ha−1) and the whole root biomass and root carbon stocks of alder trees (55.5 and 25.2 Mg ha−1 respectively) were lower than the values reported from those from mangrove forests.
Only for fine <2 mm and coarse >2 mm roots together, Bulmer et al. (2016) reported for five temperate mangrove forests in New Zealand that total root C stocks varied from 17 Mg ha-1 to 27.9 Mg C ha-1, while root N stocks ranged from 0.3 to 1.3 Mg N ha−1. In the present study, with the exclusion of the root ball, the average total root C and N stocks of ash trees and alder trees (19.2 Mg ha−1 and 13.8 Mg ha−1 respectively) were within the ranges for ash or near for alder reported from the temperate mangrove forests, but lower in root N stocks (0.628 Mg N ha−1 and 0.439 Mg ha−1 respectively) than the ranges reported by Bulmer et al. (2016) from mangrove forests.
In different forest ecosystems including tropical, temperate, and boreal forests, coarse root of individual trees and stands was mostly estimated using the root excavation methods (e.g., Brassard et al., 2011; Lima et al., 2012). However, most of those studies were limited to young plantations (e.g., Miller et al., 2006; Resh et al., 2003). For example, Aosaar et al. (2013) reported from south-eastern part of Estonia that mean whole root biomass (root ball, coarse roots > 10 mm and fine roots < 10 mm) was 22.3 Mg ha−1 for Alnus incana L. aged 17 years old. Niiyama et al. (2010) excavated 121 root systems of various species (78) with different age groups in a tropical primary forest in Malaysia and found that below-ground whole root biomass including root balls ranged from 78 to 118 Mg ha−1 (average 95.9 Mg ha−1). As for much older forest ecosystems, using root-excavation methods, the estimate of whole root biomass of Scots pine forests in Turkey aged from 95 and 125 was reported to vary from 51.5 to 65.7 Mg per hectare by Çömez (2010). In present study, the whole root biomass of ash (mean age 86 years old) and alder (mean age 49 years old) trees obtained from Karacabey floodplain forest was much higher for ash trees, but within the ranges reported from those of younger, tropical and temperate forests. Such differences can be explained by the effects of location (climate), tree species, forest type, age, soil structure, moisture regime, and chemical composition (Fortier et al., 2019; Makkonen & Helmisaari, 1998), which are considered key factors affecting the formation of root systems.
In the present study, the results showed that the percentage of different root size classes to the whole root biomass varied with tree species. The percentage of the root ball, larger (> 40 mm), medium roots (10–40 mm), and small roots (< 10 mm) of ash trees was 75.4%, 19.9%, 3.0%, and 1.6% respectively of whole root biomass, while they were 54.7%, 29.4%, 9.9%, and 1.4% respectively for alder trees. In literature, the contribution of roots of different size classes to the total biomass is variable, and comparisons among locations are difficult due to the variations in number of replicates, size of roots sampled, and sampling depth (Moser et al., 2010; Wakawa, 2016). In the present study, the average coarse root biomass (> 10 mm including root ball) of ash tree and alder trees was greater or within the ranges reported from other forest ecosystems, whereas the responsibility of small-root biomass (< 10 mm) to the whole root biomass was very low. With the root-excavation method, Niiyama et al. (2010) reported that the percentage of coarse roots (including root balls) was 86% of below ground biomass while the small roots (< 5 mm) was 14%. In Alnus incana stands (under 10 years of age), Bārdulis et al. (2015) showed that the majority, 92%, of total root biomass was composed of coarse roots (> 5 mm) (64%) and the root ball (28%), while the small roots < 5 mm were only 8%. Aosaar et al. (2013) reported for the same alder species (Alnus incana L. Moench) aged 17 years old that the root ball (40%) and the coarse roots > 10 mm (35%) constituted 75% of the total root mass, and the small roots < 10 mm were 25%. In mangrove forests, however, a number of authors found that fine roots (< 2 mm) were accounted for 44–66% of the total root biomass (Komiyama et al., 1987). In contrary, others showed that the roots of > 5 to 20 mm were mostly responsible for total root biomass (Adame et al., 2014). Similarly, coarse roots more than 3 mm were shown to be accounted for 65–95% of the total root biomass in young mangrove forest (Lang’at et al., 2013). The variations in the contribution of different root diameter class attributed to the differences in productivity and decay rates among the root diameter classes, especially fine versus coarse roots (Tamooh et al., 2008).
