Abstract
Coastal lakes and estuaries are considered economic drivers for coastal communities by delivering invaluable economic and ecosystem services. The coastal ecosystems are facing recurrent hypoxia events (dissolved oxygen; DO < 2.0 mg L−1) and are emerging as a major threat to ecosystem structure and functioning. The Ashtamudi Lake, (area = 56 km2), is one of the Ramsar sites in the State of Kerala and located on the SW coast of India. The waterways are extensively used for backwater tourism and for fishery activities. This paper discusses the spatio-temporal variation of water quality attributes with emphasis on hypoxia during non-monsoon and monsoon seasons. The extent of hypoxia on fishery diversity was discussed. The Southern Zone, adjacent to the urban area, shows the hypoxic condition with higher concentration of BOD, NO3-N, and NH4-N. The hypoxic condition is largely limited to the Southern Zone in both seasons. The occurrence of low DO in the lake is highly related to salinity and organic load in the lake system. The tracking of pollution sources in the lake system was also done through identification of pollution potential zones and found that catchments adjacent to Southern and Western Zones (urban regions) are the major source of pollution. The study suggests that hypoxia is chiefly attributed to anthropogenic interventions in the form of discharge of wastes into the lake causing overloading of nutrients and organic effluents, decrease in the freshwater supply, the absence of proper freshwater mixing or dilution, and effluent discharge from nearby urban centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the modern era of urbanization, emerging low coastal lands studded with lagoons and estuaries constitute a significant proportion of coastal wetlands, providing essential goods and services to humankind as well as sustainable habitat for innumerable species of organisms (Chilton et al., 2021; NOAA, 2017a; Zapata et al., 2018). Moreover, estuaries are often hubs of economic activity for coastal communities and zones for international commerce, recreation, and other cultural services (Barbier, 2020; Bowd et al., 2012; NOAA, 2017b). Above all, estuaries render important regulating services such as water purification and carbon sequestration, while the associated salt marshes and mangrove forests act as biological filters (NOAA, 2017a), i.e., salt marshes and mangrove vegetations have a purification capability of contaminated water and sediments passing through their habitat (Chen et al., 2020; NOAA, 2017b; USEPA, 1993; Xie et al., 2022). In general, fall in dissolved oxygen (DO) in the coastal ocean and coastal lagoons also called coastal hypoxia is manifested in most (but not all) areas of the world (Diaz & Rosenberg, 2008; Breitburg et al., 2018; Rabalais & Turner, 2019) and has emerged as a major threat to coastal ecosystems globally and especially of estuaries (Friedrich et al., 2014; Jenny et al., 2016; Joshua et al., 2022; Lee et al., 2018; Rabalais & Turner, 2019; Tian, 2020; Rashid, 2021), where DO drops to < 2.0 mg L−1, and Nixon (2009), Diaz et al. (2010), Conley et al. (2011), and Diaz (2016) designate a DO of < 0.2 mg L−1 as anoxia. In aquatic organisms, hypoxia is measured as a primary limiting factor and temperature which controls the key factor for aerobic and metabolic capacity (Keeling et al., 2010; Pörtner & Lannig, 2009; Roman et al., 2019; Xiong et al., 2022). The estuarine and coastal lakes are currently facing severe ecological damage along with habitat loss due to recurring hypoxic condition by nutrient loading especially nitrogen compounds as a result of urbanization and absence of proper pollution abatement systems (Du et al., 2018; Howarth et al., 2011; Najjar et al., 2010). In aquatic ecosystems, hypoxic condition is considered the most severe due to eutrophication (DO < 2.0 mg L−1) (Zille´n & Conley, 2010; HELCOM, 2013; Hale et al., 2016). In poorly mixed bottom waters, the hypoxic and anoxic conditions were developed as a result of eutrophication along with high primary production, which also emphasize eutrophication by enhancing recycling of both nitrogen and phosphorus (Fennel & Testa, 2018; Zhu et al., 2017).
In recent decades, rapid increase in global temperature under rising carbon dioxide (CO2) increases the global and regional climate change (like El Nino or Southern Oscillation); furthermore, anthropogenic activities potently translate coastal and marine ecosystems to hypoxic/anoxic states. Researchers attributed the escalation in occurrence, extent, and dreadful of hypoxia to extreme anthropogenic loading of nutrients into aquatic systems—freshwater, brackish, and marine water bodies (Du et al., 2018; Jenny et al., 2016). Incidence of eutrophication due to increased nutrients (particularly N & P) input from non-point and point sources on aquatic systems steers to algal and phytoplankton blooms, whose death subsequently and consequently deplete the DO content in the aquatic ecosystem (Jiang et al., 2014, 2015; Pitcher & Probyn, 2011). Hypoxia drives loss and damage to aquatic biodiversity and results in extensive mortality events (Thronson & Quigg, 2008; Vaquer-Sunyer & Duarte, 2008; Rabalais et al., 2010; Rabalais & Turner, 2019). The arising of “dead zones” originated due to the process of eutrophication in coastal and marine ecosystems were increased tremendously in the last few years (Altieri & Gedan, 2014; Du et al., 2018).
Kerala’s coastal zone in India is unique with the presence of large number of backwaters (a.k.a kayals), exceptional with rich biological and genetic diversity (Thomson, 2001). Ashtamudi Lake (ASL), a coastal lagoon (wetland) in the SW coast of India, and one of three Ramsar sites in Kerala, receives the water and total load delivered by the Kallada River (basin area = 1699 km2 and length = 121 km) originating in the Western Ghats to the east and is characterized by a high level faunal and floral diversity (ENVIS, 2019; WRIS, 2016). The fishery resources from Ashtamudi using the gear castanet consist of 21 species of fish belonging to 14 families, and it also contains 2 species of crabs (Biju Kumar et al., 2012). Furthermore, crustaceans comprise of nine species, including eight of shrimp species and one species of prawn that was also reported from ASL, and Etroplus suratensis and Mugilidae support good fishery particularly in the central part of ASL (Vimal Raj et al., 2014). The estuarine regions support on the species Oxyurichthys tentacularis. The molluscan fishery resources in Ashtamudi Lake are dominated by clams and oysters, and Paphia malabarica (Ashtamudi short-necked yellow clam) is a significant species of the molluscan fauna in ASL (Mohamed et al., 2013).
Understanding that organism-specific physiological responses to environmental stressors, such as hypoxia, provide a mechanistic link between environmental change on the one hand and population-level effects on the other is perhaps a key to predicting trends of ecological impairments in the near future (Graham et al., 2004; O¨sterblom et al., 2007; Chown, 2012; Seebacher & Franklin, 2012; Cooke et al., 2013; Claireaux & Chabot, 2016; Almeida et al., 2017; Abdel-Tawwab et al., 2019). Various morphological changes in the ASL, adversely affecting the biodiversity of clams and productivity of ASL, were reported by Jim and Appukuttan (2016). Babu et al. (2010) pointed out how the freshwater discharge from the Kallada River plays a significant role in modulating the DO status and overall nutrient dynamics in ASL. Rabalais et al. (1998) highlighted the influence of freshwater inflow, nutrient load, residence time, stratification, turbidity, nutrient dilution, etc. in the ASL. The pollution potential zones of Akkulam-Veli Lake basin were identified and classified as very high or high pollution potential zones (say hypereutrophication zone) (Sheela et al., 2016). The contaminated water discharge drains originating from the very high pollution potential zone are mainly responsible for the hypereutrophic condition of lake. The factors responsible for the very high pollution potential in Akkulam-Veli Lake is high urban population density, extent of industrial and commercial activity, and land use with mixed tree crop, level, and gentle slope (Sheela et al., 2016).
There is an urgent need to identify the source of pollution and to provide abatement measures for avoiding pollution in the inland water bodies. As there is an emergent need for improved understanding of the current state and trends, in the present paper, we report the outcomes of a water quality monitoring program especially on the state of dissolved oxygen (DO) in the Ashtamudi Lake (ASL), one of the Ramsar sites in the State of Kerala in the SW coast of India. The classification of the lake based on prominent factors has also been made. The lake is also divided based on prominent factors. The assessment of lake catchment was done by multi-thematic overlay analysis with different characteristics to recognize various pollution potential zones using GIS. The results will provide useful insights into the spatio-temporal variability of changes and their drivers, as well as threats these impose to the local coastal and marine environment. Hopefully, the results would help the managers and planners with sufficient knowledge to sustainably utilize the ASL.
Study area
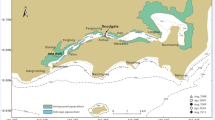
The Ashtamudi Lake (8°53′ to 9°02′N lat. and 76°31′ to 76°41′E long.), a shore-perpendicular (Joseph & Thrivikramji, 2002), brackish water body (max. length = 12 km; width = 2.5 km; area = 56 km2), is the second largest estuary after Vembanad Lake in Kerala, India. The name Ashtamudi is attributed to the rough resemblance of the lake shoreline to an eight-leaved clover in plan view (Fig. 1). The Ashtamudi Lake (ASL), connected to the Laccadive Sea through an inlet at Neendakara and an important fishing harbor, is one of the deepest among Kerala’s Kayals/estuaries (NCESS, 2017) and above all is a rich source for many fish varieties. Kollam City, a fast-growing urban area, is situated on the SW part of the ASL (Fig. 1). During monsoon, the basin experiences maximum rainfall from June to September.
Lake basin
The major river, River Kallada, is discharging into the Ashtamudi Lake at Peringalam near Kollam. Kallada River originates from the Western Ghats and discharges to the Ashtamudi Lake after traversing about 121 km. During the course of travel of river, it is not passing through any city, and the major portion of river passes through the forest area. The basin registers a mean rainfall of 2800 mm and a mean streamflow of 3374.86 Mm3 annually (ENVIS, 2019). The basin experiences moderate and uniform wind velocity throughout the year. Generally, it varies from 8.5 to 13.6 km/h (WRIS, 2016).
Freshwater inflow from the Kallada River enters at the NE of ASL, and strong tidal influence from the coastal sea at the west helps flush contaminants out and dilute the pollution load. Kerry and Baba (2001) divided the ASL into six subzones (Fig. 2), viz., Southern (Zone I), Estuarine (Zone II), Western (Zone III), Central (Zone IV), Riverine (Zone V), and Eastern Zone (Zone VI). In this study, in respect of DO levels and distribution, we follow the scheme of Ekau et al. (2010).
Sample collection and analysis
In the field campaign, water samples were collected from both surface and bottom from 58 locations (Fig. 1) at low tide during the early hours of non-monsoon (NMN-month of May) and monsoon (MN, September) seasons of 2017. The sampling stations have been identified based on previous research reports and publications (Babu et al., 2010; Kerry & Baba, 2001; Sujatha et al., 2009). Samples of both surface and bottom waters were collected (using a standard Niskin sampler—General Oceanics, 5L capacity) from 58 locations in ASL. Sampling spanned over 3 days onboard a mechanized boat in the early morning hours during the low tide. The surface water samples were collected from 0.30 m below the water surface and bottom water samples from above 0.30–0.50 m from the lakebed and subzones of Kerry and Baba (2001). Surface water was sampled using a clean steel bucket, while bottom water was collected using Niskin Water Sampler (capacity of 2L). The water transparency was measured on site by Secchi disc. The samples for DO were fixed onboard and brought to the laboratory for further analysis, and the remaining water samples were collected in acid cleaned and pre-rinsed polythene bottles and carried to the laboratory under refrigeration for subsequent analysis. Temperature, pH, conductivity (Horiba: LAQUAact 100 Series), and transparency (with Sechi disc) (Strickland & Parsons, 1972) were determined in the field itself. DO after fixing in the collection site in the field was determined by modified Winklers method (Strickland & Parsons, 1972). Salinity was measured by Mohr-Knudsen titration method (Grasshoff et al., 1983). Nutrients, viz., ammonia–nitrogen (NH4-N), nitrate-nitrogen (NO3-N), nitrite-nitrogen (NO2-N), and inorganic phosphate phosphorus (PO4-P) were estimated by colorimetric methods on filtered samples (0.45-μm Millipore) using a double-beam spectrophotometer (Systronics, Model AU-2701) after the standard method (Grasshoff et al., 1983; Parson et al., 1984; Strickland & Parsons, 1972; Zhang & Fisher, 2006).
