Abstract
Microplastics (MPs) pollution is an emerging global environmental concern. Considering the high fraction of time people spend indoors, the human population can be directly exposed to this contamination through indoor dust. This preliminary study evaluates MPs’ abundance and human health risk assessment in the deposited indoor dust. A total of forty dust samples (n = 20) were collected from homes in two different cities (Pakistan) in steel mesh pouches using the vacuum cleaner. The identification and quantification of MPs were conducted with a stereo microscope, whereas the qualitative assessment was performed with Fourier transform infrared spectroscopy (FTIR). The US EPA parameters to calculate the human health risk assessment were used to determine MPs’ risk per-day/month/year. Overall, microfibers were the dominant category, followed by microfilms, micro-fragments, and nurdles. The chemical categorization of MPs was revealed as polyester, polyethylene, copolymers of polypropylene, and polyurethane. In Lahore, an average abundance of 241.45 (items/m2) MPs were observed compared to Sahiwal, with 162.1 (items/m2). More than 90% of the identified MPs were microfibers, with higher detection frequency and abundance in Lahore than Sahiwal. The human health risk assessment revealed high exposure risk because of indoor MPs. Moreover, toddlers were more vulnerable as compared to adults at both low and high exposure risk scenarios. There is an imminent need to conduct in-depth risk assessment focusing on the respirable fraction of MPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics are ubiquitous in the environment. In the recent past, the global production scale of plastics has surged from 1.5 to 335 million tonnes (Garside, 2019). More than 80% produced plastics belong to six categories: polyethylene, polypropylene, polyvinyl chloride, polyurethane, PE terephthalate, and polystyrene (PlasticsEurope, 2016). Although the environmental awareness has increased the recycling rate of plastic waste, still the remaining proportion are released into the environment either through direct degradation or as a by-product of various inefficient waste treatment procedures (Liu et al., 2018). Generally, the plastic fragments are categorized according to their size as macro, meso, micro, and nano-plastics (˃ 2 cm, 5 mm, − 2 cm, < 5 mm, and < 1 μm, respectively) (Horton et al., 2017). Among all the plastic fragments, microplastics (MPs) got serious attention because of their lethal impacts reported in various studies on marine, freshwater lakes, and rivers’ organisms (Al-Thawadi, 2020; Bellasi et al., 2020; Thompson, 2015).
Since 1970, researchers have been sporadically reporting the presence of MPs; however, a pioneering study led by Richard Thompson effectively initiated the in-depth research related to MPs (Thompson, 2004). As the physical, chemical, and biological processes in terrestrial and freshwater environments are different from those in the oceans, it is important to separately investigate the profile distribution, spatio-temporal trends, and the effects of MPs in those media (Rochman, 2018). So far only a few studies have been carried out regarding the birds, soil, dust, and air (Zubris & Richards, 2005; Rillig, 2012; Abbasi et al., 2017; Huerta et al., 2017; Kole et al., 2017; Li et al., 2018a, b, c; Liu et al., 2019a, b; Zhang et al., 2020a, b), which demands further investigation in this area (Hurley & Nizzetto, 2018).
Indoor environment is a potential source of MPs. It poses high risk to humans (Prata, 2018), because in a modern world, 90% of people in the urban region are living a sedentary lifestyle and spend a large span of their time remained indoors (Lucattini et al., 2018). Since industrialization, the design of houses and buildings have advanced massively. Now, many synthetic polymers and chemicals are being used for interior attractiveness, efficiency, and cost-effectiveness (Pluschke & Schleibinger, 2018). Humans could be exposed to MPs via (1) ingestion, (2) inhalation, and (3) dermal exposure. Among them ingestion and inhalation are of major concern. MPs can adversely affect human health through direct exposure or indirectly by carrying other pollutants such as phthalates and bisphenol A (Liu et al., 2020).
There are two main sources of MPs: (1) primary sources and (2) secondary sources. Primary MPs are those that manufacturers produce intentionally for different purposes such as microbeads (cosmetics) and microfibers (textile). These MPs are released from the utilization of such products, whereas the secondary MPs are released from the degradation of large plastic particles because of photochemical, chemical, and mechanical processes (Galafassi et al., 2019). In the recent past, research studies by Prata et al. (2018), Catarino et al. (2018), Liu et al. (2019a, b), Cox et al. (2019), Zhang et al. (2020a, b), and Soltani et al. (2021) studied the household dust as potential source of microplastics. These studies have reported indoor environment as a potential exposure pathway for humans. However, it is very important not to generalize the study results, and region-specific research investigations should be conducted to have better understanding of the microplastics hazard.