The results of our study also showed that whole root biomass and root C and N stocks had significant correlation with the tree characteristics (mostly dbh, age, and volume of the tree), but the correlation also varied with the root diameter classes. We found almost linear increase or decrease of whole root biomass per tree and unit basis. The root-ball biomass and root ball C and N stocks increased with increasing dbh, age, and volume of trees, whereas the small, medium, and larger root biomass and root C and N stocks decreased. The majority of root biomass studies published using coniferous or deciduous tree species are generally in agreement that root biomass (especially fine roots < 2 mm) increases to a peak at canopy closure, after which it gradually declines in maturing stands. However, the effect of stand age/sizes on root biomass (total or fine roots) is still controversial. For example, Yuan and Chen (2010), based on the published literature, stated that fine root biomass changed with stand development and continued to increase in stands up to 70 and 90 years old for deciduous and coniferous stands, respectively. Similarly, Çömez (2010) also showed that total root biomass of Scots pine (Pinus sylvestris L.) forests continued to increase in stands at 125 years (65.7 Mg ha−1) compared to the stands at 95 years (51.5 Mg ha−1). However, Claus and George (2005) observed fine root biomass development in Fagus sylvatica and Quercus cerris forest chronosequences and reported that fine root biomass reached a maximum at an approximate age of 25 and then declined to a steady state, as forests approached maturity. The total root biomass increased with tree age in plantation mangrove forest was also reported by a number of researchers. For example, total root biomass of replanted Rhizophora apiculata was 23.1 Mg ha−1 at the age of 5 years old, while it increased to 35.6 Mg ha−1 at the age of 25 years old (Alongi & Dixon, 2000). Similarly, the mean total root biomass of R. mucronata plantations was 7.5 Mg ha−1 at 6 years old, while at 12 years, the root biomass was 24.9 Mg ha−1 and at natural stands, it was 35.8 Mg ha−1 (Tamooh et al., 2008).
Many authors have stated that the excavation method is much better to be used in order to accurately quantify root biomass in individual trees and whole stands (Addo-Danso et al., 2016; Niiyama et al., 2010; Ouimet et al., 2008). Especially, it is so useful to directly harvest the roots concentrated mostly around the base of the stem (Bledsoe et al., 1999; Macinnis-Ng et al., 2010). Higher larger root biomass was also reported for Quercus douglasii stands by Millikin and Bledsoe (1999) using the excavation method compared to soil pit and soil core methods. In our study, the results indicated that the root-excavation method was better to determine the smaller and also larger root biomass in floodplain forests. The results showed that the mean smaller root biomass (< 1 cm) of ash trees determined by the root-excavation method and the soil core methods was similar (2.82 and 2.72 Mg ha−1 respectively), while it was two-fold lower (0.813 Mg ha−1) than the soil-core method (2.05 Mg ha−1) used for alder trees in the same stands. A number authors have reported that the excavation method may result in sampling error as roots become broken or lost during excavation (Addo-Danso et al., 2016). Even under ideal conditions, complete recovery of entire root systems is difficult, especially for large trees which usually have extensive and deep root systems (Bledsoe et al., 1999). Some limitations of the soil core methods were also reviewed by Addo-Danso et al. (2016): (1) soil compaction due to using soil core, (2) the difficult in cleaning fine root from organic debris, and (3) the need for more soil core samples to have accurate results. The limitations of the excavation method could be responsible for having much lower fine root biomass of alder tree compared to the root-excavation method, but no changes for ash tree. However, our study was not intended to investigate all those limitations here.