Methodologies adopted
Criteria of classification
The classification of lake water is done based on the scheme of Ekau et al. (2010). The class is “hypoxic” when DO level is < or = 2 mg L−1, anoxia or “no-oxygen” is for DO < 0.2 mg L−1, while DO between 2 and 4 mg L−1 is “low oxygen” condition. In “oxygenated” condition, DO is > 4.0 mg L−1.
Quality check and statistical analysis
The quality of obtained data is analyzed by deriving relative difference percentage (RPD) from the data set. All physical parameters estimated in the period had a relative difference % ranges up to 0.5–5%. The relative difference of % ranges of nutrient parameters is 4.5–9.0. The general relationship and their degree of relationship between variables, in the surface and bottom water samples, were obtained through principal component analysis (PCA) with Varimax, Kaiser normalization with rotated matrix and Pearson Correlation analysis of data provided an insight into physic-chemical anatomy of ASL waters during the study period. The Euclidean distance method and Ward’s method are used for the hierarchical cluster analysis (HCA).
Statistical analysis
The statistical analyses were done by IBM SPSS-V.20 and R studio software; spatial modeling (ArcGIS v. 10.8) of data provided an insight into physico-chemical framework of ASL. The pollution potential zones in the Ashtamudi Lake have been identified based on the study conducted for Akkulam-Veli Lake (Sheela et al., 2016).
Results and discussion
Variation of water quality in the lake system
Water quality variability in the freshwater systems depends on the various physico-chemical parameters, sources of pollution, activities (microbial, degradation process, nutrient cycling, etc.) going-on inside the systems. Table 1 summarizes the physico-chemical data of MN and NMN water samples of ASL. Box plots (Figs. 3 and 4) depict seasonality of variations and averages of data for surface and bottom samples. Water depth ranged between 1.0 and 10.5 m, and the average depth is 2.50 ± 1.41 m during NMN and is 4.18 ± 1.57 m during MN. The minimum depths in NMN were reported from the Southern Zone (Zone I: S13 and S33) and Central Zone (Zone III: S35), while maximum depth occurred in the Eastern Zone (Zone VI: S53) agreeing very closely with Kerry and Baba (2001), Babu et al. (2000), and Babu et al., 2010. The shallow nature in Southern Zone may be due to urban discharge from Kollam City and that in Central Zone may be due to intrusion of mud with tidal influx.
The habitat, diversity, and assemblage of phytoplankton community structure in aquatic systems were controlled by the water transparency which depends on the incoming river discharge, depth of the water column, mixing and stratification, and also resuspension mechanism of sediments (Savage et al., 2010; Lehtinen & Laamanen, 2012). The average water transparency was 0.57 ± 0.21 m during NMN and 0.57 ± 0.16 m during MN seasons. During NMN, the minimum transparency (0.20 m) was observed in the Southern Zone (Zone I), mirroring the free entry of wastewater from the urban tracts of Kollam City. The maximum value of 1.30 m occurred in the Eastern Zone (Zone VI). But, in MN, the minimum value was observed in the Riverine Zone (Zone V) adjacent to the Kallada River mouth (S41). The observed low transparency in the river discharge regions, which shows a significant linear relationship between distance to the open water to the transparency of water column due to the incoming river storm discharge, was confirmed by Wu et al. (2015) and Fabricius et al. (2016).
The pH of surface and bottom waters for both seasons were in similar ranges, i.e., from neutral to mildly alkaline. For example, during NMN, the average pH of surface water was 7.5 ± 0.5, and in MN, it was 7.3 ± 0.45. A slight reduction in pH value was observed during MN. In the present study, pH is minimum in Riverine Zone and maximum in Estuarine Zone, and similar results were also reported by Babu et al. (2000) in ASL. Comparatively lower pH in Southern Zone is due to urban discharge from Kollam City. The organic matter decomposition results in the formation of biogenic carbon dioxide, and it reduces the pH condition of aquatic system (LaRowe et al., 2020). The bottom water showed an average pH of 7.90 ± 0.5 in NMN and 7.7 ± 0.40 during MN. In ASL, the bottom water shows higher pH than surface water. Sujatha et al. (2009) reported similar conditions in ASL, and the pH distribution pattern is in agreement with an earlier study by Kerry and Baba (2001). According to Babu et al. (2000), the incursion of highly saline sea water through the bottom layer as evidenced by the high salinity values in ASL.
The salinity fluctuated in surface waters in NMN season from 12.6 to 32.4 PSU, and in MN season, it fluctuated from 11.80 to 31.20 PSU. The salinity of surface waters remains higher throughout the year with a mean salinity of 22.82 ± 4.73 PSU in NMN and 22.60 ± 4.77 PSU for MN season. During NMN, average salinity concentration in surface water was 22.82 ± 7.73 PSU, whereas, for bottom water, it was 24.15 ± 4.5 PSU. However, during MN, average salinity concentration for surface water was 22.60 ± 4.77 PSU and for bottom water was 22.82 ± 4.86 PSU. The Southern, Estuarine, and Western Zones show higher salinity compared to other zones. The riverine discharge from Kallada River is the reason for the comparatively lower salinity in the Central, Riverine, and Eastern Zones.
The Kallada River discharge plays a significant role in the distribution of salinity in ASL in both seasons. The river discharge into ASL showed a slight increase from 42.68 (NMN) to 57. 83 m3/s (MN) during 2017, yet did not make any significant dilution of salinity. Later, Jennerjahn et al. (2008) observed that the riverine region of ASL remained brackish (i.e., 18.0 PSU) in October 2003 with a mean monthly discharge of 60.0 m3/s, while the estuarine region stayed above 30 PSU, which is similar to our results. Similar observations of steady brackish water presence in the lake throughout the year were recorded by Babu et al. (2010) and Salahudeen et al. (2018). The depth of lake also holds a significant role in the distribution of salinity gradient of bottom waters, especially vouched by the structural make up of biota in aquatic ecosystems (Ysebaert et al., 2003; Basset et al., 2013; Cloern et al., 2017; Cañedo-Argüelles et al., 2018).
The freshwater zone could not be found even in the MN. This is a serious condition to be looked into. This may be due to the high rate of evaporation, low freshwater input, and strong bottom currents in the lake water. This is in line with the observation of Divya (2015). According to Nair et al. (1983), there were 97 species of fish, comprising 68 species of finfish (24 marine, 9 freshwaters, 4 estuarine, transient forms), crabs with 5 species, and also consists with mollusks and prawns with 9 species. Salinity in the lake could have an impact on the existence of freshwater fishes in ASL.
The temperature of coastal lake waters is strongly influenced by variations in river water input and tidal exchange, annual precipitation rate, solar radiation flux, and atmospheric pressure (Govindasamy, 2000; Senthilkumar et al., 2002; Sukumaran et al., 2013; Balakrishnan et al., 2017). During MN and NMN seasons, surface water temperature stood higher than that of bottom water. For example, in NMN, the average surface water temperature was 28.10 ± 0.79 °C, while that of bottom water was 27.20 ± 1.25 °C. During MN, temperatures of surface and bottom waters were 29.8 ± 1.11 °C and 28.8 ± 1.14 °C, respectively. Temperature trend in the ASL is in agreement with earlier studies of Sujatha et al. (2009), Babu et al. (2010), and Antony and Ignatius (2015). The low bottom water temperature in Ashtamudi Lake is most probably due to the intrusion of dense and comparatively cooler sea water through bottom layers and the high salinity values of bottom water (Babu et al., 2000, 2010). The high surface water temperature in lake may be due to lower riverine discharge. This is in line with the finding of Krishnan et al. (2021) regarding the rise in sea surface temperature in Arabian Sea.
The average concentration of nitrate-nitrogen (NO3-N) in the surface water samples fluctuated from 0.09 to 4.45 mg L−1 (av. 1.15 ± 0.97 mg L−1) and 0.03 to 2.49 mg L−1 (av. 0.63 ± 0.50 mg L−1) in NMN and MN seasons, respectively. The NO3-N concentration in the bottom waters spans from 0.12 to 6.12 mg L−1 (av. 1.60 ± 1.40 mg L−1) and 0.06 to 3.69 mg L−1 (av. 1.01 ± 0.78 mg L−1) in NMN and MN, respectively. Nitrite-nitrogen (NO2-N) concentration in NMN had an average value of 0.08 ± 0.06 mg L−1 and 0.18 ± 0.1 mg L−1 in surface and bottom waters, respectively. In MN, it averaged 0.04 ± 0.04 mg L−1 and 0.11 ± 0.04 mg L−1 in surface and bottom water samples, respectively.
The dissolved inorganic ammoniacal nitrogen (NH4-N) concentration highly fluctuated between the surface and bottom water regions in both seasons. NH4-N concentration in the surface water and bottom water samples had an average of 1.85 ± 1.06 and 1.00 ± 0.67 mg L−1 in NMN and MN, respectively. The concentrations in bottom water samples had a higher average value of 3.01 ± 1.70 and 1.71 ± 1.28 mg L−1 in NMN and MN, respectively.
The biological oxygen demand (BOD) in the surface water samples ranged from 1.63 to 30.36 mg L−1 (av.8.60 ± 6.82 mg L−1) in NMN and in it varied from 1.03 to 22.76 mg L−1 (av. 5.44 ± 4.50 mg L−1) during MN. In bottom waters, the average concentration of BOD was average 15.92 ± 5.12 mg L−1 during NMN and was 14.46 ± 3.89 mg L−1 in MN.
Organic matter (BOD) concentration was higher in location S1 (30.36 and 35.06 mg L−1) in the Southern Zone. The content of organic matter (BOD) nitrate, nitrite, and ammoniacal nitrogen was also observed to be maximum in the Southern Zone. Comparatively higher concentrations were also observed in Estuarine and Western Zones, followed by Central Zone. Still higher concentrations were observed in the bottom water. This research brings to light the overload of inorganic nutrients such as nitrate-nitrogen, nitrite-nitrogen, and ammonia–nitrogen in the NMN, both in surface and bottom waters of ASL and especially in the southern region due to its adjacency to Kollam urban tract in that samples from this southern region reported higher levels of all the aforesaid nutrients in surface and bottom water during NMN and MN, respectively.
The concentration of inorganic phosphate phosphorus (PO4-P) in NMN had an average of 0.32 ± 0.09 and 0.38 ± 0.10 mg L−1 in both surface and bottom water samples. The phosphate concentrations in MN had an average of 0.17 ± 0.07 and 0.21 ± 0.09 mg L−1 in surface and bottom waters, respectively. The locations with minimum phosphate concentrations in the surface waters for both seasons were observed at the Estuarine Zone, and the maximum concentrations were observed at the Riverine Zone. Comparatively higher concentrations were observed in Riverine and Eastern Zones.