So far, in Pakistan, only few studies have been conducted in different environmental compartments such as in soil (Rafique et al., 2020), surface water (Irfan et al., 2020a, b), and sediments (Irfan et al., 2020a, b). Previously, to the best of our knowledge, no study was conducted regarding the presence of MPs in the indoor environment of the studied locations. Hence, this is the pioneer study to investigate the contamination of MPs in the urban indoor settings of Pakistan, using floor dust as an investigation matrix.
Materials and methods
Study area and sampling method
In March 2020, a sampling campaign was carried out in Punjab (Lahore and Sahiwal), Pakistan, as shown in Fig. 1. The sampling locations were categorized as urban (Lahore) and rural (Sahiwal). Lahore is a metropolitan city and the capital of Punjab province, which is situated between 31°32′59″N and 74°20′37″E. It is the second most populous city of Pakistan, with a total area of 1,772 km2, whereas Sahiwal lies between 30°39′40″N and 73°6′30″E, covering an average area of 60 km2. It is ranked as the 21st most populous region of Pakistan and is present 180 km away from Lahore (PCTPL, 2020).
Study area map showing the sampling cities (Punjab Maps, 2021)
A total of forty samples (n = 40) were collected, with twenty samples (n = 20) from each city. Several following factors were considered during the selection of residential sites and collection of the deposited dust. As1) for homogeneity, the single-story houses with an average of five occupants were selected, 2) dusty floors were sampled in the morning before cleaning, 3) dust sampling was carried out in the living rooms (Wang et al., 2013; Aslam et al., 2021). Figure S1 illustrates the sampling gears used for the collection of floor dust. The filter bag of portable vacuum cleaner (Electrolux) was replaced with a tailored steel mesh pouch (0.026 μm) used to avoid contamination, as polyvinyl or cotton bags could be direct sources of microplastics. A representative composite dust sample was collected by vacuuming a 1 m2 area from three different points in one house (Cao et al., 2015). The floor was vacuumed for 2 min per 1m2 area (Cristale et al., 2018). After vacuuming, the steel mesh pouch was carefully removed and wrapped in an aluminum foil to avoid (1) the loss of dust and (2) contamination and kept inside the pre-labeled zipped bags. All sampling gears were cleaned with methanol before each dust sample collection (Soltani et al., 2021). Moreover, while not using, all sampling gears were adequately covered with the aluminum foil. The collected samples were transported to the Environmental Toxicology Laboratory, University of the Punjab, Lahore, Pakistan, and stored at 4° C before further analysis.
Extraction and cleanup
Dust sample preparation, extraction, and cleanup
The dust samples were extracted by a previously mentioned oxidative extraction approach, with some modifications (Abbasi et al., 2019; Liu et al., 2019a, b). A schematic visual overview is provided in Fig. 2. Briefly, manual sorting of the indoor dust samples was carried out with a tweezer to remove hair and larger debris. Dust aliquot was added in a 250-mL beaker and digested with 50 mL of 30% H2O2 for 2 days (until the bubbling ends). For residual H2O2 removal, membrane-filtration assembly coupled with MCE (mixed cellulose ester) filter paper (0.45 μm) was used, followed by washing with distilled water. Next, the filter paper was removed and placed in a pre-washed beaker for density separation, with NaCl saturated solution (density 1.20 g/cm3). The NaCl salt was used for cost-effectiveness and environmental friendliness.
First, 100 mL saturated solution was added to the beaker, followed by magnetic stirring. That would allow dust to detach from the filter paper and mix with the brine solution. Next, 10-min magnetic stirring was followed by pouring the mixture into a density separation flask. After settling for 120 min, the top fraction was homogenized through centrifugation for 6 min at 2000 rpm. Finally, the homogenized solution was vacuum filtered. Subsequently, the gridded filter paper was dried in a desiccator before the examination, using the stereo microscope and FTIR. To calculate the density, Eq. (1) was used (Quinn et al., 2017).
Quality control and quality assurance (QA/QC)
Strict quality control measures were followed throughout the analysis. The pre-cleaned glassware was sonicated and placed in an oven at 80 °C for 6 h before use. A laboratory blank was tested with each batch of sample extraction (10 samples) (Table S2). For the laboratory blank, a pre-cleaned Petri dish filled with distilled water was placed in the middle of the working area and later was subjected to all the steps of sample extraction with other dust samples. The final results were blank corrected. The graded filter papers were marked into four equal quarters for error-free counting. Moreover, it was made sure that the Petri dishes were covered with aluminum foil while not counting. The disk of the FTIR instrument was cleaned with methanol wipes before every sample analysis to avoid contamination and error in the reading.