Conclusions
The results presented in this study have provided valuable insight into the whole root biomass and root carbon and nitrogen stocks of ash and alder tree species which are the most dominant tree species in the floodplain forest ecosystems. Although our dataset was limited, the results indicated that the whole root biomass and different size root classes varied greatly with tree species and tree characteristics (dbh, age, and volume of trees). Ash tree stands had higher total root biomass and root carbon and nitrogen stocks than alder stands. We conclude that young trees with small dbh and volume can have higher small, medium, and larger root biomass and root C and N stocks in floodplain forest, whereas older trees with larger dbh and volume can have higher root ball biomass and root ball C and N stocks. In order to better understand the short-term pattern of C and N allocation in response to flooding, precipitation, and temperature, tree and stand level root productivity data should be considered in future studies. We also conclude that sampling approach has an impact on root biomass estimations, with lower root biomass values for alder trees estimated from the root-excavation method than the soil-core method, while no differences for the ash trees. However, as stated in literature, excavation and tree root removal seriously disturb soil a long distance from the target tree and so minimizing site disturbance is a priority.
Data availability
All data produced from this study are available from the corresponding author on reasonable request.
References
Abdul Malak, D., Marin, A. I., Trombetti, M., & San Roman, S. (2021). Carbon pools and sequestration potential of wetlands in the European Union. European Topic Centre on Urban, Land and Soil Systems, Viena & Malaga. ISBN 978-3-200-07433-0.
Adame, M. F., Teutli, C., Santini, N. S., Caamal, J. P., Zaldívar-Jiménez, A., Hernández, R., & Herrera-Silveira, J. A. (2014). Root biomass and production of mangroves surrounding a karstic oligotrophic coastal lagoon. Wetlands 34, 479–488. https://doi.org/10.1007/s13157-014-0514-5
Adame, F. M., Cherian, S., Reef, R., & Stewart-Koster, B. (2017). Mangrove root biomass and the uncertainty of belowground carbon estimations. Forest Ecology and Management, 403, 52–60. https://doi.org/10.1016/j.foreco.2017.08.016
Addo-Danso, S. D., Prescott, C. E., & Smith, A. R. (2016). Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. Forest Ecology and Management, 359, 332–351. https://doi.org/10.1016/j.foreco.2015.08.015
Ahmed S., Kamruzzaman M., Azad M. S., & Khan M. N. I. (2021). Fine root biomass and its contribution to the mangrove communities in three saline zones of Sundarbans, Bangladesh. Rhizosphere, 17, 100294. https://doi.org/10.1016/j.rhisph.2020.100294
Allen, S. E. (1989). Chemical analysis of ecological materials (2nd ed.). Blackwell Scientific Publications.
Alongi, D. M. (2014). Carbon cycling and storage in mangrove forests. Annual Review of Marine Science, 6(1), 195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Alongi, D. M., & Dixon, P. (2000). Mangrove primary production and above- and below-ground biomass in Sawi Bay, southern Thailand. Phuket Marine Biological Center Special Publication, 22, 3l–38.
Aosaar, J., Varik, M., Lõhmus, K., et al. (2013). Long-term study of above- and below-ground biomass production in relation to nitrogen and carbon accumulation dynamics in a grey alder (Alnus incana (L.) Moench) plantation on former agricultural land. European Journal of Forest Research, 132, 737–749. https://doi.org/10.1007/s10342-013-0706-1
Baker III, T. T., Conner, W. H., Lockaby, B. G., Stanturf, J., & Burke, M. K. (2001). Fine root productivity and dynamics on a forested floodplain in South Carolina. Soil Science Society of America Journal, 65, 545–556. https://doi.org/10.2136/sssaj2002.6710
Bārdulis, A., Lazdiņa, D., Daugaviete, M., Bārdule, A., Daugavietis, U., & Rozītis, G. (2015). Above ground and below ground biomass in grey alder (Alnus incana (L.) Moench). young stands on agricultural land in central part of Latvia. Agronomy Research, 13(2), 277–286.
Bledsoe, C., Fahey, T. J., Ruess, R., & Day, F. P. (1999). Measurement of static root parameters-biomass, length, distribution. In G. P. Robertson, C. S. Bledsoe, D. C. Coleman, & P. Sollins (Eds.), Standard Soils Methods for Long-term Ecological Research (pp. 413–435). Oxford University Press.