Table 2 is the correlation matrix of estimated parameters of surface and bottom waters of ASL in NMN and MN seasons. Salinity shows significant strong positive correlation of surface waters in both seasons with EC (r = 0.976, r = 0.984) and TDS (r = 0.987, r = 0.987) and significant positive correlation with pH (r = 0.579, r = 0.388). Dissolved oxygen shows negative correlation with EC (− 0.445, − 0.419), TDS (− 0.443, − 0.389), and salinity (− 0.425, − 0.362) in surface waters due to the consumption during oxidation of organic matter and stratification by salinity. DO of water declines with increase in salt level, and for a given temperature and pressure conditions, DO holding capacity of freshwater is about 20% higher than that of saline (Sherwood et al., 1991; Palmer et al., 2008; Cañedo-Argüelles et al., 2018; Anifowose et al., 2020). The growth rate of certain microbial communities in the aquatic ecosystem was controlled by physicochemical gradients of numerous parameters such as pH, salinity, and DO arose due to the mixing between freshwater and saline water (Goñi-Urriza et al., 2007; Spiteri et al., 2008; Basil George et al., 2012; Spietz et al., 2015).
In NMN season, DO shows strong significant negative correlation with BOD (r = − 0.849, − 0.848), NO3-N (r = − 0.825, − 0.838), and NH4-N (r = − 0.844, − 0.871) (p = 0.001) with surface and bottom waters (Table 2). In MN season, DO also shows strong significant negative correlation with BOD (r = − 0.867, − 0.961), NO3-N (r = − 0.840, − 0.838), and NH4-N (v-0.839, − 0.900) (p = 0.001) with surface and bottom waters. NH4-N shows significant positive correlation with BOD (r = 0.912, 0.863), NO3-N (r = 0.980, 0.944), and NO2-N (r = 0.912, 0.874) in surface waters of both NMN and MN seasons. The present study reveals that the DO concentration in the surface and bottom water significantly shows strong negative correlation with NO3-N, NO2-N, and NH4-N (DIN) in both NMN and MN seasons, indicating strong DIN loading in the ASL throughout the year.
Status of dissolved oxygen condition in the lake water
Dissolved oxygen (DO) plays a significant role in the metabolism of aquatic organisms in wetland ecosystems (Antony & Ignatius, 2015). The distribution, abundance, assemblage, and structure of phytoplankton species are related to water quality especially DO concentration (Bhateria & Jain, 2016; Gammal et al., 2017; Sisodia & Chaturbhuj, 2006). Dissolved oxygen level ranges between 1.63 and 5.76 mg L−1 (av. 4.13 ± 0.88 mg L−1) in surface water and 0.81 to 3.38 mg L−1 (av. 2.78 ± 0.60 mg L−1) in bottom waters, respectively, during NMN. But a small rise in DO content was observed during MN. DO ranges from 1.86 to 5.9 mg L−1 (av. 4.40 ± 0.80 mg L−1) and 0.99 to 4.63 mg L−1 (av. 3.13 ± 0.63 mg L−1) in surface and bottom samples, respectively. The surge in mixing and water flow in aquatic systems due to rainfall increases oxygenation and DO fluctuations in the water column in tropical lake systems (Richards, 2008; Singh et al., 2019; Liu et al., 2020). When the saturation of oxygen becomes < 40% (i.e., DO < 4 mg L−1), it also intensifies concentration of CO2 in the water up to 10 mg L−1.
Table 3 and Figs. 5 and 6 demonstrate the zonal and temporal variation of DO and BOD in surface and bottom waters of ASL. DO was maximum in Riverine Zone (Zone V) and minimum in Southern Zone in both NMN and MN seasons. The Southern Zone (Zone I) in particular shows the minimum DO in both surface (av. 3.31 ± 0.68 and 3.50 ± 0.69 mg L−1) and bottom waters (av. 2.32 ± 0.84 and av. 2.54 ± 0.64 mg L−1) during NMN and MN seasons, respectively. The Southern Zone closer to Kollam urban tract registered a lower DO corresponding to acute hypoxic state in both seasons. Bottom waters in both seasons showed low DO (viz., 2–4 mg L−1) which is close to hypoxia, especially for the bottom feeders in that part of ASL. According to Nair et al. (1984), the southern portion of the lake is affected by waste disposal from urban wastes from Kollam City including from hospitals, boat building yards, and oil spillages. In Estuarine (Zone II) and Western Zones (Zone III), DO was comparatively lower in surface and bottom waters of NMN season. According to Rabalais and Turner (2019), such DO levels may negatively affect habitat of a host of bottom dwellers (viz., fish and benthic organisms) and pelagic fishery diversity, furthering mortality, predation, reduced food resources, altered trophic energy transfer, modified bioenergetics (i.e., physiological, development, growth, and reproductive norms) and migration patterns.
Spatio-temporal variation and classification (Ekau et al., 2010) of dissolved oxygen (DO) in the water samples of Ashtamudi Lake in a surface water of NMN season, b Bottom water of NMN season, c surface water of MN season, and d bottom water of MN seasons
Specifically, during NMN season, the Estuarine Zone (Zone II) has a trend of low oxygen in the surface (av. 3.71 ± 0.20 mg L−1) as well as bottom (av. 3.04 ± 0.27 mg L−1) waters, while during the MN, the surface water recorded a rise in DO (av. 4.12 ± 0.27 mg L−1). In contrast, still the bottom water in the Estuarine Zone in MN (av. 2.62 ± 0.38 mg L−1) exhibited low DO conditions in the Estuarine Zone. Again, the Western Zone (Zone III) also has a similar DO trend as that of Estuarine Zone. The Southern, Estuarine, and Western Zones are on the seaward part of the ASL, and the Estuarine Zone especially is the passage way directly linked to the sea. The decline in the DO level on the seaward side is similar to the observations done by Sherwood et al. (1991) that DO of water declines with an increase in salt level, and for a given pressure and temperature conditions, saltwater holds about 20% less DO than freshwater. Moreover, the mean seasonal DO of surface waters in the Central (Zone IV; 4.47 ± 0.67 mg L−1 and 4.89 ± 0.49 mg L−1), Riverine (Zone V; 5.24 ± 0.43 mg L−1 and 5.51 ± 0.45 mg L−1), and Eastern Zones (Zone VI; 4.67 ± 0.47 mg L−1 and 5.00 ± 0.50 mg L−1) were not in low DO state (2–4 mg L−1) during NMN and MN seasons. However, the bottom waters in these zones were in deficient of DO state similar to the other zones. The MN season improves the DO of bottom waters in Central, Eastern, and Riverine Zones.
The low DO concentration in ASL water associated with the turbid conditions even in the MN seasons diminishes the nitrification process, and it leads to the higher concentration of ammonium ions in both surface and bottom waters, especially in the bottom water. Unionized ammonia may cause toxicity to bacterium such as Nitrosomonas sp. and Nitrobacter sp. hindering the nitrification process (Grommen et al., 2002). The increase in water temperature and pH intensifies toxicity of unionized ammonia (NH3) as exponentially to aquatic organisms, especially fishes (Hargreaves & Tucker, 2004; EPA, 2013; Yan et al., 2019). The central nervous system of fishes is affected by the acute toxicity of ammonia (Kim et al., 2019; Xu et al., 2021). The dissolved ammonia concentration causes mass mortality of fishes and species like Oreochromis mossambicus in the aquatic system when its concentration raised up to 7.40 mg L−1 (Benli & Koksal, 2005).
Extent of hypoxic, low oxygen and oxygenated condition in the lake
Figure 5 shows the areal classification of DO in the lake during MN and NMN seasons. The hypoxic condition exists in 2% (1.13 km2), and low oxygen condition exists in 39.04% (21.50 km2) and oxygenated condition to the tune of 58.96% (33.54 km2) area in the lake during NMN. During MN, hypoxic condition decreases to 0.19% (0.11 km2), low oxygen area in 16.21% (8.92 km2), and oxygenated condition in 83.60% (46.05 km2). In ASL during MN season, the surface waters show an enhancement in water quality. The stress in fish population can be observed during NMN season because of the existence of low oxygen conditions in the lake. The Southern Zone, Estuarine Zone, and Western Zone are affected areas. Further, in the bottom waters, the extent of hypoxic conditions is 9.46% (5.39 km2) in NMN and low oxygen 90.54% (50.61 km2). During MN, the hypoxic state covered is 7.25% (4.06 km2), low oxygen condition is 92.75% (51.94 km2), and oxygenated condition stood at 0.98% (0.54 km2).
The DO, essential for all aquatic living organisms, in that fishes are the most sensitive to hypoxia, among aquatic organisms and fishery populations causes severe loss and damage due to hypoxic events (Bernard et al., 2014; Chamberlain et al., 2020; Hrycik et al., 2017; Roman et al., 2019). The species existing as benthic organisms required a minimum concentration of DO (1–6 mg L−1); while there is a much higher DO (4–15 mg L−1), concentration is required for shallow water fish species (Tomasetti et al., 2018). Later, Vaquer-Sunyer and Duarte (2008) reported that thresholds of hypoxia span over a broad range between DO of 0.29 to 4 mg L−1, but on average, when DO level drops below 2.61 mg L−1, sub-lethal effects on marine benthic macro-organisms occur while lethal conditions dawn at 2.05 mg L−1, yet many taxa of crustaceans and fish demonstrate negative impacts of hypoxia near 4 mg L−1. Piontkovski et al. (2012) reported DO of < 2.0 mg L−1 leads to an incidence of fish kill, and while DO plunges to < 3.0 mg L−1, fish population is stressed. In an aquatic ecosystem that has eutrophic and hypereutrophic status experiences, most severe cases of hypoxia and anoxia associated with fish kill incidents to both invertebrates and vertebrates species (Misra & Chadurvedi, 2016; Lu et al., 2018; Budnik et al., 2021). The formation of different reduced compound species like H2S and ammonia–nitrogen due to a decline in DO concentration results in added adverse effects on aquatic fauna (Wetzel, 2001; Breitburg, 2002; Pajares & Ramos, 2019).
The fishery catch of ASL is dominated by Etroplus, and shrimp species from the Central, Estuarine, and some part of Southern Zones were reported by Sinclair et al. (2021). The brackish water shrimps and crab species had a threshold level DO concentration of 3–3.5 mg L−1 (Allison et al., 2018; Dean & Richardson, 1999). Below the threshold level, high mortality to the shrimp species will occur especially for juvenile species, and in hypoxic conditions between 1 and 2 mg L−1, 80% mortality of adult shrimps will occur (Dean & Richardson, 1999; Mugnier & Soyez, 2005; Mugnier et al., 2008). In the present study, the surface and bottom waters from Southern, Estuarine, Western, and Central Zones in both NMN and MN seasons were below 3.5 mg L−1, and some of the locations in Southern Zone completely fall under hypoxic conditions. The bottom water in ASL shows a severe decline of DO especially in the Southern Zone which may affect the diversity and abundance of crab and benthic species in ASL. The Riverine and Eastern Zones show comparatively higher DO concentrations between 4 and 5 mg L−1. The carb species are mostly associated along with the mangrove vegetation which is located in the Estuarine and Southern Zones of ASL. Vimal Raj et al. (2014) observed a decline of fishery diversity of ASL especially crabs and shrimps from 2 species of crabs and 9 species of shrimps from 8 species of crabs and 15 species of shrimps in 1981.
There was a decline in the fishery diversity among the marine, estuarine, and freshwater finfish, crabs, and shrimps of ASL from 1980 to 2018 (Suseelan & Kathirvel, 1982; Nair et al., 1983; Biju Kumar et al., 2012; Raju, 2011; Vimal Raj et al., 2014; Venkatesan et al., 2015). The hypoxic condition along with steady low oxygen condition in the surface and bottom waters especially the Southern Zone directly affects the fishery diversity and its abundance in ASL along with the nutrient pollution.