Microscopic analysis of MPs
The residential dust samples were identified using a stereo microscope (Euromex – StereoBlue – SB.1402) with head (magnification × 20/ × 40) and zoom objective (magnification × 7 to × 45). The criteria opted to identify the anthropogenic MPs is described elsewhere in detail (Dris et al., 2015). Briefly, while identifying it was assessed that the fibers should be equally thick in their entire length and straight. Additionally, careful consideration was made to distinguish the synthetic fibers from the cellular and organic structures. Figure 3 illustrates the collage of different types of MPs identified during the microscopic screening of the dust samples. The identified MPs were marked on the pre-prepared MPs’ datasheet against the different types and colors mentioned in the literature. The template of the datasheet is given in the SI. Overall, the microfibers were detected in a more significant proportion in all different colors. Comparatively, the other MPs such as films/sheets, fragments, foams, and nurdles were primarily detected in transparent categories in somewhat less proportion.
FTIR examination of MPs
Different chemical compositions of MPs could have different toxic effects on the environment. Therefore, it is essential to categorize them according to their chemical nature (Mattsson et al., 2017; Alimi et al., 2021). Furthermore, the accuracy of visual assessment results could fluctuate depending on several different factors, such as human and instrumental errors (Catarino et al., 2018). Therefore, to further elucidate the visual assessment results, a Fourier transform infrared (FT-IR) coupled with an ATR (attenuated total reflectance) add-on was used for chemical classification (Model: Agilent Cary 630 FTIR) (Dris et al., 2017). Microplastics were analyzed on a wavelength range of (IR = 650 to 4000 cm−1) (Zhang et al., 2020a, b). It is a widely used instrumental approach to determine the chemical categorization of microplastics with a 50-µm size detection limit (Dris et al. 2016; Shim et al. 2017; Soltani et al., 2021).
For analysis, because of the large proportion of fibers, a sub-sample of fibers = 15 was randomly selected from all identified colors (Soltani et al., 2021) to differentiate natural from synthetic fibers (Dris et al., 2016) (Fig. 4). Next, sheets, fragments, and nurdles were analyzed. The sample spectra were compared with the already in-built Agilent MicroLab FTIR Software Library of more than 9000 spectra. Moreover, as previously practiced by Soltani et al. (2021), to comply with the QA/QC standards, automatic outcome of the software was only considered when the spectrum match was > 70%; otherwise, manual integration of the resultant spectrum was performed with the given library. The graphical outcome of Agilent MicroLab Spectra Software is shown in Fig. 5.
Human health risk assessment
For MPs, dust inhalation and ingestion are considered two main exposure routes (Dris et al., 2015; Liu et al., 2019a, b). Indoor dust can provide a reasonable estimation for the contaminant’s exposure, including MPs (Wright & Kelly, 2017). The human exposure of MPs (per-day and per-year) was calculated for the general public, including toddlers and adults, using Eq. (2) (Guo & Kannan, 2011).
where EDI is an estimated daily intake, MA is the microplastics abundance in the indoor dust (fibers/m2/day), F is the fraction of daily time spent indoors, IR is the inhalation and ingestion rates, and M represents the body weight. The detail of adopted assumed values for F, IR, and M is given elsewhere (EPA, 2011, 2014, 2017). Moreover, based on the daily fraction of time spent indoors, the per-month and per-years days are adjusted for both adults and toddlers. A brief description of parameters is given in S3.
Data analysis
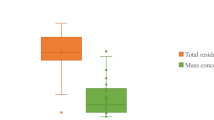
R studio and Microsoft Excel were used for the statistical data analysis. One-way ANOVA was performed to validate the variation between two data sets of Lahore and Sahiwal. Whisker boxplots were used to understand the distribution of microfibers in Lahore and Sahiwal. Furthermore, QGIS 3.2.0 was used to show the spatial distribution pattern of MPs.
Results and discussion
Table 1 depicts the descriptive statistics for the sampling stations Lahore and Sahiwal. For each sampling site, detailed Tables 2 and 3 demonstrate the sample code, mesh pore size, color, shape, and abundance of MPs in Lahore and Sahiwal, respectively. Figure 6 illustrates the geographical distribution of MPs in the study areas, with a detailed table of coordinates given in Table S3. MPs were identified in all the studied samples, showing indoor dust as a significant MPs source. The result of the ANOVA test was significant (p value < 0.05) for the difference between the data sets of Lahore and Sahiwal.
Lahore
Overall, in Lahore the mean abundance of different microplastics was as fibers (234.05 ± 148.41), films/sheets (3.20 ± 6.45), fragments (0.75 ± 2.24), and nurdles (3.45 ± 6.67). The range of fibers, sheets, fragments, and nurdles was 48–636, ND–27, ND–15, and ND–20, respectively. The highest MPs were detected at the sampling site 14 with 638 items/m2 dust, whereas the least MPs were reported at the sampling site 10, with 48 items/m2 dust.