Brack, C. (2006). Tree crown: Forest measurement and modelling. http://sres-associated.anu.edu.au/mensuration/crown.htm
Brassard, B. W., Chen, H. Y., Bergeron, Y., & Paré, D. (2011). Coarse root biomass allometric equations for Abies balsamea, Picea mariana, Pinus banksiana, and Populus tremuloides in the boreal forest of Ontario, Canada. Biomass and Bioenergy, 35, 4189–4196. https://doi.org/10.1016/j.biombioe.2011.06.045
Brunner, I., & Godbold, D. L. (2007). Tree roots in a changing world. Journal of Forest Research, 12(2), 78–82. https://doi.org/10.1007/s10310-006-0261-4
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analysis of soils. Agronomy Journal, 54, 464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Bulmer, R. H., Schwendenmann, L., & Lundquist, C. J. (2016). Carbon and nitrogen stocks and below-ground allometry in temperate mangroves. Frontiers in Marine Science, 3, 150. https://doi.org/10.3389/fmars.2016.00150
Cierjacks, A., Kleinschmit, B., Kowarik, I. Graf, M., & Lang F. (2011). Organic matter distribution in floodplains can be predicted using spatial and vegetation structure data. River Research & Application, 27, 1048–1057. https://doi.org/10.1002/rra.1409
Claus, A., & George, E. (2005). Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Canadian Journal of Forest Research, 35, 1617–1625. https://doi.org/10.1139/x05-079
Çömez, A. (2010). Determination of carbon sequestration in Scots pine (Pinus sylvestris L.) stands on Sündiken mountain. İstanbul University, PhD Thesis. 233 p.
Dantas, D., de Terra, M., & C. N. S., Pinto, L. O. R., Calegario, N., & Maciel, S. M. (2020). Above and belowground carbon stock in a tropical forest in Brazil Acta Scientiarum. Agronomy, 43(1), e48276. https://doi.org/10.4025/actasciagron.v43i1.48276
Dayathilake, D. D. T. L., Lokupitiya, E. & Wijeratne, V. P. I. S. (2020). Estimation of aboveground and belowground carbon stocks in urban freshwater wetlands of Sri Lanka. Carbon Balance Manage, 15, 17. https://doi.org/10.1186/s13021-020-00152-5
de Assis, R. L., Wittmann, F., Bredin, Y. K., Schöngart, J., Quesada, C. A. N., Piedade, M. T. F., & Haugaasen, T. (2019). Above-ground woody biomass distribution in Amazonian floodplain forests: Effects of hydroperiod and substrate properties. Forest Ecology & Management, 432, 365–375. https://doi.org/10.1016/j.foreco.2018.09.031
Fortier, J., Truax, B., Gagnon, D., et al. (2019). Abiotic and biotic factors controlling fine root biomass, carbon and nutrients in closed-canopy hybrid poplar stands on post-agricultural land. Scientific Reports, 9, 6296. https://doi.org/10.1038/s41598-019-42709-6
From, A. S., Krauss, K. W., Noe, G. B., Cormier, N., Stagg, C. L., Moss, R. F., et al. (2021). Belowground productivity varies by assessment technique, vegetation type, and nutrient availability in tidal freshwater forested wetlands transitioning to marsh. PLoS ONE, 16(7), e0253554. https://doi.org/10.1371/journal.pone.0253554
Gardner, R. C., & Finlayson, C. M. (2018). Ramsar convention on Wetlands. Global wetland outlook: State of the world’s wetlands and their services to people. Gland, Switzerland: Ramsar Convention Secretariat.