Spatial distribution of water quality assessment using cluster analysis
The dendrogram prepared with HCA for the surface waters of NMN and MN season on the basis of water quality sampling sites is presented in Fig. 7. The locations consisting of surface waters are grouped into four major clusters (I–IV). The first cluster of both NMN and MN (Fig. 7a, c) consists of locations in the Riverine, Eastern, and a few locations in Central Zone adjacent to Riverine Zone (S38-S58). The first cluster consists of a wide area of lake with maximum depth with comparatively low salinity, NO3-N, NO2-N, NH4-N, and BOD. It is also observed that regions are sufficient with DO concentration. The freshwater river discharge to the lake is the reason for the sufficient dilution of salinity and DIN concentration in these regions. The river water discharge plays a significant role in DO concentration in ASL. The freshwater discharge from Kallada River plays a significant role in modulating the DO status and overall nutrient dynamics in ASL (Babu et al., 2010). According to this analysis, Riverine Zone and some part of Central Zone are having low saline and organic influential areas and can be considered oxygenated areas.
The second cluster of both NMN and MN (Fig. 7a, c) seasons consists of locations belonging to the highly urbanized Southern Zone (S1-4, S11) and the water way towards the regions of Estuarine Zone; it belongs to the narrow portion of ASL, with low water depth. The higher concentrations of NO3-N, NO2-N, NH4-N, and BOD were observed in this cluster with low DO concentration. The high NO3-N indicates the oxidation of NH4-N to NO3-N and high loading of nutrients and organic matter which causes the high concentration of BOD and low DO by the microbial respiration due to the organic matter decomposition. Southern Zone is organic polluted area. This cluster can be termed as urban region cluster.
Cluster III of NMN and MN seasons consists of locations that belong to the Estuarine Zone (S16-S24, S33) having high salinity, which are dominated by the strong tidal influx from sea. Estuarine Zone can be considered high saline area. The cluster IV consists of the location that belongs to the different zones (Zones I, II, III, and IV) having intermediate salinity and DO concentration with sufficient nutrient concentrations. A similar classification was observed for bottom waters also (Fig. 7b, d).
Assessment of water quality using factor analysis
In NMN season, factor 1 shows strong positive loading of BOD (0.948), NO3-N (0.968), NO2-N (0.953), and NH4-N (0.973) along with strong negative loading of DO (− 0.863) (Tables 4 and 5). In NMN season, bottom waters show strong positive loading of DIN (NO3-N, NO2-N, and NH4-N) along with the strong negative loading of DO. Almost the same trend exists during MN. The study reveals that factor 1 can be termed as the urban waste factor which occurred due to the discharge of urban wastes including solid and liquid wastes from the city area in the catchment of the lake on the Southern Zone (Zone 1). The strong positive loading with BOD, NO3-N, NO2-N, and NH4-N indicates the discharge of organic matter and DIN in this part of the lake. The eutrophication and DO depletion in aquatic bodies were driven by nutrient loads and BOD concentration (Alam et al., 2020; Bargu et al., 2019). Figure 8 shows the spatial distribution of urban waste factors in the surface water samples of NMN season. Urban waste factor dominates in the Southern Zone with an area of 8.86 km2 and 8.1 km2 in surface waters during both seasons respectively, which is more than 90% of the area that belongs to Southern Zone (area = 9.75 km2). During NMN, the spreading of urban waste factor in the Estuarine Zone (Zone 2) can be observed for an area of 0.78 sq. km. The higher concentrations of NO3-N, NO2-N, and NH4-N were observed at the Southern Zone in both surface and bottom samples and in the two seasons. The DIN with higher numerical values compared to previous studies (e.g., Sujatha et al., 2009; Babu et al., 2010; Venkatesan et al., 2015, Santu et al., 2016) have been observed, and similar behavior at par with Salahudeen et al. (2018) has also been observed. In the past decades, DIN higher values in Coastal Zone chiefly by anthropogenic actions led to the deterioration of water quality and ecosystem degradation. The ASL data analysis reveals that Southern Zone (Zone I), skirted by the urban tract of Kollam City, has an overload of organic matter from various anthropogenic sources (Aswathy et al., 2021; Babu et al., 2010; Reshmi et al., 2015; Sujatha et al., 2009). In coastal, marine, and estuarine ecosystems organic matter accumulation from different sources such as in the form of municipal sewage, industrial and agricultural effluents are the factors contributing NO3-N (Babu et al., 2010; Chen et al., 2012; Philips et al., 2002; Razumov & Tyutyunova, 2001).
The bottom DO has a significant negative loading along with the positive loading of DIN but not with the DIP. This indicates the increased mineralization of organic detritus. The various non-point and point sources of organic matter rich discharge from different sources such as municipal solid wastes, surface runoff from agricultural lands, livestock operations, septage, and sewage effluents are the major sources of origin of BOD. The high concentration of BOD associated with depleted DO concentration also induces high productivity of phytoplankton species due to the enrichment of nutrients like DIN (Granéli & Turner, 2006; Bricker et al., 2008; Heisler et al., 2008; Wu et al., 2016; Zhao et al., 2020). The intense anthropogenic activities adjacent to the coastal lakes and wetlands such as urbanization and aquaculture attribute DIN loads in water especially ammonium (Antony & Edward, 2019; Hao et al., 2021; Li et al., 2014).
This research brings to light the overload of inorganic nutrients such as NO3-N, NO2-N, NH4-N, and PO4-P in the NMN, both in surface and bottom waters of ASL and especially in the Southern Zone I due to its adjacency to Kollam urban tract in that samples from this Southern Zone reported higher levels of all the aforesaid nutrients in surface and bottom water during NMN and MN, respectively. The study reveals that Factor 2 shows strong positive loading with EC (0.968, 0.984), TDS (0.962, 0.970), and salinity (0.979,0.982) and a negative loading with DO (− 0.331, − 0.247) and PO4-P (− 0.934, − 0.962) with a variance of 39% and 37%. This factor is termed as Sea Saline factor. Figure 8 shows its spatial distribution in the surface water samples during both seasons. Sea Saline factor covers the locations belonging to the Estuarine Zone (Zone II) completely (area = 4.75 km2) in NMN and MN seasons. Sea saline factor covers an area of 10.63 km2 and 10.14 km2 Central Zone (Zone IV) in both seasons. An area of 3 km2 in Southern Zone (Zone I) is also affected by Sea Saline factor in both seasons.
The bivariate loading plots of F1 vs F2 reveals two major groups, viz., anthropogenic sources from the urban stretches and saline water of ASL, which controls the hydrochemistry of ASL. Among these, saline water interaction is the dominant factor which derives from the adjacent sea and Estuarine Zone (Zone II) but having comparatively higher DO than the Southern Zone (Zone I) with polyhaline condition during study period and high discharge of freshwater and sewage to the ASL which hinders the exchange of dissolved oxygen between surface and bottom regions due to the salinity stratification (Janout et al., 2020; Kuo & Nielson, 1987; Pein et al., 2021). The thin surface water layer will flow as a coherent layer, exhibiting little mixing with the denser bottom water layer due to stratification. The little mixing with bottom water layers, the dissolved oxygen in surface layers of water remains unchanged. The Riverine (Zone V) and Eastern Zones (zone VI) show strong negative values in factor analysis in Urban waste factor and Sea saline factor. The freshwater discharge from the Kallada River plays a significant role in the DO concentration in these regions.
The increase in nutrient concentrations in Southern Zone (Zone I) compared to other parts of the lake reveals that the enrichment of nutrients is due to the input from TS canal (Trivandrum Shornur Canal)—a shore parallel canal carrying effluents and various forms of discharges from urbanized regions of Southern Kerala. The higher concentrations of NO3-N, NO2-N, and NH4-N were observed at the station “TS canal joining region (S2)” in both surface and bottom samples and in the two seasons.
The effluent discharge enriched with organic matter from different pathways accumulates in the southern and south-western regions of ASL. The hypoxic (< 2.0 mg L−1) condition is evolved, when organic matter oxidation is occurred by using dissolved oxygen in ASL, especially during NMN season (Dutton et al., 2018; Jessen et al., 2017; Ra. et al., 2014). In the south-west regions (Southern and Estuarine Zone) of ASL, there are a large number of point and non-point municipal solid waste and sewage discharge sources. The population status of Kollam Corporation, which was 0.32 million during 2001, has increased to 0.39 million (2011) in the last 10 years, and the waste generation is also raised from 74 tons per day to 104 tons per day without sufficient waste collection, treatment facilities, and solid waste management plants (Census of India, 2011; KSPCB, 2020; Sitaram, 2014). The coastal, estuarine, and marine ecosystems facing the ecological and ecosystem damages due to the widespread hypoxic events arise from anthropogenic activities, especially nutrient loading in the absence of sustainable pollution, waste monitoring management (Howarth et al., 2011; Malone & Alice, 2020; Kelly et al., 2021).
Anthropogenic pressures led to manifest reduction or shrinkage of the water spread area due to reclamation, hike in supply of fertilizer residue (nutrient) load due to intensive farming in the Kallada basin, extensive in-stream sand mining, and increased fishery activity. This is in agreement with the findings that dumping of industrial and municipal wastewaters of Kollam City and other settlements of the hinterland has caused the environmental degradation of the lake (Aswathy et al., 2021; Sinclair et al., 2021; Sreedevi et al., 2016). The goal of improvement in ecosystem health and services is achieved only through managing and reducing the hypoxic conditions with reducing nutrient load in the aquatic ecosystem (Conley et al., 2009; Angela et al., 2015). The factor analysis also reveals that the locations in the southern, estuarine, riverine, and eastern regions show similar physico-chemical characteristics which are agreeing with the geographical divisions done by the present study followed by Kerry and Baba (2001).
Tracking of pollution in Ashtamudi lake system
In Ashtamudi Lake basin, the pollution potential zones were derived from the basin characteristics such as lithology, geomorphology, soil type, land use, and population density of different sub-basins. The zones were identified with the help of multi-thematic overlay analysis using GIS, and the pollution potential zones were classified into various classes such as very high, high, medium, and low (Sheela et al., 2016).
Lake basin characteristics
Geomorphology: The lake basin consists of plateau, pediplain, floodplain, and coastal plain (Fig. 9a). The area covered by plateau is maximum with an extent of 180.82 km2, followed by coastal plain 82.074 km2, then pediplain 39.611 km2, and floodplain 0.039 km2. Water body covers about 58.73 km2.
Soil: The soil in the lake basin consists of gravelly clay, clay, and sand. Gravelly covers an area of 208.3 sq. km, followed by clay 41.8 km2 and sand covers 0.74 km2 (Fig. 9b).
Lithology: Lithology of basin consists of sandstone and clay with lignite, sand and silt, charnockite, and khondalite rock. Sandstone and clay with lignite cover an area of 135.4 km2, followed by sand and silt (Fig. 9c).
Slope: A majority of the basin falls in the slope of 0–10%, especially near coastal area. Figure 9d is the slope map of the basin. Low slope is located in the land area close to the shore. This includes Kollam City areas surrounding the Southern Zone, the land areas covering Western Zone, and land areas surrounding Riverine zone. The stagnation of water flow can be observed in these land areas.
Land use: The study revealed that the land use is mainly built up and agriculture (Fig. 10a). Built up area is maximum for Kollam Corporation (24.22 km2), which is in the Southern Zone catchment, followed by Western Zone catchment (5.88 km2) and minimum in Riverine Zone catchment (2.07 km2).
Population: The population is maximum in Kollam Corporation region surrounded by Southern Zone, followed by catchment of Western Zone and Estuarine Zone. The minimum population was observed in the catchment of Riverine Zone.