Sahiwal
Largely, in Sahiwal the average abundance of MPs was observed in the following order as fibers (159.02 ± 79.65) > films/sheets (0.95 ± 1.39) > fragments (0.10 ± 0.31) > nurdles (2.00 ± 4.57). The range of fibers, sheets, fragments, and nurdles was 29–341, ND–5, ND–1, and ND–17, respectively. The highest and lowest MPs were detected at the sampling site 5 and 8, with 344 items/m2 and 32 items/m2 of the dust, respectively.
Abundance and profile distribution of MPs
Overall, the geographical distribution of detected MPs (fibers, films, fragments, and nurdles) showed higher abundance in the urban region of Lahore as compared to the rural area of Sahiwal (Fig. 6). In both Sahiwal and Lahore, the microfibers were accounted for the highest proportion and made up approximately 90% of all the different types of MPs detected (Fig. 7). The detection frequency of microfibers in Lahore was higher (180 to 350 items/m2) than Sahiwal (100–150 items/m2). The second most frequent category of MPs was the micro-films/sheets, with average percentage composition of 2.5%, followed by nurdles and fragments. There is clear evidence from the results of two different cities of Punjab, Pakistan, that MPs are prevalent in the indoor settings, with possible sources from clothes, furniture, curtains, sofas, and quilt (Dris et al., 2017; Zhang et al., 2020b). Previously, Rafique et al. (2020) studied the distribution of MPs in the outdoor soil of Lahore, Pakistan. Comparatively, the high proportion of microfibers in the present study is consistent with the previous findings. However, in the current studied areas, it has been observed that the indoor sources of MPs were imperative to determine the distribution of MPs in the residential settings, which resulted in a very high proportion of the microfibers.
Overall, the urban region of Punjab (Lahore) exhibited 67.14% more MPs than the rural area (Sahiwal). Previously, Abbasi et al. (2017) and Zhang et al. (2020a, b) reported similar results in Iran and 12 different countries of the world, respectively, where they found that the abundance of MPs was more significant in the urban region as compared to the rural states. This difference could be attributed to the lifestyle of residents as it had been observed that the residents of Lahore were living a more lavish lifestyle and the modern interior was common in the houses as compared to the inhabitants of Sahiwal, which were living a relatively simple and modest lifestyle. Moreover, we observed a high density of shops, markets, traffic load, and industrial activity in the metropolitan area of Lahore as compared to Sahiwal, where there was more greenery and less traffic load.
Comparatively, in the present study, for Lahore and Sahiwal, the per-day deposition of MPs was lower as compared to the deposition rate reported by Daris et al. (2017) in Paris’ apartments, which was 1600–11,000 particles/day/m2. Similarly, the observed abundance of MPs in settled dust was many folds lower to the research study conducted by Zhang et al. (2020a, b) in China that reported 99,000 particles/day/m2 in the university dormitory. This difference could be attributed to geographical difference, lifestyle, and methodology. Moreover, as emphasized by Ageel et al. (2022), in case of microplastics, there is a huge hindrance in comparison because of varying methodologies and units of MPs.
Color distribution of MPs
Traditionally, microplastic fibers are classified according to their color distribution (Dris et al., 2017; Zhang et al., 2020a, b; Soltani et al., 2021). In the present study, the microfibers were classified into seven categories: white/transparent, red/pink, blue/purple, yellow/brown, green, black/gray, and orange. Overall, the color distribution of the identified MPs is given in Fig. 4. In Lahore, the average percentage composition of different colors of microfibers was white/transparent (43.97%), red/pink (19.96%), blue/purple (14.65%), yellow/brown (8.59%), green (3.92%), black/gray (6.30%), and orange (2.62%), whereas in Sahiwal, the color distribution was found as white/transparent (39.94%), red/pink (20.54%), blue/purple (19.69%), yellow/brown (9.61%), green (5.12%), black/gray (2.28%), and orange (2.82%). White/transparent MPs were found in the highest proportion among the seven color classes. These results are consistent with previous findings, which reported white/transparent microfibers as the most abundant in the indoor environment (Abbasi et al., 2019; Narmadha et al., 2020; Yukioka et al., 2020; Zhang et al., 2020a, b). Additionally, in the present study, red/pink and blue/purple fibers were also found.
This color variation could be attributed to the washing/drying laundry (Dris et al., 2017) and sewing the cloths in indoor settings. Comparatively, black and gray MPs were abundant in Lahore as compared to Sahiwal. It could be because Lahore is a commercial hub, and the chances of drift in MPs from the wear and tear of vehicle tires are higher (Wang et al., 2019). In the past, research studies from the urban regions in Shanghai and Sydney also found black color fibers as the most abundant (Liu et al., 2019a, b; Soltani et al., 2021).