Girardin, C. A. J., Aragão, L. E. O. C., Malhi, Y., Huasco, W. H., Metcalfe, D. B., Durand, L., Mamani, M., Silva-Espejo, J. E., & Whittaker, R. J. (2013). Fine root dynamics along an elevational gradient in tropical Amazonian and Andean forests. Global Biogeochemical Cycles, 27, 1–13. https://doi.org/10.1029/2011GB004082
Feng, H., Chen, Q., Hu, Y., Du, Z., Lin, G., Wang, C., & Huang, Y. (2021) Estimation of forest aboveground biomass by using mixed-effects model. International Journal of Remote Sensing, 42(22), 8675–8690. https://doi.org/10.1080/01431161.2021.1984611
Huynh, T., Lee, D. J., Applegate, G., & Lewis, T. (2021). Field methods for above and belowground biomass estimation in plantation forests. MethodsX, 8, 101192. https://doi.org/10.1016/j.mex.2020.101192
Jagodzinski, A. M., Jędrzej, Z., Aleksandra, W., Hubert, P., & Gordon, L. E. (2016). Tree age effects on fine root biomass and morphology over chronosequences of Fagus sylvatica, Quercus robur and Alnus glutinosa stands. PLoS ONE, 11(2), e0148668. https://doi.org/10.1371/journal.pone.0148668
Kaçar, B. (2016). Physical and chemical soil analysis. Nobel Academic Publications.
Komiyama A, Ogino K, Aksornkoae S, & Sabhasri S. (1987). Root biomass of a mangrove forest in southern Thailand. 1. Estimation by the trench method and the zonal structure of root biomass. Journal of Tropical Ecology, 3, 97108. https://doi.org/10.1017/S0266467400001826
Lang’at, J. K. S., Kirui, B. K. Y., Skov, M. W., Kairo, J. G., Mencuccini, M., & Huxham, M. (2013). Species mixing boosts root yield in mangrove trees. Oecologia, 172(1), 271–278. https://doi.org/10.1007/s00442-012-2490-x
Lima, A. J. N., Suwa, R., de Mello Ribeiro, G. H. P., Kajimoto, T., dos Santos, J., da Silva, R. P., de Souza, C. A. S., de Barros, P., Noguchi, H., Ishizuka, M., & Higuchi, N. (2012). Allometric models for estimating above-and belowground biomass in Amazonian forests at São Gabriel da Cachoeira in the upper Rio Negro, Brazil. Forest Ecology & Management, 277, 163–172. https://doi.org/10.1016/j.foreco.2012.04.028
Liu, Y., Ni, H., Zeng, Z., & Chai, C. (2013). Effect of disturbance on carbon cycling in wetland ecosystem. Advanced Materials Research. https://doi.org/10.4028/www.scientific.net/AMR.610-613.3186
Macinnis-Ng, C., Fuentes, M. O. S., O’Grady, A. P., Palmer, A. R., Taylor, D., Whitley, R. J., Yunusa, I., Zeppel, M. J. B., & Eamus, D. (2010). Root biomass distribution and soil properties of an open woodland on a duplex soil. Plant and Soil, 327, 377–388. https://doi.org/10.1007/s11104-009-0061-7
Makkonen, K., & Helmisaari, H.-S. (1998). Seasonal and yearly variations of fine-root biomass and necromass in a Scots pine (Pinus sylvestris L.) stand. Forest Ecology & Management, 102, 283–290. https://doi.org/10.1016/S0378-1127(97)00169-2
Miller, A. T., Allen, H. L., & Maier, C. A. (2006). Quantifying the coarse-root biomass of intensively managed loblolly pine plantations Canadian. Journal of Forest Research, 36, 12–22. https://doi.org/10.1139/x05-229
Moser, G., Leuschner, C., Röderstein, M., Graefe, S., Soethe, N., & Hertel, D. (2010). Biomass and productivity of fine and coarse roots in five tropical mountain forests stands along an altitudinal transect in southern Ecuador. Plant Ecology & Diversity, 3(2), 151–164. https://doi.org/10.1080/17550874.2010.517788
Njana, M. A., Eid, T., Zahabu, E., & Malimbwi, R. (2015). Procedures for quantification of belowground biomass of three mangrove tree species. Wetlands Ecology & Management, 23, 749–764. https://doi.org/10.1007/s11273-015-9417-3
Neumann, M., Godbold, D. L., Hirano, Y., & Finér, L. (2020). Improving models of fine root carbon stocks and fluxes in European forests. Journal of Ecology, 108(2), 496–514. https://doi.org/10.1111/1365-2745.13328