Identification of pollution potential zones
Pollution potential area of the lake basin has been identified using GIS-enabled, multi-thematic overlay analysis (Fig. 10b). The study revealed that 42.91% of lake basin falls in the category of very high pollution potential region (near to Southern Zone), followed by 22.7% of high (near to Western Zone) and 21% moderate pollution potential area. Kollam Corporation, having 0.40 million population with a population density of 6250/km2 and 0.14 million households and a built up area of 25 km2, lies in the catchment area of Southern Zone facing very high pollution potential. The TS canal is passing through the densely populated land area of Southern Zone. The municipal waste water consisting of sewage, sullage, and garbage is mainly responsible for high BOD in the lake water and having low DO in the lake and thereby causing stress to the aquatic organisms. Thus, urbanization is mainly responsible for the degradation of lake water quality. The Western Zone region observed as high level pollution potential zone analysis has a built up area of 2.5 km2 with a population of 42,655. The activities in this region include boat manufacturing and repairing, fishing processing units. The Eastern and Riverine Zones show medium to low levels of pollution due to low population and low built-up area. The study revealed that the improper waste management in the catchment area near to Southern Zone and Western Zone, especially Kollam Corporation and local bodies lying on the southern catchment, is mainly responsible for water quality degradation. Urgent attention is to be given to the implementation of pollution control measures in the high-value pollution potential zones.
Conclusions
The study has been carried out to assess the water quality and hypoxia status of Ashtamudi Lake (ASL) and also the tracking of pollution in the lake system. The lake is divided into six zones (I–VI), viz., Southern, Estuarine, Western, Central, Riverine, and Eastern Zone. The shallow nature is observed in the Southern and Central Zones of the lake. The maximum depth exists in the Eastern Zone, but lower transparency exists in the Riverine Zone due to incoming runoff. The high content of BOD, nitrate, and ammoniacal nitrogen has been observed in the Southern Zone, whereas salinity is high in the Estuarine and Western Zones. The high content of ammoniacal nitrogen was also observed in the Southern, Central, Estuarine, and Western Zones. DO is maximum in the Riverine and Eastern Zones. Hypoxic condition (0.2–2%) exists over a smaller area (< 1%) and a comparatively low oxygen region (2–4%) exists in 69% of lake area during non-monsoon season. On the other hand, in the bottom water, the hypoxic condition prevailed over a smaller area (2.8%) and low oxygen region in a larger area (97%). But some improvement has occurred during the monsoon season. Oxygenated condition in the lake rises to 83.64% during monsoon in surface water. The hypoxic condition is largely limited to the Southern Zone in both seasons. Low oxygen state (2–4 mg L−1) in the Southern and Westerns Zones of ASL and also approximately in one-half of the Central Zone may affect the fishery resources (fishes, crabs, and molluscs) including marine, estuarine, and freshwater organisms. The study suggests that hypoxia is chiefly attributed to anthropogenic interventions causing discharge of wastes into the lake causing overloading of organic matter and nutrients, decrease in the freshwater supply, the absence of proper freshwater mixing or dilution, effluent discharge from nearby urban centers, etc.
The rise in the spread of salinity is posing a threat to freshwater species in the lake system. The occurrence of low DO in the lake is highly related to salinity and organic load in the lake system. Urban waste factor, sea saline factor, and natural factor have been identified as the quality controlling factors in the lake. Urban waste factor predominates in the Southern Zone. Sea Saline factor predominates in the Western and Central Zone. The current status, as experimentally observed in this study, may ultimately lead to impairment of ecosystem, as well as reduction of fish diversity and productivity, impacting the socio-economic status of folks, directly and indirectly, depending on ASL and its environmental sustenance. The tracking of pollution in the lake system was also done and found that catchments adjacent to Southern and Western Zones (covering Kollam Corporation and adjacent panchayaths) are the major source of pollution. The study calls for urgent solid and liquid pollution mitigatory measures for eco-restoration and sustainable management of the lake.
Availability of data and materials
There are no data, codes, and images of any individual person included in the manuscript.
References
Abdel-Tawwab, M., Monier, M. N., Hoseinifar, S. H., & Faggio, C. (2019). Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiology and Biochemistry, 45, 997–1013. https://doi.org/10.1007/s10695-019-00614-9
Alam, M. S., Bangshuai, H., Amy, G., & John, P. (2020). Nitrate and biochemical oxygen demand change in a typical Midwest stream in the past two decades. H2Open Journal, 3, 519–537. https://doi.org/10.2166/h2oj.2020.054
Allison, M. S., David, H. K., Raffaela, M, & Richard, J. H. (2018). A review of dissolved oxygen requirements for key sensitive species in the Delaware Estuary. The Patrick Center for Environmental Research Academy of Natural Sciences of Drexel University.
Almeida, L. Z., Guffey, S. C., Sepulveda, M. S., & Höök, T. O. (2017). Behavioral and physiological responses of yellow perch (Perca flavescens) to moderate hypoxia. Comparative Biochemistry and Physiology - Part a: Molecular & Integrative Physiology, 209, 47–55. https://doi.org/10.1016/j.cbpa.2017.04.009
Altieri, A. H., & Gedan, K. B. (2014). Climate change and dead zones. Global Change Biology Bioenergy, 21, 1395–1406. https://doi.org/10.1111/gcb.12754
Angela, M., Caballero-Alfonso, J., & C., & Daniel, J. C. (2015). Biogeochemical and environmental drivers of coastal hypoxia. Journal of Marine System, 141, 190–199. https://doi.org/10.1016/j.jmarsys.2014.04.008
Anifowose, F. A., Ajayi, O., & Fagorite, V. I. (2020). Seasonal variability of dissolved oxygen in relation to temperature and salinity in Gulf of Mexico, South of Cocodrie, Louisiana. American Journal of Innovative Research & Applied Sciences, 10, 237–241.
Antony, F. D., & Edward, L. L. (2019). Ammonia toxicity and adaptive response in marine fishes – A review. Indian Journal of Geo-Marine Sciences. http://nopr.niscair.res.in/handle/123456789/47054
Antony, M. M., & Ignatius, J. (2015). A Hydrological study of Ashtamudi lake, Kerala, India with special reference to its ecological difference. International Journal of Science and Research, 8, 1841–1846.
Aswathy, T. S., Achu, A. L., Shincy, F., Girish, G., Shijo, J., Surendran, U., & Sunil, P. S. (2021). Assessment of water quality in a tropical ramsar wetland of southern India in the wake of COVID-19. Remote Sensing Applications: Society and Environment. https://doi.org/10.1016/j.rsase.2021.100604
Babu, K. N., Ouseph, P. P., & Padmalal, D. (2000). Interstitial water–sediment geochemistry of N, P and Fe and its response to overlying waters of tropical estuaries: A case from the south west coast of India. Environmental Geology, 39, 633–640. https://doi.org/10.1007/s002540050475
Babu, N. K., Omana, P. K., & Mohan, M. (2010). Water and sediment quality of Ashtamudi estuary, a Ramsar site, south west coast of India-A statistical appraisal. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-009-0947-0
Balakrishnan, S., Chelladurai, G., Mohanraj, J., et al. (2017). Seasonal variations in physico-chemical characteristics of Tuticorin coastal waters, southeast coast of India. Applied Water Science, 7, 1881–1886. https://doi.org/10.1007/s13201-015-0363-2
Barbier, E. B. (2020). Estuarine and coastal ecosystems as defense against flood damages: An economic perspective. Frontiers in Climate. https://doi.org/10.3389/fclim.2020.594254
Bargu, S., Justic, D., White, J. R., Lane, R., Day, J., Paerl, H., & Raynie, R. (2019). Mississippi River diversions and phytoplankton dynamics in deltaic Gulf of Mexico estuaries: A review. Estuarine, Coastal and Shelf Science, 221, 39–52. https://doi.org/10.1016/j.ecss.2019.02.020
Basil George, J. I., Kumar, N., & Rita, N. K. (2012). Study on the influence of hydro-chemical parameters on phytoplankton distribution along Tapi estuarine area of Gulf of Khambhat India. The Egyptian Journal of Aquatic Research, 38, 157–170. https://doi.org/10.1016/j.ejar.2012.12.010
Basset, A. E., Barbone, E., M., Li, B.L., Jorgensen, S.E., Lucena-Moya, P., et al. (2013). A unifying approach to understanding transitional waters: Fundamental properties emerging from ecotone ecosystems. Estuarine, Coastal and Shelf Science, 132, 5–16. https://doi.org/10.1016/j.ecss.2012.04.012
Benli, K. A., & Koksal, G. (2005). The acute toxicity of ammonia on tilapia (Oreochromis niloticus L.) larvae and fingerlings. Turkish Journal of Veterinary and Animal Sciences., 29, 339–344.
Bernard, M., Zukowski, S., Whiterod, N., Vilizzi, L., Beesley, L., & King, A. (2014). Hypoxic blackwater event severely impacts Murray crayfish (Euastacus armatus) populations in the Murray River. Australia. Austral Ecology, 39, 491–500. https://doi.org/10.1111/aec.12109
Bhateria, R., & Jain, D. (2016). Water quality assessment of lake water: A review. Sustainable Water Resources Management, 2, 161–173. https://doi.org/10.1007/s40899-015-0014-7
Biju Kumar, A., Smrithy, R., & Sathasivam, K. (2012). Dolphin-assisted cast net fishery in the Ashtamudi Estuary, south-west coast of India. Journal of Fisheris, 59, 143–148. https://www.researchgate.net/publication/263280106
Bowd, R., Nevil, Q., Donovan, C., Kotze, D., & G., & Hay, M. M. (2012). The identification of potential resilient estuary-based enterprises to encourage economic empowerment in South Africa: A toolkit approach. Ecology and Society, 17, 15. https://doi.org/10.5751/ES-04840-170315
Breitburg, D. (2002). Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries, 25, 767–781. https://doi.org/10.1007/BF02804904
Breitburg, D., Levin, L. A., Oschlies, A., Grégoire, M., Chavez, E. P., Conley, D. J., et al. (2018). Declining oxygen in the global ocean and coastal waters. Science. https://doi.org/10.1126/science.aam7240
Bricker, S. B., Longstaff, B., Dennison, W., Jones, A., Boicourt, K., Wicks, C., & Woerner, J. (2008). Effects of nutrient enrichment in the nation’s estuaries: A decade of change. Harmful Algae, 8, 21–32. https://doi.org/10.1016/j.hal.2008.08.028
Budnik, R. R., Steinhart, G. B., Conroy, J. D., Dillon, R. A., Zweifel, R. D., & Ludsin, S. A. (2021). Effects of hypoxia on habitat quality of reservoir largemouth bass, saugeye, and white crappie. Transactions of the American Fisheries Society, 150, 75–88.