Chemical categorization of MPs
According to the FTIR spectroscopy results, overall, the chemically most abundant microfibers were polyester followed by polyethylene-terephthalate (PET), a copolymer of polypropylene (ethylene-polypropylene), polyethylene, and polyurethane. These results are consistent with commonly distributed types of MPs. According to the literature, polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), polyurethane (PUR), polyvinyl chloride (PVC), and polycarbonate (PC) are the most widely manufactured MPs (Li et al., 2016). Among microfibers, approximately 70% had a natural origin, mainly from cotton in the form of cellulose. Around 20% of microfibers were anthropogenic with a petrochemical origin, whereas 10% were unidentified. The results of the present study indicate textile products as the dominant source of microfibers in the indoor environment; Dris et al. (2016) categorized microfibers as completely natural (cellulose), synthetic (polyester, polyethylene-terephthalate, and polyurethane), or a mixture of both. These findings are also supported by the fact that the textile industry is very active in Pakistan and uses both natural and synthetic fibers, especially polyester (Abbas & Halog, 2021). Polyethylene sheets, fiberglass (fiber-reinforced plastic) fragments (Rodrigues et al., 2019), and polyurethane nurdles were also observed in minor abundance. Some other sources of microplastics could be interior paints and by-products resulting from the weathering of plastic materials (Verschoor et al., 2017). According to an estimate, in Pakistan, 55 billion polyethylene shopping bags are being used every year (The News, 2018).
Risk via inhalation and ingestion
The two imperative exposure routes to determine the human health risk assessment via indoor dust contaminants are (1) inhalation and (2) ingestion (Kim et al., 2018; Wright & Kelly, 2017). In the current study, the non-dietary exposure was estimated for adults and toddlers, considering the median concentration of microfibers. A detailed table of results is given in S3. Here, only particles/year values have been discussed. In Lahore, for adults and toddlers, the inhalation risk was 9402.27 and 58,184.38, whereas the ingestion risk was 17,629.25 and 290,921.88, respectively. In Sahiwal, for adults, the inhalation and ingestion risks were observed as 7619.50 and 14,286.56, which was 47,152.01 and 235,760.07 for toddlers, respectively. Toddlers are severely exposed to MPs compared to adults because of their low body weight and frequent hand-to-mouth playing activities.
Concerning global comparison, per-annum exposure of MPs through inhalation was found lower for adults but higher for toddlers compared to what was reported by Cox et al. (2019) for American children and adults as 37,781–55,099 particles/year, respectively. Catarino et al. (2018) conducted a study in the UK, which reported risk as 13,731–68,415 particles/year for adults and toddlers, respectively. Comparatively, in the current research study, the risk was found lower for both adults and toddlers. Apart from the geographical variation, there could be other reasons for this difference, such as lifestyle and the sampling methodology. It is pertinent to mention here that this is not a true comparison because, in literature, there have been several different models to calculate the human health risk assessment of MPs, which are not identical to each other.
Conclusion
The results revealed a high abundance and prevalence of microfibers in Pakistan’s residential indoor dust samples. They were found in various colors, with transparent/white being the dominant one. Approximately 70% of microfibers showed natural origin (cellulose), whereas 20% were recognized as synthetic fibers. Comparatively, other categories of MPs (films, fragments, and nurdles) were observed in fewer proportions in both the studied sites. The results showed a greater abundance and detection frequency of MPs in the urban region (Lahore) than in the rural area (Sahiwal). It was observed that in the current study, the significant contributing sources of MPs were textile/clothing, house interior (curtains, sofas, paint), toys, shoes, lifestyle, and weathering of the plastic products. However, to a lesser extent, the abundance of MPs is also influenced by ambient activities such as industries, traffic load, and drift-in of MPs contaminants. There is no inclusive method to study the human health risk assessment, making comparison very difficult. Therefore, in the future, it is imperative to work on a human health risk assessment model that has more in-depth parameters and could give a global perspective regarding the risk that humans could face because of MPs contamination.
Change history
10 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10661-023-11677-w
References
Abbas, S., & Halog, A. (2021). Analysis of Pakistani textile industry: Recommendations towards circular and sustainable production. Circular economy (pp. 77–111). Springer Singapore. https://doi.org/10.1007/978-981-16-3698-1_3
Abbasi, S., Keshavarzi, B., Moore, F., Delshab, H., Soltani, N., & Sorooshian, A. (2017). Investigation of microrubbers, microplastics and heavy metals in street dust: A study in Bushehr city, Iran. Environmental Earth Sciences, 76(23). https://doi.org/10.1007/s12665-017-7137-0
Abbasi, S., Keshavarzi, B., Moore, F., Turner, A., Kelly, F., Dominguez, A., & Jaafarzadeh, N. (2019). Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environmental Pollution, 244, 153–164. https://doi.org/10.1016/j.envpol.2018.10.039
Ageel, H. K., Harrad, S., & Abdallah, M. A. (2022). Occurrence, human exposure, and risk of microplastics in the indoor environment. Environmental Science-Processes & Impacts, 24(1), 17–31. https://doi.org/10.1039/D1EM00301A
Alimi, O. S., Fadare, O. O., & Okoffo, E. D. (2021). Microplastics in African ecosystems: current knowledge, abundance, associated contaminants, techniques, and research needs. Science of the Total Environment, 755, 142422.