Niiyama, K., Kajimoto, T., Matsuura, Y., Yamashita, T., Matsuo, N., Yashiro, Y., Ripin, A., Kassim, A. R., & Noor, N. S. (2010). Estimation of root biomass based on excavation of individual root systems in a primary dipterocarp forest in Pasoh Forest Reserve, Peninsular Malaysia. Journal of Tropical Ecology, 26, 271–284. https://doi.org/10.1017/S0266467410000040
Ouimet, R., Camiré, C., Brazeau, M., & Moore, J.-D. (2008). Estimation of coarse root biomass and nutrient content for sugar maple, Jack pine, and black spruce using stem diameter at breast height. Canadian Journal of Forest Research, 38, 92–100. https://doi.org/10.1139/X07-134
Raich, J. W., Clark, D. A., Schwendenmann, L., & Wood, T. E. (2014). Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS ONE, 9(6), e100275. https://doi.org/10.1371/journal.pone.0100275
Resh, S. C., Battaglia, M., Worledge, D., & Ladiges, S. (2003). Coarse root biomass for eucalypt plantations in Tasmania, Australia: Sources of variation and methods for assessment. Trees, 17, 389–399. https://doi.org/10.1007/s00468-003-0250-6
Shupe, H. A., Hartmann, T., Scholz, M., Jensen, K., & Ludewig, K. (2021). Carbon stocks of hardwood floodplain forests along the middle Elbe: The influence of forest age, structure, species, and hydrological conditions. Water, 13(5), 670. https://doi.org/10.3390/w13050670
Sun, T., Dong, L., Mao, Z., & Li, Y. (2015). Fine root dynamics of trees and understory vegetation in a chronosequence of Betula platyphylla stands. Forest Ecology & Management, 346, 1–9. https://doi.org/10.1016/j.foreco.2015.02.035
Tamooh, F., Huxham, M., Karachi, M., Mencuccini, M., Kairo, J. G., & Kirui, B. (2008). Below-ground root yield and distribution in natural and replanted mangrove forests at Gazi bay. Kenya. Forest Ecology & Management, 256(6), 1290–1297. https://doi.org/10.1016/j.foreco.2008.06.026
Valiela, I., & Cole, M. (2002). Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems, 5, 92–102. https://doi.org/10.1007/s10021-001-0058-4
Vesterdal, L., & Raulund-Rasmussen, K. (1998). Forest floor chemistry under seven tree species along a soil fertility gradient. Canadian Journal of Forest Research, 28(11), 1636–1647. https://doi.org/10.1139/x98-140
Vogt, K. A. Vogt, D. J. & Bloomfield, J. (1998). Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. in root demographics and their efficiencies in sustainable agriculture, grasslands, and forest ecosystems, Ed. by J. E. Box (Kluwer, Dordrecht), pp. 687–721.
Wakawa, L. D. (2016). Biomass estimation in forest ecosystems-A review. Journal of Research in Forestry, Wildlife & Environment, 8, 126–144.
Yuan, Z. Y., & Chen, H. Y. H. (2010). Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. Critical Reviews in Plant Sciences, 29(4), 204–221. https://doi.org/10.1080/07352689.2010.483579
Acknowledgements
General Directorate of Forestry, Department of International Relations, Education and Research, for granting permission to work in Karacabey Floodplain Forest protected by the International Ramsar Agreement.
Funding
This research was funded by The Scientific and Technological Research Council of Turkey (TUBITAK) with the project number 219O146.
Author information
Authors and Affiliations
Contributions
TS designed the study, TS and MT collected the soil and root samples; SP measured stand characteristics; MT prepared the soil and root samples for analysis. TS, SP, and MT prepared the tables and figures. TS wrote the manuscript. All the authors contributed to editing and agreed on the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sariyildiz, T., Tani, M. & Parlak, S. First assessment of root biomass and root carbon and nitrogen stocks in Turkish floodplain forests. Environ Monit Assess 195, 148 (2023). https://doi.org/10.1007/s10661-022-10758-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10758-6