Cañedo-Argüelles, M., Kefford, B., & Schäfer, R. (2018). Salt in freshwaters: Causes, effects and prospects - introduction to the theme issue. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 374, 1764. https://doi.org/10.1098/rstb.2018.0002
Census of India. (2011). Data on disability. Office of the Registrar General & Census Commissioner, New Delhi. Retrieved December 27, 2013, from http://www.disabilityaffairs.gov.in
Chamberlain, D. W., Knight, C. T., Kraus, R. T., Gorman, A. M., Xu, W., & Collingsworth, P. D. (2020). Hypoxia augments edge effects of water column stratification on fish distribution. Fisheries Research, 231, 105684. https://doi.org/10.1016/j.fishres.2020.105684
Chen, K., Cai, M., Wang, Y., Chen, B., Li, X., Qiu, C., Huang, S., Sun, J., Liu, X., Qian, B., & Ke, H. (2020). Organochlorine pesticides in sediment of Zhang River estuary mangrove national natural reserve: The implication of its source change in China’s mangroves. Sustainability, 12, 3016. https://doi.org/10.3390/su12073016
Chen, W., Tong, A. H., & Liu, H. (2012). Effects of nitrate on nitrite toxicity to Microcystis aeruginosa. Marine Pollution Bulletin, 64, 1106–1111. https://doi.org/10.1016/j.marpolbul.2012.04.006
Chilton, D., Hamilton, D. P., Nagelkerken, I., Perran, C., Hipsey Matthew, R., Robert, R., Marcus, S., Waltham, N. J., & Brookes, J. (2021). Environmental flow requirements of estuaries: Providing resilience to current and future climate and direct anthropogenic changes. Frontiers in Environmental Science. https://doi.org/10.3389/fenvs.2021.764218
Chown, S. L. (2012). Trait-based approaches to conservation physiology: Forecasting environmental change risks from the bottom up. OnLine Journal of Biological Sciences, 367, 1615–1627. https://doi.org/10.1098/rstb.2011.0422
Claireaux, G., & Chabot, D. (2016). Responses by fishes to environmental hypoxia: Integration through Fry’s concept of aerobic metabolic scope. Journal of Fish Biology, 88, 232–251. https://doi.org/10.1111/jfb.12833
Cloern, J. E., Jassby, A. D., Schraga, T. S., Nejad, E., & Martin, C. (2017). Ecosystem variability along the estuarine salinity gradient: Examples from long-term study of San Francisco Bay. Limnology and Oceanography, 62, S272–S291. https://doi.org/10.1002/lno.10537
Conley, D. J., Carstensen, J., Aigars, J., Axe, P., Bonsdorff, E., Eremina, T., Haahti, B. M., Humborg, C., Jonsson, P., Kotta, J., Lännegren, C., Larsson, U., Maximov, A., Rodriguez Medina, M., Lysiak-Pastuszak, E., Remeikaité-Nikiené, N., Walve, J., Wilhelms, S., & Zillén, L. (2011). Hypoxia is increasing in the coastal zone of the Baltic Sea. Environmental Science and Technology, 45, 6777–6783. https://doi.org/10.1021/es201212r
Conley, D. J., Carstensen, J., Vaquer-Sunyer, R., & Duarte, C. M. (2009). Ecosystem thresholds with hypoxia. Hydrobiologia, 629, 21–29. https://doi.org/10.1007/s10750-009-9764-2
Cooke, S. J., Donaldson, M. R., O’Connor, C. M., Raby, G. D., Arlinghaus, R., Danylchuk, A. J., Hanson, S. G., Hinch, K. C., Clark, T., & Patterson, D. A. (2013). The physiological consequences of catch-and-release angling: Perspectives on experimental design, interpretation, extrapolation, and relevance to stakeholders. Fisheries Management and Ecology, 20, 268–287. https://doi.org/10.1111/j.1365-2400.2012.00867.x
Dean, T. L., & Richardson, J. (1999). Responses of seven species of native freshwater fish and a shrimp to low levels of dissolved oxygen. New Zealand Journal of Marine and Freshwater Research, 33, 99–106. https://doi.org/10.1080/00288330.1999.9516860
Diaz, R. J. (2016). Anoxia, Hypoxia, And Dead Zones. In: Kennish M. J. (eds) Encyclopedia of estuaries. Encyclopedia of Earth Sciences Series. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8801-4_82
Diaz, R., Selman, M., & Chique, C. (2010). Global eutrophic and hypoxic coastal systems. World Resources Institute. Eutrophication and Hypoxia: Nutrient Pollution in Coastal Waters. http://www.wri.org/project/eutrophication
Diaz, R. J., & Rosenberg, R. (2008). Spreading dead zones and consequences for marine ecosystems. Science, 321, 926–929. https://doi.org/10.1126/science.1156401
Divya, S. R. (2015). Salinity fluctuations of upstream and downstream waters of Ashtamudi estuary. International Journal of Fisheries and Aquatic Studies, 6, 26–330.
Du, J., Shen, J., Park, K., Wang, Y. P., & Yu, X. (2018). Worsened physical conditions due to climate change contributes to the increasing hypoxia in Chesapeake Bay. Science of the Total Environment, 630, 707–717.
Dutton, C. L., Subalusky, A. L., Hamilton, S. K., et al. (2018). Organic matter loading by hippopotami causes subsidy overload resulting in downstream hypoxia and fish kills. Nature Communications, 9, 1951. https://doi.org/10.1038/s41467-018-04391-6
Ekau, W., Auel, H., Portner, H., & Gilbert, D. (2010). Impacts of hypoxia on the structure on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences Discussions, 7, 1669–1699. https://doi.org/10.5194/bgd-6-5073-2009
ENVIS. (2019). WII-ENVIS Centre on Wildlife & Protected Areas. http://www.wiienvis.nic.in/
EPA. (2013). Aquatic life ambient water quality criteria for ammonia-freshwater 2013. Office of Science and Technology.
Fabricius, K. E., Logan, M., Weeks, S. J., Lewis, S. E., & Brodie, J. (2016). Changes in water clarity in response to river discharges on the Great Barrier Reef continental shelf: 2002–2013. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2016.03.001
Fennel, K., & Testa, J. M. (2018). Biogeochemical controls on coastal hypoxia. Annual Review of Marine Science, 11, 105–130. https://doi.org/10.1146/annurev-marine-010318-095138
Fleming-Lehtinen, V., & Laamanen, M. (2012). Long-term changes in Secchi depth and the role of phytoplankton in explaining light attenuation in the Baltic Sea. Estuarine, Coastal and Shelf Science, 102, 1–10. https://doi.org/10.1016/j.ecss.2012.02.015
Friedrich, J., Janssen, F., Aleynik, D., Bange, H. W., Boltacheva, N., Çagatay, M., Dale, A. W., Etiope, G., Erdem, Z., Geraga, M., et al. (2014). Investigating hypoxia in aquatic environments: Diverse approaches to addressing a complex phenomenon. Biogeosciences, 10, 12655–12772. https://doi.org/10.5194/bg-11-1215-2014
Gammal, M. A. M. E., Nageeb, M., & Al-Sabeb, S. (2017). Phytoplankton abundance in relation to the quality of the coastal water – Arabian Gulf Saudi Arabia. The Egyptian Journal of Aquatic Research, 43, 275–282. https://doi.org/10.1016/j.ejar.2017.10.004
Goñi-Urriza, M. S., Point, D., Amouroux, D., Guyoneaud, R., Donard, O. F. X., Caumette, P., & Duran, R. (2007). Bacterial community structure along the Adour estuary (French Atlantic coast): Influence of salinity gradient versus metal contamination. Aquatic Microbial Ecology, 49, 47–56. https://doi.org/10.3354/ame01128
Govindasamy, C., Kannan, L., & Jayapaul, A. (2000). Seasonal variation in physico-chemical properties and primary production in the coastal water biotopes of Coromandel coast India. Journal of Environmental Biology, 21, 17.
Graham, B. A., Chan, F., Nielsen, K. J., Fox, D. S., Barth, J. A., Huyer, A., et al. (2004). Upwelling-driven nearshore hypoxia signals ecosystem and oceanographic changes in the northeast Pacific. Nature, 429, 749–751. https://doi.org/10.1038/nature02605
Granéli, E., & Turner, J. (2006). Ecology of harmful algae. Springer-Verlag.
Grasshoff, K., Ehrhardt, M., & Kremling, K. (1983). Methods of seawater analysis. Second, Revised and Extended Edition. https://doi.org/10.1002/iroh.19850700232
Grommen, R., Hautenghum, I. V., Wambeke, M. V., & Verstracte, W. (2002). An improved nitrifying enrichment to remove ammonia and nitrite from freshwater aquaculture systems. Aquaculture, 211, 11–124.
Hale, S. S., Cicchetti, G., & Deacutis, C. F. (2016). Eutrophication and hypoxia diminish ecosystem functions of benthic communities in a New England estuary. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2016.00249
Hao, Y. Y., Zhu, Z. Y., Fang, F. T., Novak, T., Čanković, M., Hrustić, E., Ljubešić, Z., Li, M., Du, J. Z., Zhang, R. U., & Gašparović, B. (2021). Tracing nutrients and organic matter changes in eutrophic Wenchang (China) and oligotrophic Krka (Croatia) estuaries: A comparative study. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2021.663601
Hargreaves, J. A., & Tucker, C. S. (2004). Managing ammonia in fish ponds. Southern Regional Aquaculture Center, Stoneville.
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., Dortch, Q., Gobler, C. J., Heil, C. A., Humphries, E., Lewitus, A., Magnien, R., Marshall, H. G., Sellner, K., Stockwell, D. A., Stoecker, D. K., & Suddleson, M. (2008). Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae, 8, 3–13. https://doi.org/10.1016/j.hal.2008.08.006
HELCOM. (2013). Approaches and methods for eutrophication target setting in the Baltic Sea region. Baltic Sea Environmental Proceedings No. 133. http://www.helcom.f
Howarth, R., Chan, F., Conley, D. J., Garnier, J., Doney, S. C., Marino, R., & Billen, G. (2011). Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems, Frontiers in Ecology and the Environment, 9, 18–26. https://doi.org/10.1890/100008
Hrycik, A. R., Almeida, L. Z., & Höök, T. O. (2017). Sub-lethal effects on fish provide insight into a biologically-relevant threshold of hypoxia. Oikos, 126, 307–317. https://doi.org/10.1111/oik.03678
Janout, M. A., Hölemann, J., Laukert, G., Smirnov, A., Krumpen, T., Bauch, D., & Timokhov, L. (2020). On the variability of stratification in the freshwater-influenced Laptev Sea Region. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2020.543489
Jennerjahn, T. C., Soman, K., Ittekkot, V., et al. (2008). Effect of land use on the biogeochemistry of dissolved nutrients and suspended and sedimentary organic matter in the tropical Kallada River and Ashtamudi estuary, Kerala, India. Biogeochemistry, 90, 29–47. https://doi.org/10.1007/s10533-008-9228-1
Jenny, P., Francus, P., Normandeau, A., Lapointe, F., Perga, M. E., Ojala, A., Schimmelmann, A. B., & Zolitschka, N. (2016). Global spread of hypoxia in freshwater ecosystems during the last three centuries is caused by rising local human pressure. Global Change Biology Bioenergy, 22, 1481–1489. https://doi.org/10.1111/gcb.13193
Jessen, G. L., Lichtschlag, A., Ramette, A., Pantoja, S., Rossel, P. E., Schubert, C. J., Struck, U., & Boetius, A. (2017). Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Science Advances, 3(2), e1601897. https://doi.org/10.1126/sciadv.1601897
Jiang, Z. B., Liu, J. J., Chen, J. F., Chen, Q. Z., Yan, X. J., Xuan, J. L., et al. (2014). Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Research, 54, 1–11. https://doi.org/10.1016/j.watres.2014.01.032
Jiang, Z., Chen, J., Zhou, F., Shou, L., Chen, Q., Tao, B., et al. (2015). Controlling factors of summer phytoplankton community in the Changjiang (Yangtze River) Estuary and adjacent East China Sea shelf. Continental Shelf Research, 101, 71–84. https://doi.org/10.1016/j.csr.2015.04.009
Jim, A., & Appukuttan, K. K. (2016). MSC Sustainable Fisheries Certification, Off-Site Surveillance Visit - Report for Ashtamudi Estuary Short Necked Clam Fishery.
Joseph, S., & Thrivikramji, K. P. (2002). Kayals of Kerala coastal land and implication to quaternary sea level changes. Memoir No.49. Late Quaternary Geology of India and Sea Level Changes, (Eds) Narayana AC, Geological society of India, ISBN No: 81–85867–51–8, 51–64.
Joshua, M. T., Nicholas, I. K., Benjamin, S. L., David, C., Tomas, O. H, & Paris, D. C. (2022). Widespread prevalence of hypoxia and the classification of hypoxic conditions in the Laurentian Great Lakes. Journal of Great Lakes Research, 48, 13–23. https://doi.org/10.1016/j.jglr.2021.11.004
Keeling, R. F., Körtzinger, A., & Gruber, N. (2010). Ocean deoxygenation in a warming world. Annual Review of Marine Science, 2, 199–229. https://doi.org/10.1146/annurev.marine.010908.163855
Kelly, N. E., Sabaniel, J. G., & Zimmerman, R. (2021). Anthropogenic nitrogen loading and risk of eutrophication in the coastal zone of Atlantic Canada, Estuarine. Coastal and Shelf Science, 263, 107630. https://doi.org/10.1016/j.ecss.2021.107630
Kerry, B, & Baba, M. (2001). Ashtamudi Management Plan for Ashtamudi estuary, Kollam, India. ASR Limited, Hamilton, New Zealand and Centre for Earth Sciences and Studies, Thiruvananthapuram.