Al-Thawadi, S. (2020). Microplastics and nanoplastics in aquatic environments: Challenges and threats to aquatic organisms. Arabian Journal for Science and Engineering, 45(6), 4419–4440.
Aslam, I., Mumtaz, M., Qadir, A., Jamil, N., Baqar, M., Mahmood, A., ... & Zhang, G. (2021). Organochlorine pesticides (OCPs) in air‐conditioner filter dust of indoor urban setting: Implication for health risk in a developing country. Indoor Air, 31(3), 807–817.
Bellasi, A., Binda, G., Pozzi, A., Galafassi, S., Volta, P., & Bettinetti, R. (2020). Microplastic contamination in freshwater environments: A review, focusing on interactions with sediments and benthic organisms. Environments, 7(4), 30. https://doi.org/10.3390/environments7040030
Cao, Z., Xu, F., Li, W., Sun, J., Shen, M., Su, X., & Covaci, A. (2015). Seasonal and particle size-dependent variations of hexabromocyclododecanes in settled dust: Implications for sampling. Environmental Science & Technology, 49(18), 11151–11157.
Catarino, A., Macchia, V., Sanderson, W., Thompson, R., & Henry, T. (2018). Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environmental Pollution, 237, 675–684. https://doi.org/10.1016/j.envpol.2018.02.069
Cox, K. D., Covernton, G. A., Davies, H. L., Dower, J. F., Juanes, F., & Dudas, S. E. (2019). Human consumption of microplastics. Environmental Science & Technology, 53(12), 7068–7074.
Cristale, J., Aragão Belé, T. G., Lacorte, S., de Marchi, R., & Rosa, M. (2018). Occurrence and human exposure to brominated and organophosphorus flame retardants via indoor dust in a Brazilian city. Environmental Pollution, 237, 695–703. https://doi.org/10.1016/j.envpol.2017.10.110
Dris, R., Gasperi, J., Mirande, C., Mandin, C., Guerrouache, M., Langlois, V., & Tassin, B. (2017). A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environmental Pollution, 221, 453–458. https://doi.org/10.1016/j.envpol.2016.12.013
Dris, R., Gasperi, J., Rocher, V., Saad, M., Renault, N., & Tassin, B. (2015). Microplastic contamination in an urban area: A case study in Greater Paris. Environmental Chemistry, 12(5), 592. https://doi.org/10.1071/en14167
Dris, R., Gasperi, J., Saad, M., Mirande, C., & Tassin, B. (2016). Synthetic fibers in atmospheric fallout: a source of microplastics in the environment?. Marine Pollution Bulletin, 104(1–2), 290–293.
EPA. (2011). Exposure Factors Handbook. 2011. Edition (Final Report). Washington, DC: U.S. Environmental Protection Agency, EPA/600/R-09/052F.
EPA. (2014). Child-specific exposure scenario examples (The final document). US. Washington, DC: Environmental Protection Agency, EPA/600/R-14/217F.
EPA. (2017). Update for Chapter 5 of the Exposure Factors Handbook (Soil and Dust ingestion). Washington, DC: US. Environmental Protection Agency, EPA/600/R-17/384F.
Guo, Y., & Kannan, K. (2011). Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environmental Science and Technology, 45, 3788–3794.
Galafassi, S., Nizzetto, L., & Volta, P. (2019). Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Science of the Total Environment, 693, 133499. https://doi.org/10.1016/j.scitotenv.2019.07.305
Garside, M. (2019). Global plastic production | Statista. Retrieved 10 October 2020, from https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/
Horton, A., Svendsen, C., Williams, R., Spurgeon, D., & Lahive, E. (2017). Large microplastic particles in sediments of tributaries of the River Thames, UK – Abundance, sources and methods for effective quantification. Marine Pollution Bulletin, 114(1), 218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Huerta Lwanga, E., Mendoza Vega, J., Ku Quej, V., Chi, J. D. L. A., Sanchez del Cid, L., Chi, C., ... & Geissen, V. (2017). Field evidence for transfer of plastic debris along a terrestrial food chain. Scientific Reports, 7(1), 1–7.