Kim, J. H., Kang, Y. J., Kim, K. I., Kim, S. K., & Kim, J. H. (2019). Toxic effects of nitrogenous compounds (ammonia, nitrite, and nitrate) on acute toxicity and antioxidant responses of juvenile olive flounder, Paralichthys olivaceus. Environmental Toxicology and Pharmacology, 67, 73–78. https://doi.org/10.1016/j.etap.2019.02.001
Krishnan, R., Sanjay, J., Gnanaseelan, C., Mujumdar, M., Kulkarni, A., & Chakraborty, S. (2021). Correction to: Assessment of climate change over the Indian Region. In: Krishnan R, Sanjay J, Gnanaseelan C, Mujumdar M, Kulkarni A, Chakraborty S (eds) Assessment of Climate Change over the Indian Region. Springer, Singapore. https://doi.org/10.1007/978-981-15-4327-2_13
KSPCB. (2020). Report by the KSPCB on a solid waste dump yard at Kureepuzha, Kollam district, Kerala.
Kuo, A. Y., & Nielson, B. J. (1987). Hypoxia and salinity in Virginia estuaries. Estuaries, 10, 277–282. https://doi.org/10.2307/1351884
LaRowe, D. E., Arndt, S., Bradley, J. A., Burwicz, E. B., Dale, A. W., & Amend, J. P. (2020). Organic carbon and microbial activity in marine sediments on a global scale throughout the quaternary. Geochimica Et Cosmochimica Acta. https://doi.org/10.1016/j.gca.2020.07.017
Lee, J., Park, K. T., Lim, J. H., Yoon, J. E., & Kim, I. N. (2018). Hypoxia in Korean coastal waters: A case study of the Natural Jinhae Bay and Artificial Shihwa Bay. Frontiers in Marine Science, 5, 70. https://doi.org/10.3389/fmars.2018.00070
Li, R. H., Liu, S. M., Li, Y. W., Zhang, G. L., Ren, J. L., & Zhang, J. (2014). Nutrient dynamics in tropical rivers, lagoons, and coastal ecosystems of eastern Hainan Island, South China Sea. Biogeosciences, 11, 481–506. https://doi.org/10.5194/bg-11-481-2014
Liu, M., Zhang, Y., Shi, K., et al. (2020). Effects of rainfall on thermal stratification and dissolved oxygen in a deep drinking water reservoir. Hydrological Processes, 34, 3387–3399. https://doi.org/10.1002/hyp.13826
Lu, Z., Gan, J., Dai, M., Liu, H., & Zhao, X. (2018). Joint effects of extrinsic biophysical fluxes and intrinsic hydrodynamics on the formation of hypoxia west off the Pearl River estuary. Journal of Geophysical Research: Oceans, 123, 6241–6259. https://doi.org/10.1029/2018JC014199
Malone, T. C., & Alice, N. (2020). The globalization of cultural eutrophication in the coastal ocean: Causes and consequences. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2020.00670
Misra, O. P., & Chaturvedi, D. (2016). Fate of dissolved oxygen and survival of fish population in aquatic ecosystem with nutrient loading: A model. Modeling Earth Systems and Environment, 2, 112. https://doi.org/10.1007/s40808-016-0168-9
Mohamed, K. S., Venkatesan, V., Kripa, V., Prema, D., Joseph, M., Alloycious, P. S., Jenni, B., Valsala, K. K., Saji Kumar, K. K., Ragesh, N., Bose, J., & Mohan, A. (2013). Fishery management plan for Ashtamudi Lake clam resources. CMFRI Special Publication, 114, 1–48.
Mugnier, C., & Soyez, C. (2005). Response of the blue shrimp Litopenaeus stylirostris to temperature decrease and hypoxia in relation to molt stage. Aquaculture, 244, 315–322. https://doi.org/10.1016/j.aquaculture.2004.11.010
Mugnier, C., Zipper, E., Goarant, C., & Lemonnier, H. (2008). Combined effect of exposure to ammonia and hypoxia on the blue shrimp Litopenaeus stylirostris survival and physiological response in relation to molt stage. Aquaculture, 274, 398–407. https://doi.org/10.1016/j.aquaculture.2007.11.033
Nair, N. B., Dharmaraj, K., Abdul Azis, P. K., Arunachalam, M., Krishnakumar, K., & Balasubramanian, N. K. (1984). Ecology of Indian estuaries: 8. Inorganic nutrients in the Ashtamudi Estuary, Mahasagar. http://www.ijs.nio.org/index.php/msagar/article/view/1780
Nair, N. B., Krishnakumar, K., Nair, J. R., Abdul Azis, P. K., Dharmaraj, K., & Arunachalam, M. (1983). Ecology of Indian estuaries, XI. A preliminary survey of the fishery resources of Ashtamudi estuarine system. Fishery Technology, 20, 75–83.
Najjar, R. G., Pyke, C. R., Adams, M. B., Breitburg, D., Hershner, C., Kemp, M., Howarth, R., Mulholland, M. R., Paolisso, M., Secor, D., Sellner, K., Wardrop, D., & Wood, R. (2010). Potential climate-change impacts on the Chesapeake Bay. Estuarine, Coastal and Shelf Science, 86, 1–20. https://doi.org/10.1016/j.ecss.2009.09.026
NCESS. (2017). Annual report 2016–17, ESSO- National Centre for Earth Science Studies. https://www.ncess.gov.in/images/Annual_Reports/AR_2016-17.pdf
Nixon, S. W. (2009). Eutrophication and the macroscope. Hydrobiologia, 629, 5–19. https://doi.org/10.1007/s10750-009-9759-z
NOAA. (2017a). Ocean Service education. https://oceanservice.noaa.gov/education/kits/estuaries/estuaries02_economy.html
NOAA. (2017b). Estuaries and human. https://oceanservice.noaa.gov/education/pd/estuaries/estuarieshumans.html
O¨sterblom, H., Hansson, S., Larsson, U., Hjerne, O., Wulff, F., Elmgren, R., & Folke, C. (2007). Human induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems, 10, 877–889. https://doi.org/10.1007/s10021-007-9069-0
Pajares, S., & Ramos, R. (2019). Processes and microorganisms involved in the marine nitrogen cycle: Knowledge and gaps. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2019.00739
Palmer, T., Montagna, P., & Kalke, R. D. (2008). Benthic indicators of the initial effect of opening a channel. Environmental Bioindicators. https://doi.org/10.1080/15555270802551776
Parson, T. R., Maita, Y., & Lalli, C. M. (1984). A manual biological and chemical methods for sea water analysis. Oxford, Pregamon. https://doi.org/10.1002/iroh.19850700634
Pein, J., Eisele, A., Sanders, T., Daewel, U., Stanev, E. V., van Beusekom, J. E. E., Staneva, J., & Ca, S. (2021). Seasonal stratification and biogeochemical turnover in the freshwater reach of a partially mixed dredged estuar. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2021.623714
Philips, S., Laanbroek, H. J., & Verstraete, W. (2002). Origin, causes and effects of increased nitrite concentrations in aquatic environments. Reviews in Environmental Science and Biotechnology, 1, 115–141. https://doi.org/10.1023/A:1020892826575
Piontkovski, S. A., Al-Gheilani, H. M. H., Jupp, B., Sarma, Y. V. B., & Al-Azri, A. R. (2012). The relationship between algal blooms, fish kill incidents, and oxygen depletions along the Omani coast. International Journal of Oceans and Oceanography, 6, 145–177.
Pitcher, G. C., & Probyn, T. A. (2011). Anoxia in southern Benguela during the autumn of 2009 and its linkage to a bloom of the dinoflagellate Ceratium balechii. Harmful Algae, 11, 23–32. https://doi.org/10.1016/j.hal.2011.07.001
Pörtner, H. O., & Lannig, G. (2009). Oxygen and capacity limited thermal tolerance. (Eds) Jeffrey, A. P. F., G. Richards, J. B. J. Colin. Fish Physiol. 27 Academic Press, Cambridge 143–191.
Rabalais, N. N., & Turner, R. E. (2019). Gulf of Mexico hypoxia: Past, present, and future. Limnology and Oceanography Bulletin, 28, 117–124. https://doi.org/10.1002/lob.10351
Rabalais, N. N., Díaz, R. J., Levin, L. A., Turner, R. E., Gilbert, D., & Zhang, J. (2010). Dynamics and distribution of natural and human caused hypoxia. Biogeosciences, 7, 585–619. https://doi.org/10.5194/bg-7-585-2010
Rabalais, N. N., Turner, R. E., Wiseman, W. J., Jr., & Dortch, Q. (1998). Consequences of the 1993 Mississippi River flood in the Gulf of Mexico. Regulated Rivers: Research & Management, 14, 161–177. https://doi.org/10.1002/(SICI)1099-1646(199803/04)14:2%3c161::AID-RRR495%3e3.0.CO;2-J
Raju, B. (2011). Ecobiology of Ashtamudi Estuary with special reference to the region of confluence of Kallada River with the estuary. Ph.D. Thesis University of Kerala.
Ram, A., Jiyalal Ram, M., Jaiswar, M. A., Rokade, S., Bharti, C., Vishwasrao, D., & Majithiya. (2014). Nutrients, hypoxia and Mass Fishkill events in Tapi Estuary, India. Estuarine, Coastal and Shelf Science, 148, 48–58. https://doi.org/10.1016/j.ecss.2014.06.013
Rashid, H. (2021). Effects of dissolved oxygen concentrations on growth, survival and gonadal development of freshwater pearl mussel Lamellidens marginalis Kafi MFH, Islam MH, Uddin MH, Sumon KA, Rashid H. 2021. Effects of dissolved oxygen concentrations on growth, survival and gonadal development of freshwater pearl mussel Lamellidens marginalis. Bangladesh Journal of Fisheries, 33, 99–108. https://doi.org/10.52168/bjf.2021.33.12
Razumov, V. A., & Tyutyunova, F. I. (2001). Nitrite contamination of the Moskva River: Causes and effects. Water Resources, 28, 324–334. https://doi.org/10.1023/A:1010409009477
Reshmi, R. R., Deepa Nair, K., Zachariah, E. J., & Vincent, S. G. T. (2015). Methanogenesis: Seasonal changes in human impacted regions of Ashtamudi estuary (Kerala, South India). Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2014.11.031
Richards, J. G., Sardella, B. A., & Schulte, P. M. (2008). Regulation of pyruvate dehydrogenase in the common killifish, Fundulus heteroclitus, during hypoxia exposure, American journal of physiology. Regulatory, Integrative and Comparative Physiology, 295, 979–990. https://doi.org/10.1152/ajpregu.00192.2008
Roman, M. R., Brandt, S. B., Houde, E. D., & Pierson, J. J. (2019). Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Frontiers in Marine Science. https://doi.org/10.3389/fmars.2019.00139
Salahudeen, J. H., Reshmi, R. R., Anoop, K. K., Ragi, M. S., & Vincent, S. G. T. (2018). Denitrification rates in estuarine sediments of Ashtamudi, Kerala India. Environmental Monitoring and Assessment, 190, 323. https://doi.org/10.1007/s10661-018-6698-z
Santu, K. S., Nandan, S. B., & Athira, K. (2016). Occurrence of mass swarming of family Acartiidae (Calanoid Copepods) (Zooplankton) in Ashtamudi Estuary Kerala. International Journal of Marine Science, 30, 1–8. https://doi.org/10.5376/ijms.2016.06.0030
Savage, C., Leavitt, P. R., & Elmgren, R. (2010). Effects of land use, urbanization, and climate variability on coastal eutrophication in the Baltic Sea. Limnology and Oceanography, 55, 1033–1046. https://doi.org/10.4319/lo.2010.55.3.1033a
Seebacher, F., & Franklin, C. E. (2012). Determining environmental causes of biological effects: The need for a mechanistic physiological dimension in conservation biology. Biological Sciences, 367, 1607–1614. https://doi.org/10.1098/rstb.2012.0036
Senthilkumar, S., Santhanam, P., & Perumal, P. (2002). Diversity of phytoplankton in Vellar estuary, southeast coast of India. In: Ayyappan S, Jena JK, Mohan JM. (Eds.), Proc. 5th Indian Fisheries Forum, AFSIB, Mangalore & AeA, Bhubaneswar, India, 245—248.