Hurley, R., & Nizzetto, L. (2018). Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Current Opinion in Environmental Science & Health, 1, 6–11. https://doi.org/10.1016/j.coesh.2017.10.006
Irfan, M., Qadir, A., Mumtaz, M., & Ahmad, S. (2020a). An unintended challenge of microplastic pollution in the urban surface water system of Lahore, Pakistan. Environmental Science and Pollution Research, 27(14), 16718–16730. https://doi.org/10.1007/s11356-020-08114-7
Irfan, T., Khalid, S., Taneez, M., & Hashmi, M. (2020b). Plastic driven pollution in Pakistan: The first evidence of environmental exposure to microplastic in sediments and water of Rawal Lake. Environmental Science and Pollution Research, 27(13), 15083–15092. https://doi.org/10.1007/s11356-020-07833-1
Kim, J. S., Lee, H. J., Kim, S. K., & Kim, H. J. (2018). Global pattern of microplastics (MPs) in commercial food-grade salts: Sea salt as an indicator of seawater MP pollution. Environmental Science & Technology, 52(21), 12819–12828.
Kole, P., Löhr, A., Van Belleghem, F., & Ragas, A. (2017). Wear and tear of tyres: A stealthy source of microplastics in the environment. International Journal of Environmental Research and Public Health, 14(10), 1265. https://doi.org/10.3390/ijerph14101265
Lucattini, L., Poma, G., Covaci, A., de Boer, J., Lamoree, M., & Leonards, P. (2018). A review of semi-volatile organic compounds (SVOCs) in the indoor environment: Occurrence in consumer products, indoor air and dust. Chemosphere, 201, 466–482.
Li, H., Ma, L., Lin, L., Ni, Z., Xu, X., Shi, H., et al. (2018a). Microplastics in oysters Saccostrea cucullata along the Pearl River Estuary, China. Environmental Pollution, 236, 619–625. https://doi.org/10.1016/j.envpol.2018.01.083
Li, J., Liu, H., & Paul Chen, J. (2018b). Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Research, 137, 362–374. https://doi.org/10.1016/j.watres.2017.12.056
Li, L., Arnot, J., & Wania, F. (2018c). Towards a systematic understanding of the dynamic fate of polychlorinated biphenyls in indoor, urban and rural environments. Environment International, 117, 57–68. https://doi.org/10.1016/j.envint.2018.04.038
LI, W. C., Tse, H. F., & Fok, L. (2016). Plastic waste in the marine environment: A review of sources, occurrence and effects. Science of the Total Environment, 566, 333–349.
Liu, M., Lu, S., Song, Y., Lei, L., Hu, J., Lv, W., et al. (2018). Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environmental Pollution, 242, 855–862. https://doi.org/10.1016/j.envpol.2018.07.051
Liu, C., Li, J., Zhang, Y., Wang, L., Deng, J., & Gao, Y. et al. (2019a). Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environment International, 128, 116–124. https://doi.org/10.1016/j.envint.2019a.04.024
Liu, K., Wang, X., Fang, T., Xu, P., Zhu, L., & Li, D. (2019b). Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Science of the Total Environment, 675, 462–471. https://doi.org/10.1016/j.scitotenv.2019b.04.110
Liu, P., Zhan, X., Wu, X., Li, J., Wang, H., & Gao, S. (2020). Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere, 242, 125193. https://doi.org/10.1016/j.chemosphere.2019.125193
Mattsson, K., Johnson, E. V., Malmendal, A., Linse, S., Hansson, L.-A., & Cedervall, T. (2017). Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Scientific Reports, 7(1), 11452.
Narmadha, V., Jose, J., Patil, S., Farooqui, M., Srimuruganandam, B., Saravanadevi, S., & Krishnamurthi, K. (2020). Assessment of microplastics in roadside suspended dust from urban and rural environment of Nagpur, India. International Journal of Environmental Research, 14(6), 629–640. https://doi.org/10.1007/s41742-020-00283-0
Pakistan City & Town Population List (PCTPL). (2020). Retrieved October 11, 2020, from https://Tageo.com
PlasticsEurope. (2016). Plastics—The facts 2016. An Analysis of European Plastics Production, Demand and Waste Data. Retrieved October 11, 2021, from https://plasticseurope.org/wp-content/uploads/2021/10/2016-Plastic-the-facts.pdf
Pluschke, P., & Schleibinger, H. (2018). Indoor air pollution (pp. 69–72). Berlin: Springer Berlin Heidelberg.
Prata, J. C. (2018). Airborne microplastics: Consequences to human health? Environmental Pollution, 234, 115–126. https://doi.org/10.1016/j.envpol.2017.11.043
Punjab Maps. (2021). Punjab Maps. Retrieved March 17, 2021, from https://www.freeworldmaps.net/asia/pakistan/punjab/
Quinn, B., Murphy, F., & Ewins, C. (2017). Validation of density separation for the rapid recovery of microplastics from sediment. Analytical Methods, 9(9), 1491–1498. https://doi.org/10.1039/c6ay02542k
Rafique, A., Irfan, M., Mumtaz, M., & Qadir, A. (2020). Spatial distribution of microplastics in soil with context to human activities: A case study from the urban center. Environmental Monitoring And Assessment, 192(11). https://doi.org/10.1007/s10661-020-08641-3
Rodrigues, A., Oliver, D., McCarron, A., & Quilliam, R. (2019). Colonisation of plastic pellets (nurdles) by E. coli at public bathing beaches. Marine Pollution Bulletin, 139, 376–380. https://doi.org/10.1016/j.marpolbul.2019.01.011
Rillig, M. (2012). Microplastic in terrestrial ecosystems and the soil? Environmental Science & Technology, 46(12), 6453–6454. https://doi.org/10.1021/es302011r
Rochman, C. (2018). Microplastics research—from sink to source. Science, 360(6384), 28–29. https://doi.org/10.1126/science.aar7734
Shim, W. J., Hong, S. H., & Eo, S. E. (2017). Identification methods in microplastic analysis: a review. Analytical Methods, 9(9), 1384–1391.
Soltani, N. S., Taylor, M. P., & Wilson, S. P. (2021). Quantification and exposure assessment of microplastics in Australian indoor house dust. Environmental Pollution, 283, 117064.
The News. (2018). 55bn plastic bags used in Pakistan each year, Senate told. [online] Thenews.com.pk. Available at: https://www.thenews.com.pk/print/273837-55bn-plastic-bags-used-in-pakistan-each-year-senate-told. Accessed 16 February 2022.
Thompson, R. (2004). Lost at sea: Where is all the plastic? Science, 304(5672), 838–838. https://doi.org/10.1126/science.1094559
Thompson, R. C. (2015). Microplastics in the marine environment: Sources, consequences and solutions. In Marine anthropogenic litter (pp. 185–200). Springer, Cham.
Verschoor, A., de Poorter, L., ge, R., Kuenen, J., de Valk, E., MSP, & M&V. (2017). Emission of microplastics and potential mitigation measures: Abrasive cleaning agents, paints and tyre wear. https://www.narcis.nl/publication/RecordID/oai:rivm.openrepository.com:10029%2F617930
Wang, T., Zou, X., Li, B., et al. (2019). Preliminary study of the source apportionment and diversity of microplastics: Taking floating microplastics in the South China Sea as an example. Environmental Pollution, 245, 965–974.
Wang, W., Huang, M. J., Wu, F. Y., Kang, Y., Wang, H. S., Cheung, K. C., & Wong, M. H. (2013). Risk assessment of bioaccessible organochlorine pesticides exposure via indoor and outdoor dust. Atmospheric Environment, 77, 525–533.
Wright, S. L., & Kelly, F. J. (2017). Plastic and human health: A micro issue? Environmental Science & Technology, 51(12), 6634–6647.
Yukioka, S., Tanaka, S., Nabetani, Y., Suzuki, Y., Ushijima, T., Fujii, S., et al. (2020). Occurrence and characteristics of microplastics in surface road dust in Kusatsu (Japan), Da Nang (Vietnam), and Kathmandu (Nepal). Environmental Pollution, 256, 113447. https://doi.org/10.1016/j.envpol.2019.113447
Zhang, J., Wang, L., & Kannan, K. (2020a). Microplastics in house dust from 12 countries and associated human exposure. Environment International, 134, 105314. https://doi.org/10.1016/j.envint.2019.105314
Zhang, Q., Zhao, Y., Du, F., Cai, H., Wang, G., & Shi, H. (2020b). Microplastic fallout in different indoor environments. Environmental Science & Technology, 54(11), 6530–6539. https://doi.org/10.1021/acs.est.0c00087
Zubris, K., & Richards, B. (2005). Synthetic fibers as an indicator of land application of sludge. Environmental Pollution, 138(2), 201–211. https://doi.org/10.1016/j.envpol.2005.04.013
Acknowledgements
The authors would like to thank the Higher Education Commission of Pakistan for providing funds under the National Research Program for Universities (NRPU No: 8463/Punjab/ NRPU/R&D/HEC/ 2017) to carry out this extensive research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslam, I., Qadir, A. & Ahmad, S.R. A preliminary assessment of microplastics in indoor dust of a developing country in South Asia. Environ Monit Assess 194, 340 (2022). https://doi.org/10.1007/s10661-022-09928-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-09928-3