Sheela, A. M., Letha, J., Sabu, J., Justus, J., Jobin, T., & Pramod, L. (2016). Prioritization of pollution potential zones for conservation activities of a lake system. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 21, 188–205. https://doi.org/10.1111/lre.12137
Sherwood, J. E., Stagnitti, F., Kokkinn, M. J., & Williams, W. D. (1991). Dissolved oxygen concentrations in hypersaline waters. Limnology and Oceanography, 36, 235–250. https://doi.org/10.4319/lo.1991.36.2.0235
Sinclair, M., Vishnu Sagar, M. K., Knudsen, C., Joseph, S., & Ghermandi, A. (2021). Economic appraisal of ecosystem services and restoration scenarios in a tropical coastal Ramsar wetland in India. Ecosystem Services. https://doi.org/10.1016/j.ecoser.2020.101236
Singh, P., Bagrania, J., & Haritash, A. K. (2019). Seasonal behaviour of thermal stratification and trophic status in a sub-tropical Palustrine water body. Applied Water Science, 9, 139. https://doi.org/10.1007/s13201-019-1011-z
Sisodia, R., & Chaturbhuj, M. (2006). Assessment of the water quality index of wetland Kalakho Lake, Rajasthan India. Journal of Environmental Hydrology, 14, 1–11.
Sitaram, N. (2014). Impact of urbanization on water quality parameters- A case study of Ashtamudi Lake, Kollam. International Journal of Research in Engineering and Technology, 6, 140–147.
Spietz, R. L., Williams, C. M., Rocap, G., & Horner-Devine, M. C. (2015). A dissolved oxygen threshold for shifts in bacterial community structure in a seasonally hypoxic estuary. PLoS ONE, 10, e0135731. https://doi.org/10.1371/journal.pone.0135731
Spiteri, C., Cappellen, P. V., & Regnier, P. (2008). Surface complexation effects on phosphate adsorption to ferric iron oxyhydroxides along pH and salinity gradients in estuaries and coastal aquifers. Geochimica Et Cosmochimica Acta, 72, 3431–3445. https://doi.org/10.1016/j.gca.2008.05.003
Sreedevi, B., Arya, D., Sethulekshmi, R., Subi Merin Babu, & Chithra, V. S. (2016) Water quality of Ashtamudi Lake: A tropical estuary in Southwest India. Kerala Environment Congress. 141.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of sea water analysis. Journal of the Fisheries Research Board of Canada., 167, 21–26.
Sujatha, C. H., Nify, B., Ranjitha, R., Fanimol, C. L., & Samantha, N. K. (2009). Nutrient dynamics in the two lakes of Kerala India. Indian Journal of Geo-Marine Sciences, 38, 451–456.
Sukumaran, M., Muthukumaravel, K., & Sivakami, R. (2013). Seasonal variation in physico-chemical characteristics of Agniar Estuary, Southeast Coast of India. Asia Pacific Journal of Tourism Research, 2, 108–120. http://apjor.com/files/1376844720.pdf
Suseelan, C., & Kathirvel, M. (1982). Study on the prawns of Ashtamudi backwaters in Kerala with special reference to penaeids. Fisheries, 29, 71–78.
Thomson, K. T. (2001). Economic and social issues of biodiversity loss in Cochin Backwaters, The Kerala Research Programme on local level development Centre for development studies, Trivandrum, Kerala, India
Thronson, A., & Quigg, A. (2008). Fifty-five years of fish kills in Coastal Texas. Estuaries and Coasts, 31, 802–813. https://doi.org/10.1007/sl2237-008-9056-5
Tian, R. (2020). Factors controlling hypoxia occurrence in estuaries, Chester River Chesapeake Bay. Water. https://doi.org/10.3390/w12071961
Tomasetti, S. J., Morrell, B. K., Merlo, L. R., & Gobler, C. J. (2018). Individual and combined effects of low dissolved oxygen and low pH on survival of early stage larval blue crabs Callinectes Sapidus. PLoS ONE, 13, e0208629. https://doi.org/10.1371/journal.pone.0208629
USEPA. (1993) Volunteer estuary monitoring: A methods Manual, EPA 842-B-93–004, 176. http://www.epa.gov/owow/estuaries/monitor/
Vaquer-Sunyer, & Duarte. (2008). Thresholds of hypoxia for marine biodiversity. PNAS, 105, 15452–15457. https://doi.org/10.1073/pnas.0803833105
Venkatesan, V., Vidya, R, Alloycious, P. S., Jenni, B., Sajikumar, K. K., Jestin Joy, K. M., Sheela, P. P., Abhilash, K. S., Mohan, G., & Mohamed, K. S. (2015). An assessment of the short-neck clam biomass in Ashtamudi Lake. Marine Fisheries Information Service, No. 223 & 224, 9–14.
Vimal Raj, R. V., Raju, B., Soumya, W., Shibu, A., Lekshmi, S., Shibu Vardhanan, Y., Sruthi, S., & Radhakrishnan, T. (2014). Aquatic bioresources of Ashtamudi lake, Ramsar site Kerala. Aquatic Biology and Fisheries., 2, 297–303.
Wetzel Robert, G. (2001). Limnology: Lake and river ecosystems (3rd ed.). San Diego: Academic Press.
WRIS. (2016). India WRIS wiki, Water Resources Information System of India. https://nrsc.gov.in/India%20WRIS
Wu, M. L., Hong, Y. G., Yin, J. P., et al. (2016). Evolution of the sink and source of dissolved inorganic nitrogen with salinity as a tracer during summer in the Pearl River Estuary. Scientific Reports, 6, 36638. https://doi.org/10.1038/srep36638
Wu, Z., Zhang, Y., Zhou, Y., Liu, M., Shi, K., & Yu, Z. (2015). Seasonal-spatial distribution and long-term variation of transparency in Xin’anjiang Reservoir: Implications for reservoir management. International Journal of Environmental Research and Public Health, 12, 9492–9507. https://doi.org/10.3390/ijerph120809492
Xie, Z., Zhu, G., Xu, M., Zhang, H., Yi, W., Jiang, Y., Liang, M., & Wang, Z. (2022). Risk assessment of heavy metals in a typical mangrove ecosystem - A case study of Shankou Mangrove National Natural Reserve, southern. Marine Pollution Bulletin, 178, 113642. https://doi.org/10.1016/j.marpolbul.2022.113642
Xiong, W., Zhu, Y., Zhang, P., Xu, Y., Zhou, J., Zhang, J., & Luo, Y. (2022). Effects of temperature on metabolic scaling in silver carp. Journal of Experimental Zoology, 337, 141–149. https://doi.org/10.1002/jez.2542
Xu, Z., Cao, J., Qin, X., Qiu, W., Mei, J., & Xie, J. (2021). Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and tissue structure in fish exposed to ammonia nitrogen: A review. Animals. https://doi.org/10.3390/ani11113304
Yan, Z., Fan, J., Zheng, X., Wang, S., Guo, X., Zhang, T., Yang, S., & Zhang, Y. (2019). Neglect of temperature and pH impact leads to underestimation of seasonal ecological risk of ammonia in Chinese surface freshwaters. Journal of Chemistry. https://doi.org/10.1155/2019/3051398
Ysebaert, T., Herman, P. M. J., Meire, P., Craeymeersch, J., Verbeek, H., & Heip, C. H. R. (2003). Large-scale spatial patterns in estuaries: Estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuarine, Coastal and Shelf Science., 57, 335–355. https://doi.org/10.1016/S0272-7714(02)00359
Zapata, C., Puente, A., García, A., Garcia-Alba, J., & Espinoza, J. (2018). Assessment of ecosystem services of an urbanized tropical estuary with a focus on habitats and scenarios. PLoS ONE, 13, e0203927. https://doi.org/10.1371/journal.pone.0203927
Zhang, J. Z., & Fisher, C. J. (2006). A Simplified resorcinol method for direct spectrophotometric determination of nitrate in sea water. Marine Chemistry, 99, 220–226. https://doi.org/10.1016/j.marchem.2005.09.008
Zhao, Y., Song, Y., Cui, J., Gan, S., Yang, X., Wu, R., & Guo, P. (2020). Assessment of water quality evolution in the Pearl River Estuary (South Guangzhou) from 2008 to 2017. Water, 12, 59. https://doi.org/10.3390/w12010059
Zhu, Y., McCowan, A., & Cook, P. L. M. (2017). Effects of changes in nutrient loading and composition on hypoxia dynamics and internal nutrient cycling of a stratified coastal lagoon. Biogeosciences, 14, 4423–4433. https://doi.org/10.5194/bg-14-4423-2017
Zille´n, L., & Conley, D. J. (2010). Hypoxia and cyanobacteria blooms—Are they really natural features of the late Holocene history of the Baltic Sea. Biogeoscience, 7, 2567–2580. https://doi.org/10.5194/bg-7-2567-2010
Acknowledgements
This research is part of an Indo-Israel (UGC-ISF) joint 3-year major research program (F. No. 6-7/2017(IC)). The authors thank the University Grants Commission (UGC), India, and the Israel Science Foundation (Grant No. 2751/16) for funding. The first author also expresses gratitude to the Directorate of Environment and Climate Change (DoECC), Govt. of Kerala for providing Research Fellowship (DoECC/D1/2357/2015(1)).
Funding
The work forms part of an Indo-Israel (UGC-ISF) joint 3 year’s major research program (F. No. 6–7/2017(IC)). The authors are thankful to the University Grants Commission (UGC), India, and the Israel Science Foundation (Grant No. 2751/16) for funding from the Indian and Israeli sides, respectively. The first author also expresses gratitude to the Directorate of Environment and Climate Change (DoECC), Govt. of Kerala, for providing Research Fellowship (DoECC/D1/2357/2015(1)).
Author information
Authors and Affiliations
Contributions
All the authors have contributed to the manuscript in various ways such as problem identification, sampling and analysis, interpretation, writing, editing, and proof reading. Vishnu Sagar M.K: Methodology, field work, sample analysis, draft writing. Sabu Joseph: Conceptualization, co-ordinated sampling, editing. Arunkumar P.S: Field work, sample analysis. Sheela A.M.: Co-ordinated sampling, writing and editing. Andrea Ghermandi: Writing and editing. Andrea Ghermandi: Writing and editing.
Corresponding author
Ethics declarations
Ethical approval
The authors declare that there is no experiment included in the manuscript which requires ethics approval.
Consent for publication
All the authors have given their consent for publication and no data of any individual person included in the manuscript.
Consent to participate
All the authors have given their consent for publication in this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
M.K, V.S., Joseph, S., P.S, A. et al. A coastal Ramsar site on transition to hypoxia and tracking pollution sources: a case study of south-west coast of India. Environ Monit Assess 195, 45 (2023). https://doi.org/10.1007/s10661-022-10602-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10602-x